The Detection of Vaccine Virus and Protection of a Modified Live, Intranasal, Trivalent Vaccine in Neonatal, Colostrum-Fed Calves with an Experimental Bovine Respiratory Syncytial Virus Challenge
Abstract
1. Introduction
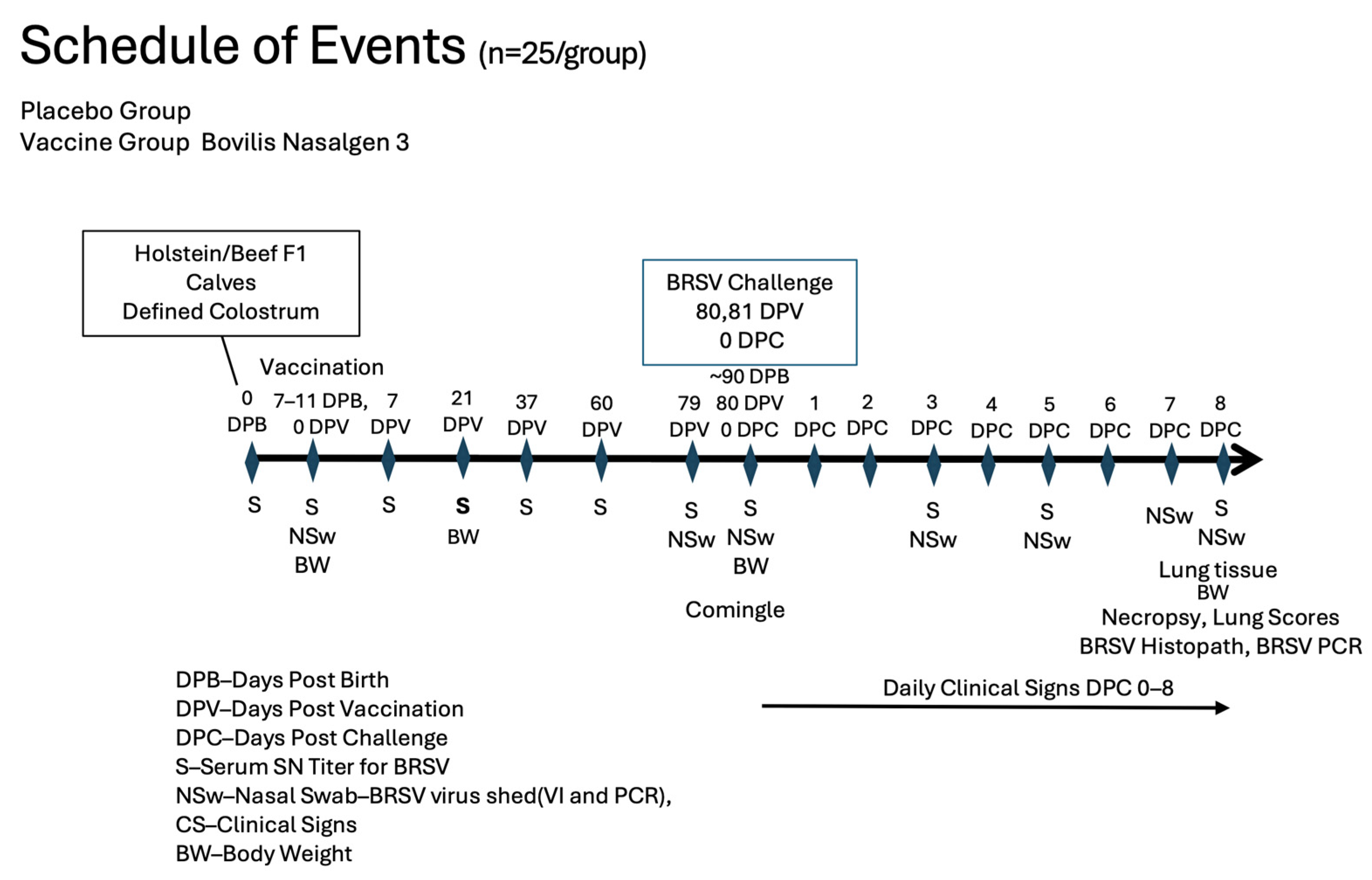
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Processing and Analysis
3. Results
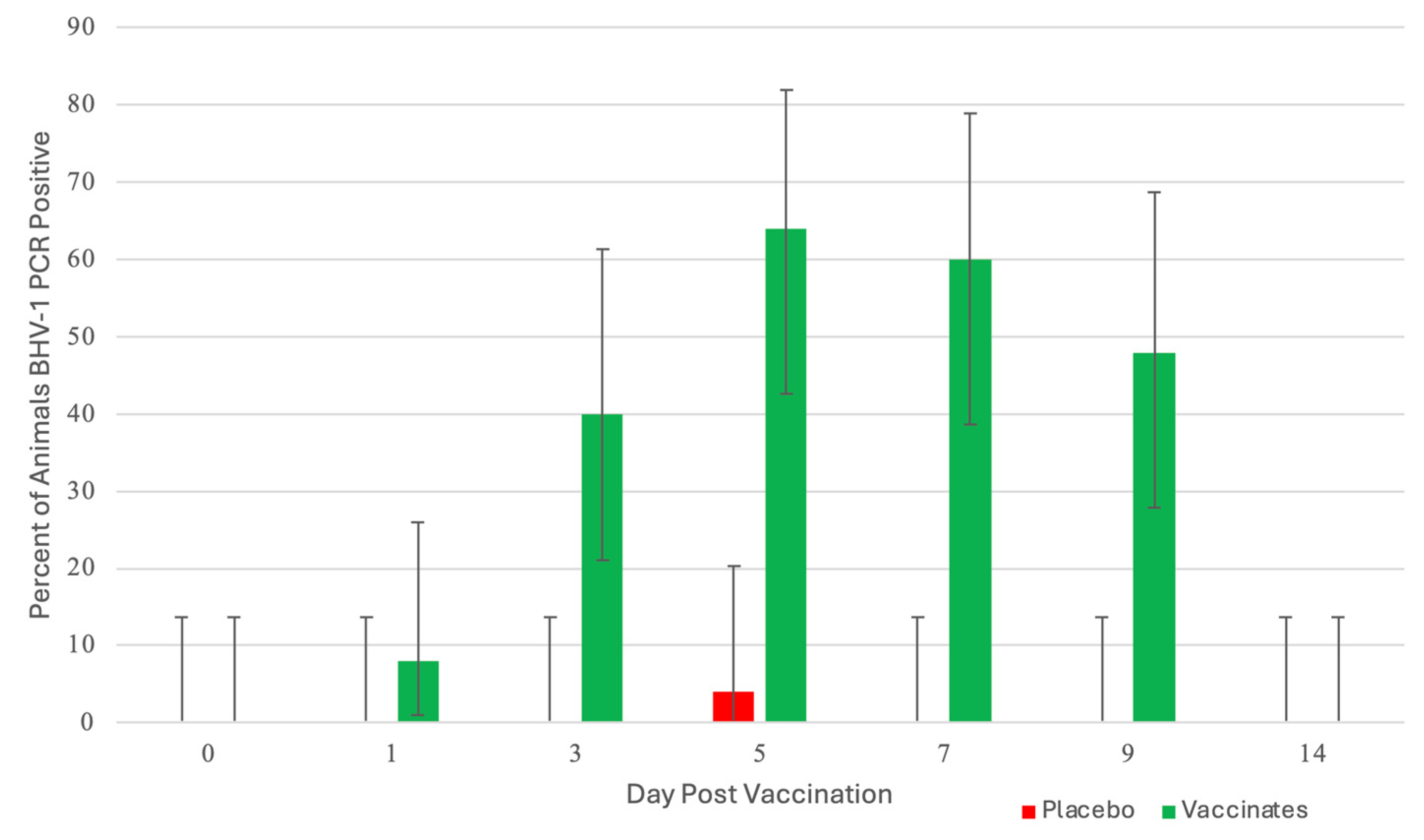
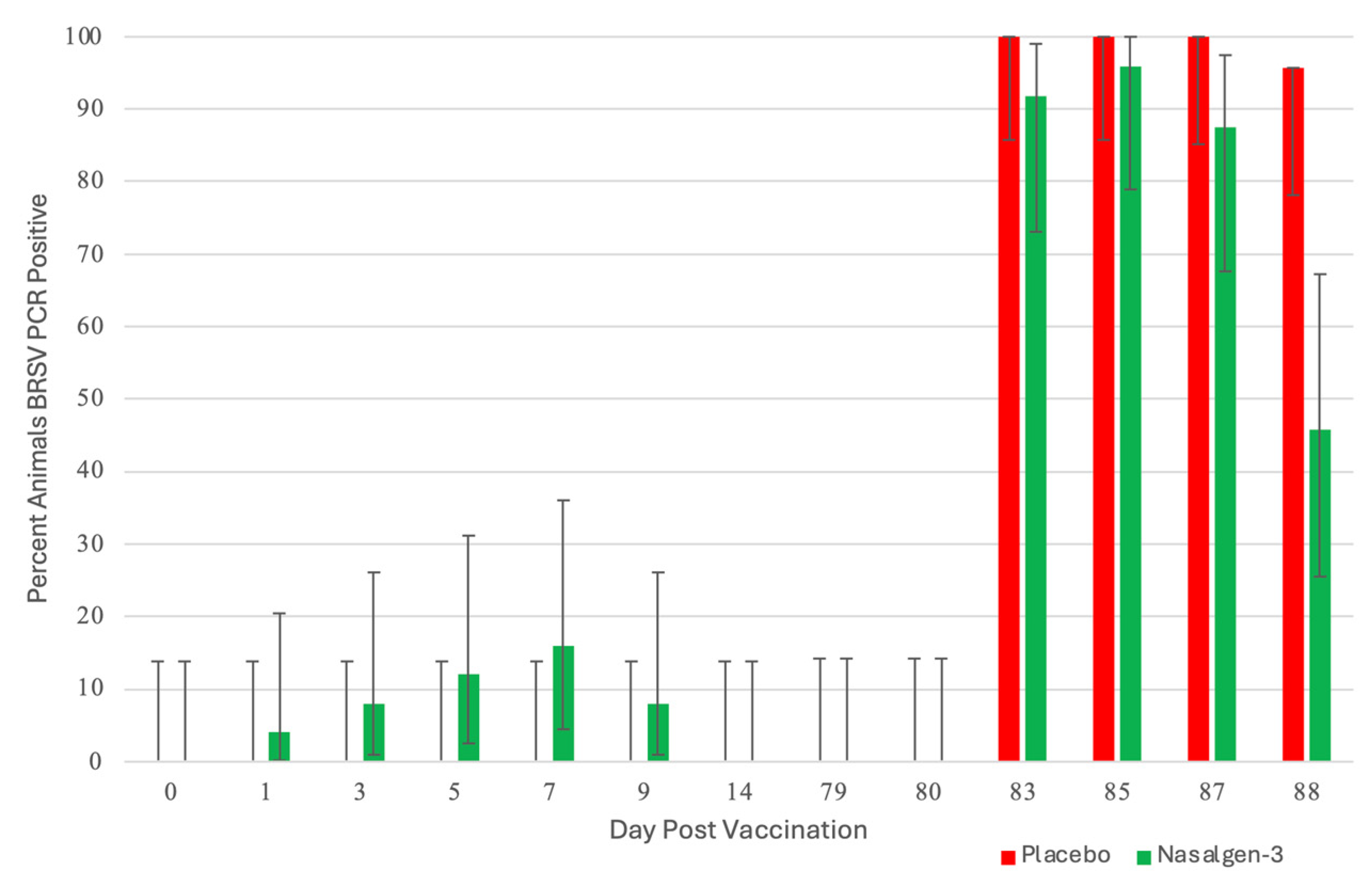
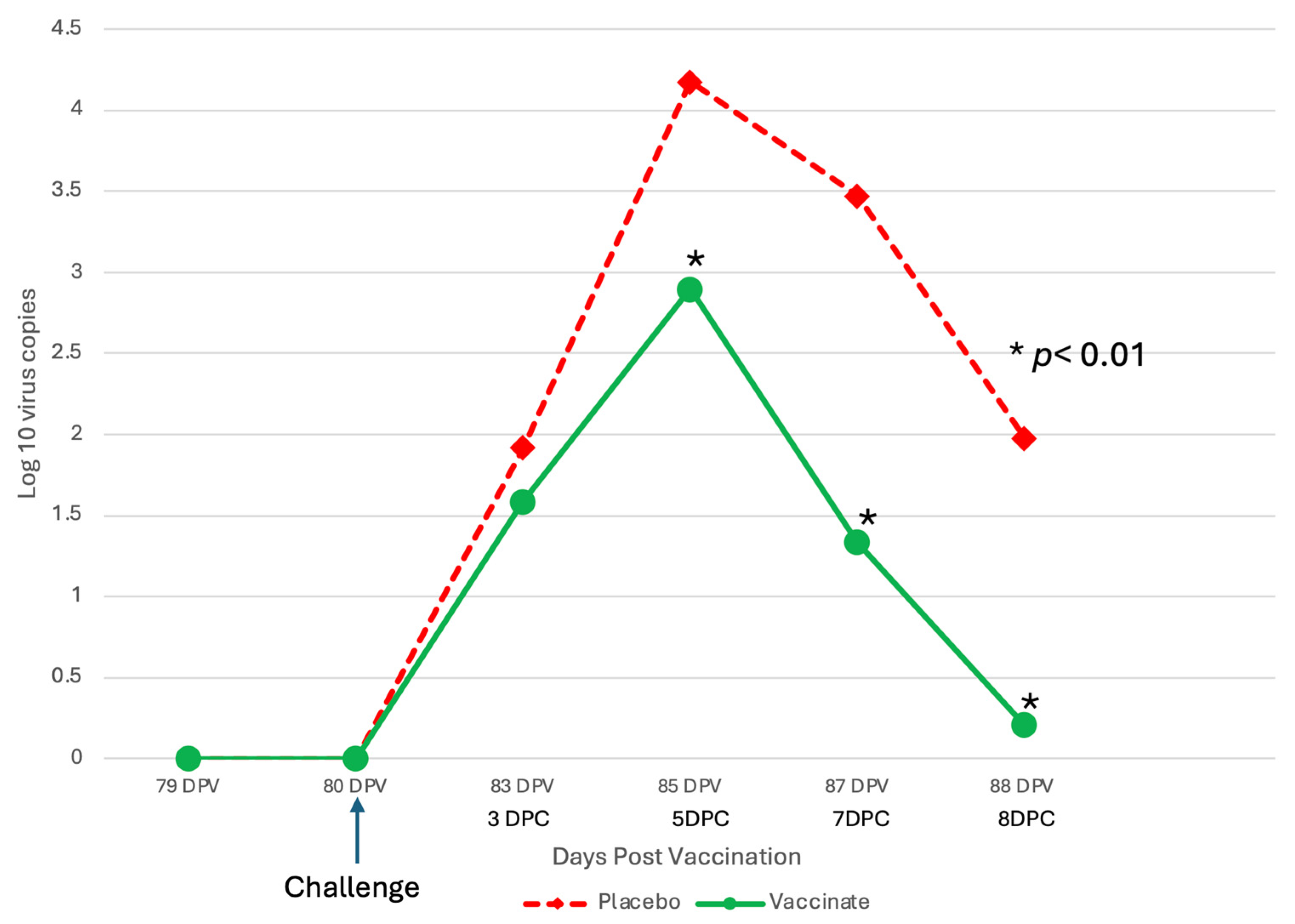
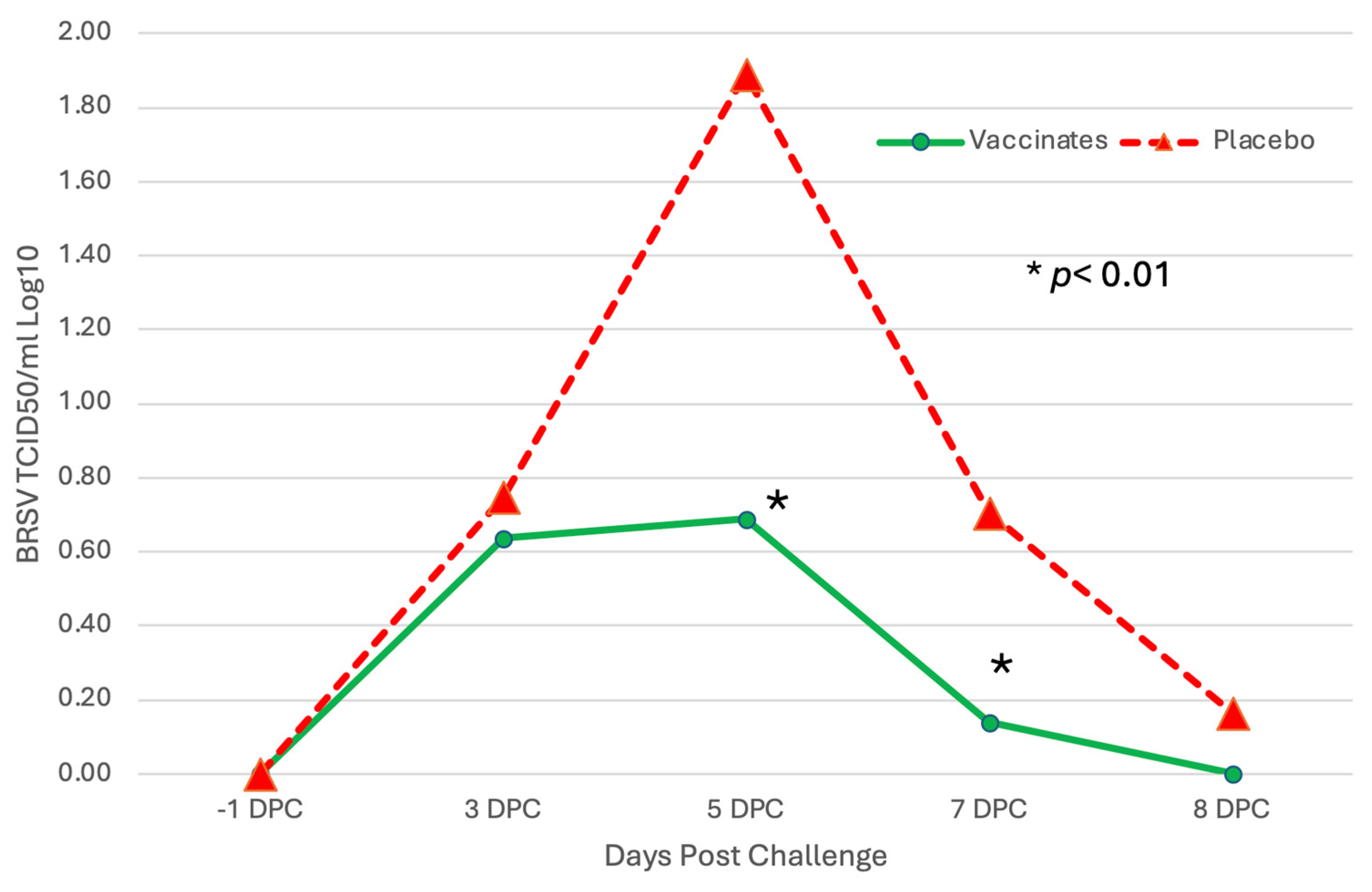
3.1. Nasal Shedding of BHV-1 or BRSV Virus Following Vaccination
3.2. Clinical Signs
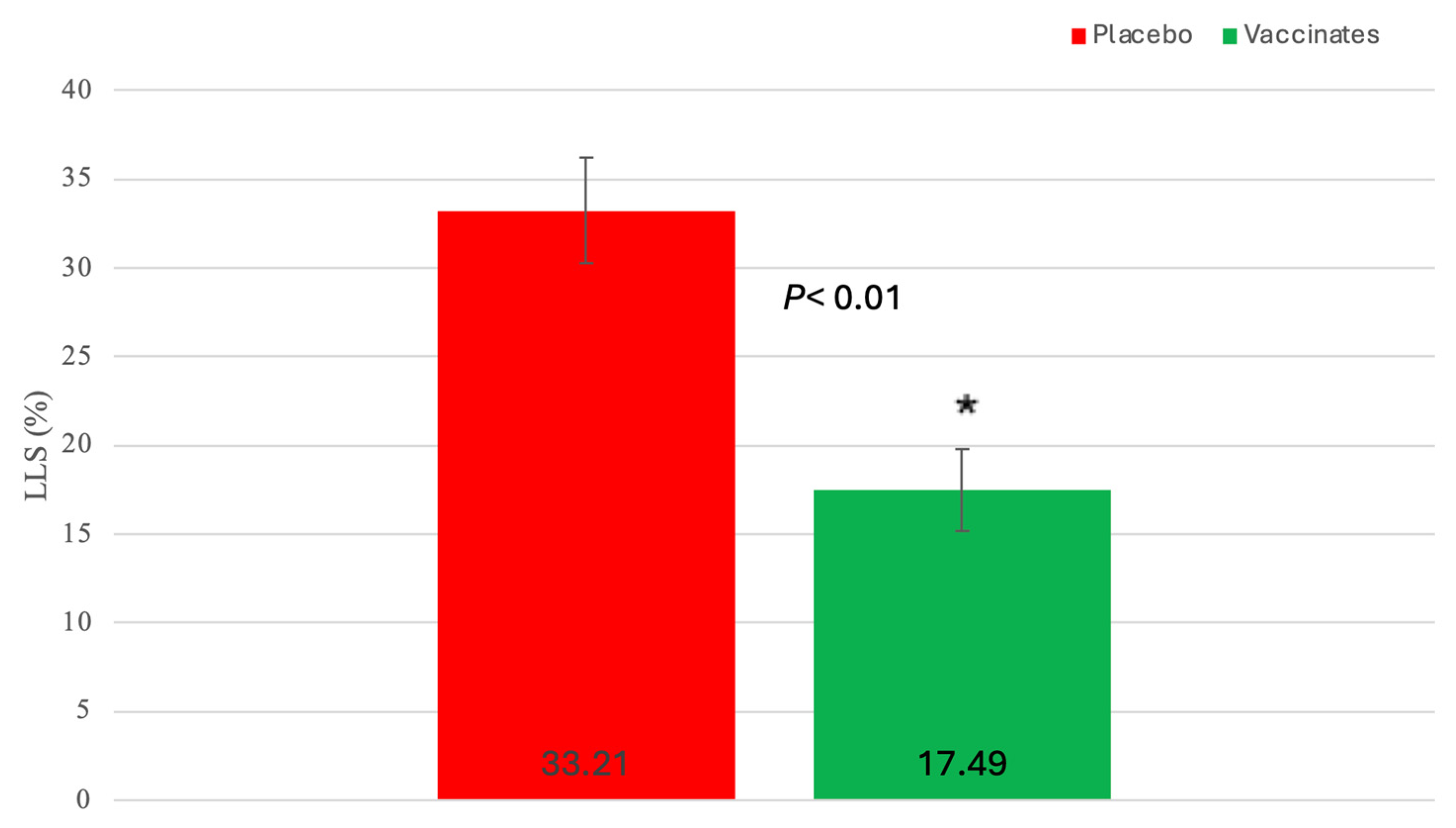
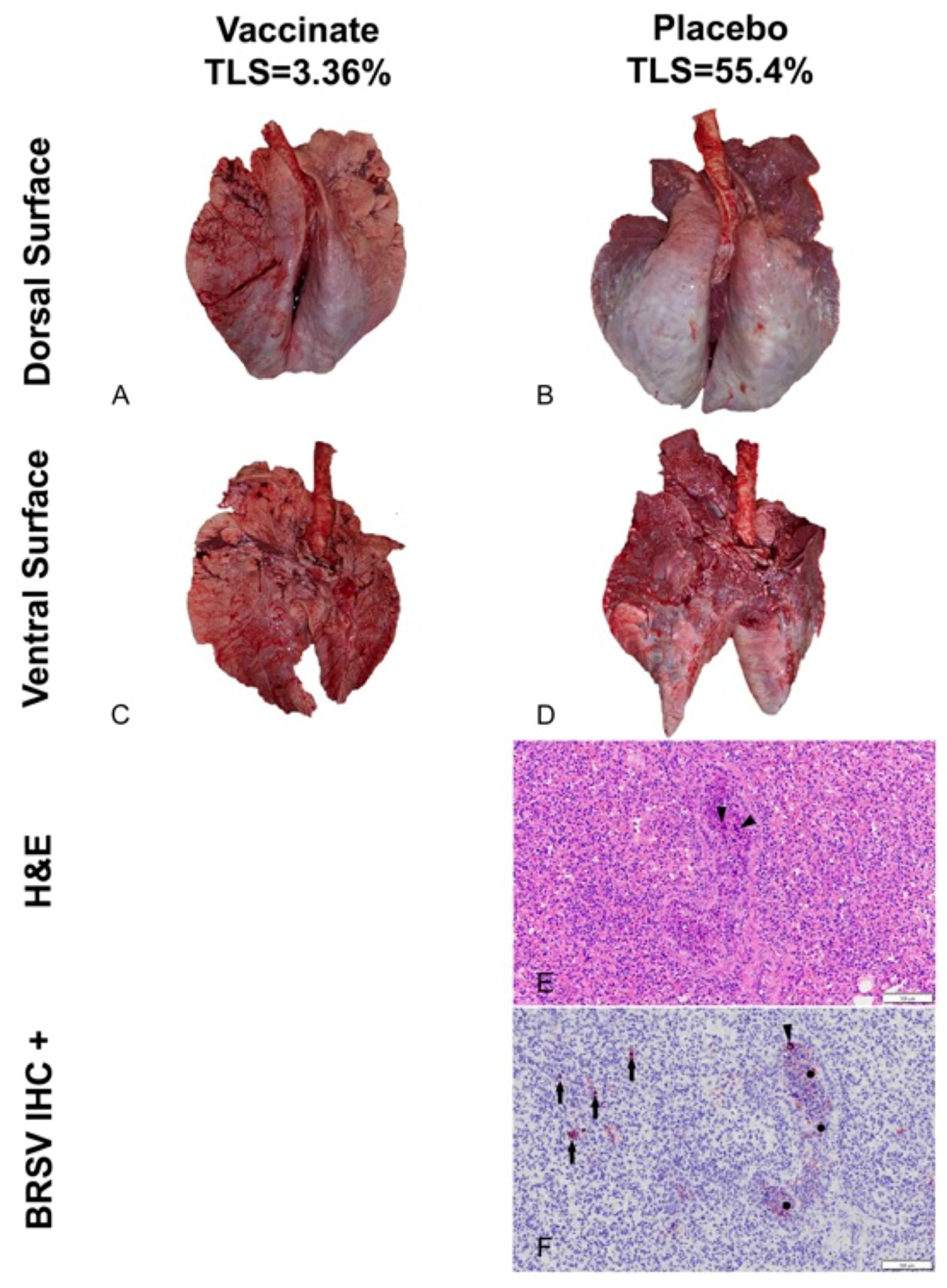
3.3. BRSV Lung Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Short, D.M.; Lombard, J.E. The National Animal Health Monitoring System’s Perspective on Respiratory Disease in Dairy Cattle. Anim. Health Res. Rev. 2020, 21, 135–138. [Google Scholar] [CrossRef]
- White, B.J.; Larson, B.L. Impact of Bovine Respiratory Disease in U.S. Beef Cattle. Anim. Health Res. Rev. 2020, 21, 132–134. [Google Scholar] [CrossRef]
- Preview: Economic Effects of Bovine Respiratory Disease. J. Anim. Sci. 2020, 98, skaa042. [CrossRef]
- Blakebrough-Hall, C.; McMeniman, J.P.; González, L.A. An evaluation of the economic effects of bovine respiratory disease on animal performance, carcass traits, and economic outcomes in feedlot cattle defined using four BRD diagnosis methods. J. Anim. Sci. 2020, 98, skaa005. [Google Scholar] [CrossRef]
- Peel, D.S. Economic Considerations of Enhanced BRD Control. Anim. Health Res. Rev. 2020, 21, 139–142. [Google Scholar] [CrossRef]
- Makoschey, B.; Berge, A.C. Review on bovine respiratory syncytial virus and bovine parainfluenza—Usual suspects in bovine respiratory disease—A narrative review. BMC Vet. Res. 2021, 17, 261. [Google Scholar] [CrossRef]
- Chase, C.; Hurley, D.J.; Reber, A.J. Neonatal Immune Development in the Calf and Its Impact on Vaccine Response. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef]
- Chase, C.C.L.; Parreno, V. In the Beginning: Development and Maximizing the Neonate Immune System. In Bovine Immunity: Making Immunology and Vaccinology Come Alive; Chase, C.C.L., Ed.; Hipra: Amer, Spain, 2022; pp. 25–28. Available online: https://bovineimmunity.hipra.com/index-en.html (accessed on 18 June 2024).
- Windeyer, M.C.; Gamsjäger, L. Vaccinating Calves in the Face of Maternal Antibodies. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 557–573. [Google Scholar] [CrossRef]
- Flynn, A.; McAloon, C.; Sugrue, K.; Fitzgerald, R.; Sheridan, C.; Cowley, B.; McAloon, C.; Kennedy, E. Investigation into the Safety, and Serological Responses Elicited by Delivery of Live Intranasal Vaccines for Bovine Herpes Virus Type 1, Bovine Respiratory Syncytial Virus, and Parainfluenza Type 3 in Pre-Weaned Calves. Front. Vet. Sci. 2024, 11, 1283013. [Google Scholar] [CrossRef]
- Ellis, J.A. How efficacious are vaccines against bovine respiratory syncytial virus in cattle? Vet. Microbiol. 2017, 206, 59–68. [Google Scholar] [CrossRef]
- Kirkpatrick, J.; Fulton, R.W.; Burge, L.J.; DuBois, W.R.; Payton, M. Passively transferred immunity in newborn calves, rate of antibody decay, and effect on subsequent vaccination with modified live virus vaccine. Bov. Pract. 2001, 35, 47–55. [Google Scholar] [CrossRef]
- Fulton, R.W.; Briggs, R.E.; Payton, M.E.; Confer, A.W.; Saliki, J.T.; Ridpath, J.F.; Burge, L.J.; Duff, G.C. Maternally derived humoral immunity to bovine viral diarrhea virus (BVDV) 1a, BVDV1b, BVDV2, bovine herpesvirus-1, parainfluenza-3 virus bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half-life studies and effect on response to vaccination. Vaccine 2004, 22, 643–649. [Google Scholar] [CrossRef]
- Vangeel, I.; Antonis, A.F.G.; Fluess, M.; Riegler, L.; Peters, A.R.; Harmeyer, S.S. Efficacy of a Modified Live Intranasal Bovine Respiratory Syncytial Virus Vaccine in 3-Week-Old Calves Experimentally Challenged with BRSV. Vet. J. 2007, 174, 627–635. [Google Scholar] [CrossRef]
- Woolums, A.R.; Brown, C.C.; Brown, J.C., Jr.; Cole, D.J.; Scott, M.A.; Williams, S.M.; Miao, C. Effects of a Single Intranasal Dose of Modified-Live Bovine Respiratory Syncytial Virus Vaccine on Resistance to Subsequent Viral Challenge in Calves. Am. J. Vet. Res. 2004, 65, 363–372. [Google Scholar] [CrossRef]
- Kolb, E.A.; Buterbaugh, R.E.; Rinehart, C.L.; Ensley, D.; Perry, G.A.; Abdelsalam, K.W.; Chase, C.C. Protection against bovine respiratory syncytial virus in calves vaccinated with adjuvanted modified live vaccine administered in the face of maternal antibody. Vaccine 2020, 38, 298–308. [Google Scholar] [CrossRef]
- Timsit, E.; Maingourd, C.; Le Dréan, E.; Belloc, C.; Seegers, H.; Douart, A.; Assié, S. Evaluation of a commercial real-time reverse transcription polymerase chain reaction kit for the diagnosis of bovine respiratory syncytial virus infection. J. Vet. Diag. Investig. 2010, 22, 238–241. [Google Scholar] [CrossRef]
- Fairbanks, K.F.; Campbell, J.; Chase, C.C.L. Rapid Onset of Protection against Infectious Bovine Rhinotracheitis with a Modified-Live Virus Multivalent Vaccine. Vet. Therap. 2004, 5, 17–25. [Google Scholar]
- Manual of Standards for Diagnostic Tests and Vaccines; Office International des Epizooties: Paris, France, 2008.
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. F Exp. Pathol. U Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Zimmerman, A.D.; Klein, A.L.; Buterbaugh, R.E.; Rinehart, C.L.; Chase, C.C.L. Protection against Bovine Herpesvirus Type 1 (BHV-1) Abortion Following Challenge 8 Months or Approximately 1 Year after Vaccination. Bov. Pract. 2013, 47, 73–81. [Google Scholar] [CrossRef]
- Achenbach, J.E.; Topliff, C.L.; Vassilev, V.B.; Donis, R.O.; Eskridge, K.M.; Kelling, C.L. Detection and quantitation of bovine respiratory syncytial virus using real-time quantitative RT-PCR and quantitative competitive RT-PCR assays. J. Virol. Methods 2004, 121, 1–6. [Google Scholar] [CrossRef]
- Jericho, K.W.; Langford, E.V. Aerosol Vaccination of Calves with Pasteurella Haemolytica against Experimental Respiratory Disease. Can. J. Comp. Med. 1982, 46, 287–292. [Google Scholar]
- Marley, M.S.D.; Givens, M.D.; Galik, P.K.; Riddell, K.P.; Stringfellow, D.A. Development of a duplex quantitative polymerase chain reaction assay for detection of bovine herpesvirus 1 and bovine viral diarrhea virus in bovine follicular fluid. Theriogenology 2008, 70, 153–160. [Google Scholar] [CrossRef]
- Ellis, J.A.; Gow, S.P.; Goji, N. Response to Experimentally Induced Infection with Bovine Respiratory Syncytial Virus Following Intranasal Vaccination of Seropositive and Seronegative Calves. J. Am. Vet. Med. Assoc. 2010, 236, 991–999. [Google Scholar] [CrossRef]
- Kimman, T.G.; Westenbrink, F.; Straver, P. Priming for Local and Systemic Antibody Memory Responses to Bovine Respiratory Syncytial Virus: Effect of Amount of Virus, Virus Replication, Route of Administration and Maternal Antibodies. Vet. Immunol. Immunopathol. 1989, 22, 145–160. [Google Scholar] [CrossRef]
- Merck Animal Health. Efficacy of the Bovine Respiratory Syncytial Virus Fraction of Nasalgen® 3-PMH in Calves 4 to 7 Days Old. In Nasalgen 3-PMH Tech Bulletin Series; Merck Animal Health: Millsboro, DE, USA, 2020; pp. 1–7. [Google Scholar]
- Merck Animal Health. Duration of Immunity of the Bovine Respiratory Syncytial Virus Fraction of Nasalgen® 3-PMH Administered to Calves 5 to 7 Days of Age. In Nasalgen 3-PHM Tech Bulletin Series; Merck Animal Health: Millsboro, DE, USA, 2020; pp. 1–10. [Google Scholar]
- Chamorro, M.F.; Walz, P.H.; Haines, D.M.; Passler, T.; Earleywine, T.; A Palomares, R.; Riddell, K.P.; Galik, P.; Zhang, Y.; Givens, M.D. Comparison of levels and duration of detection of antibodies to bovine viral diarrhea virus 1, bovine viral diarrhea virus 2, bovine respiratory syncytial virus, bovine herpesvirus 1, and bovine parainfluenza virus 3 in calves fed maternal colostrum or a colostrum-replacement product. Can. J. Vet. Res. 2012, 78, 81–88. [Google Scholar]
- Cortese, V.S.; Woolums, A.; Hurley, D.J.; Berghaus, R.; Bernard, J.K.; Short, T.H. Comparison of Interferon and Bovine Herpesvirus-1-Specific IgA Levels in Nasal Secretions of Dairy Cattle Administered an Intranasal Modified Live Viral Vaccine Prior to Calving or on the Day of Calving. Vet. Immunol. Immunopathol. 2017, 187, 35–41. [Google Scholar] [CrossRef]
- Todd, J.D.; Volenec, F.J.; Paton, I.M. Interferon in Nasal Secretions and Sera of Calves after Intranasal Administration of Avirulent Infectious Bovine Rhinotracheitis Virus: Association of Interferon in Nasal Secretions with Early Resistance to Challenge with Virulent Virus. Infect. Immun. 1972, 5, 699–706. [Google Scholar] [CrossRef]
- Daly, R.; Rozeboom, C.; Sumption, J. Assessing Passive Transfer and Respiratory Pathogen Colonization of Neonatal Beef Calves in a Confinement Operation. In SDSU Beef Day 2020 Proceedings; South Dakota State University: Brookings, SD, USA, 2020; pp. 44–49. Available online: https://openprairie.sdstate.edu/sd_beefday_2020/1/ (accessed on 18 June 2024).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkins-Oines, S.; Senevirathne, N.D.; Krafsur, G.M.; Abdelsalam, K.; Renter, D.; Meyer, B.; Chase, C.C.L. The Detection of Vaccine Virus and Protection of a Modified Live, Intranasal, Trivalent Vaccine in Neonatal, Colostrum-Fed Calves with an Experimental Bovine Respiratory Syncytial Virus Challenge. Pathogens 2024, 13, 517. https://doi.org/10.3390/pathogens13060517
Perkins-Oines S, Senevirathne ND, Krafsur GM, Abdelsalam K, Renter D, Meyer B, Chase CCL. The Detection of Vaccine Virus and Protection of a Modified Live, Intranasal, Trivalent Vaccine in Neonatal, Colostrum-Fed Calves with an Experimental Bovine Respiratory Syncytial Virus Challenge. Pathogens. 2024; 13(6):517. https://doi.org/10.3390/pathogens13060517
Chicago/Turabian StylePerkins-Oines, Stephanie, Nirosh D. Senevirathne, Greta M. Krafsur, Karim Abdelsalam, David Renter, Brent Meyer, and Christopher C. L. Chase. 2024. "The Detection of Vaccine Virus and Protection of a Modified Live, Intranasal, Trivalent Vaccine in Neonatal, Colostrum-Fed Calves with an Experimental Bovine Respiratory Syncytial Virus Challenge" Pathogens 13, no. 6: 517. https://doi.org/10.3390/pathogens13060517
APA StylePerkins-Oines, S., Senevirathne, N. D., Krafsur, G. M., Abdelsalam, K., Renter, D., Meyer, B., & Chase, C. C. L. (2024). The Detection of Vaccine Virus and Protection of a Modified Live, Intranasal, Trivalent Vaccine in Neonatal, Colostrum-Fed Calves with an Experimental Bovine Respiratory Syncytial Virus Challenge. Pathogens, 13(6), 517. https://doi.org/10.3390/pathogens13060517






