Isolation, Identification, and Characterisation of a Novel ST2378 Aeromonas hydrophila Strain from Naturally Diseased Frogs, Rana dybowskii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diseased R. dybowskii and Bacterial Isolation
2.2. Physiological and Biochemical Characterisation
2.3. Sequencing and Analysis of 16S rRNA and gyrB Genes
2.4. Experimental Infections
2.4.1. R. dybowskii Husbandry
2.4.2. Determination of Median Lethal Dose
2.4.3. Single Infection and Co-Infection
2.5. Analysis of Antimicrobial Susceptibility
2.6. Screening of Virulence Genes
2.7. Multi Locus Sequence Typing (MLST)
3. Results
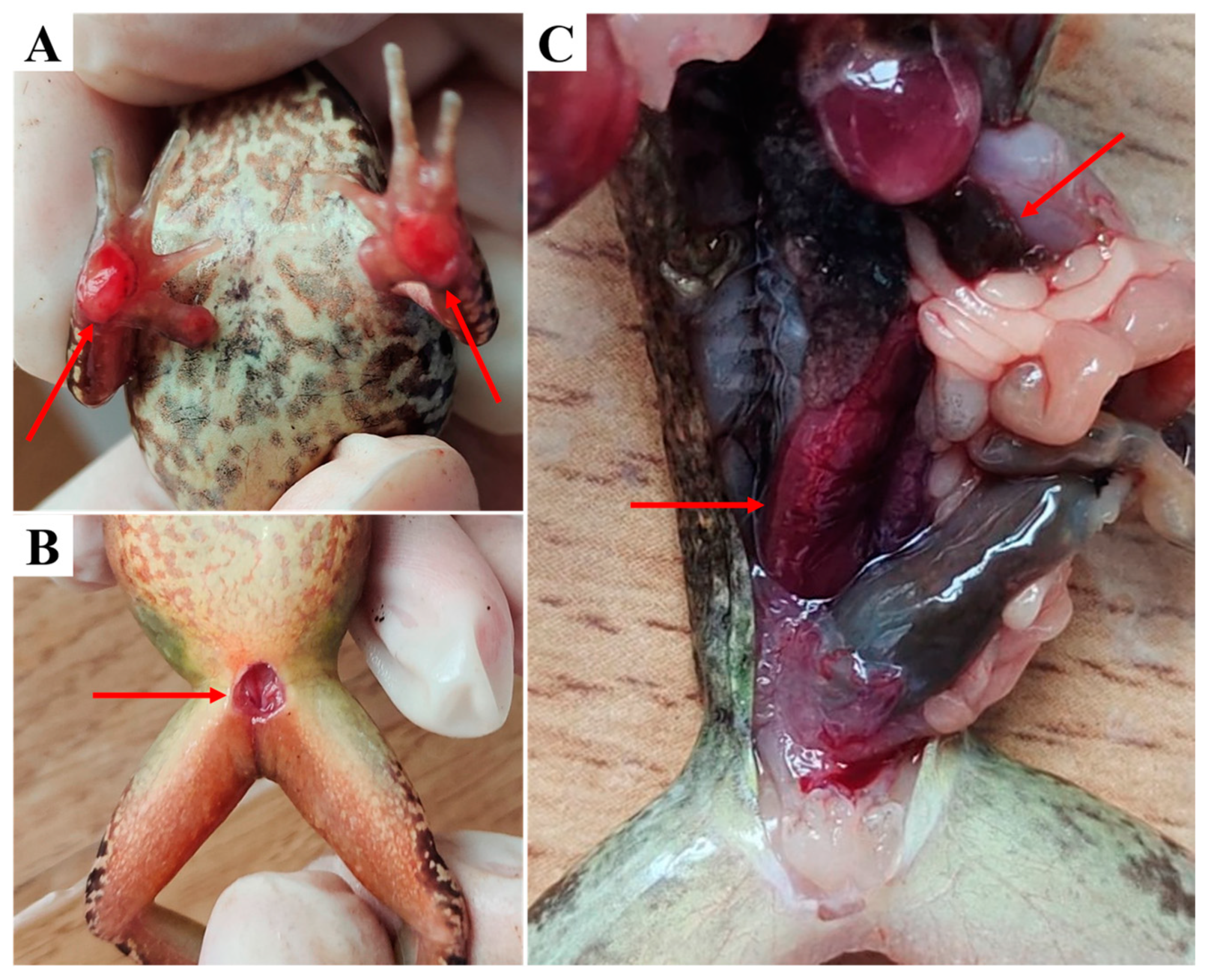
3.1. Clinical Examination
3.2. Physiological and Biochemical Characteristics
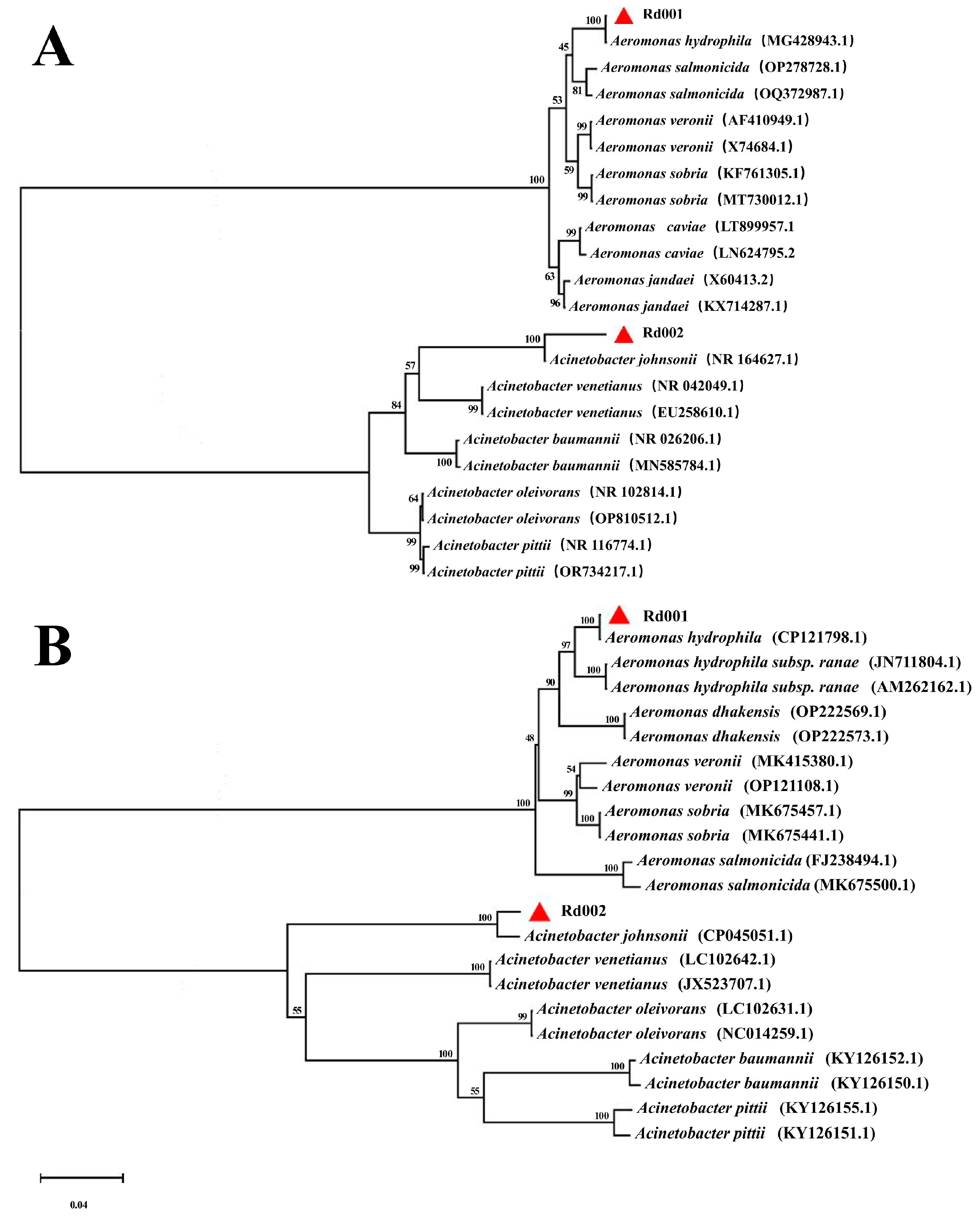
3.3. Sequence Analysis of the 16S rRNA and gyrB Genes
3.4. Experimental Infections
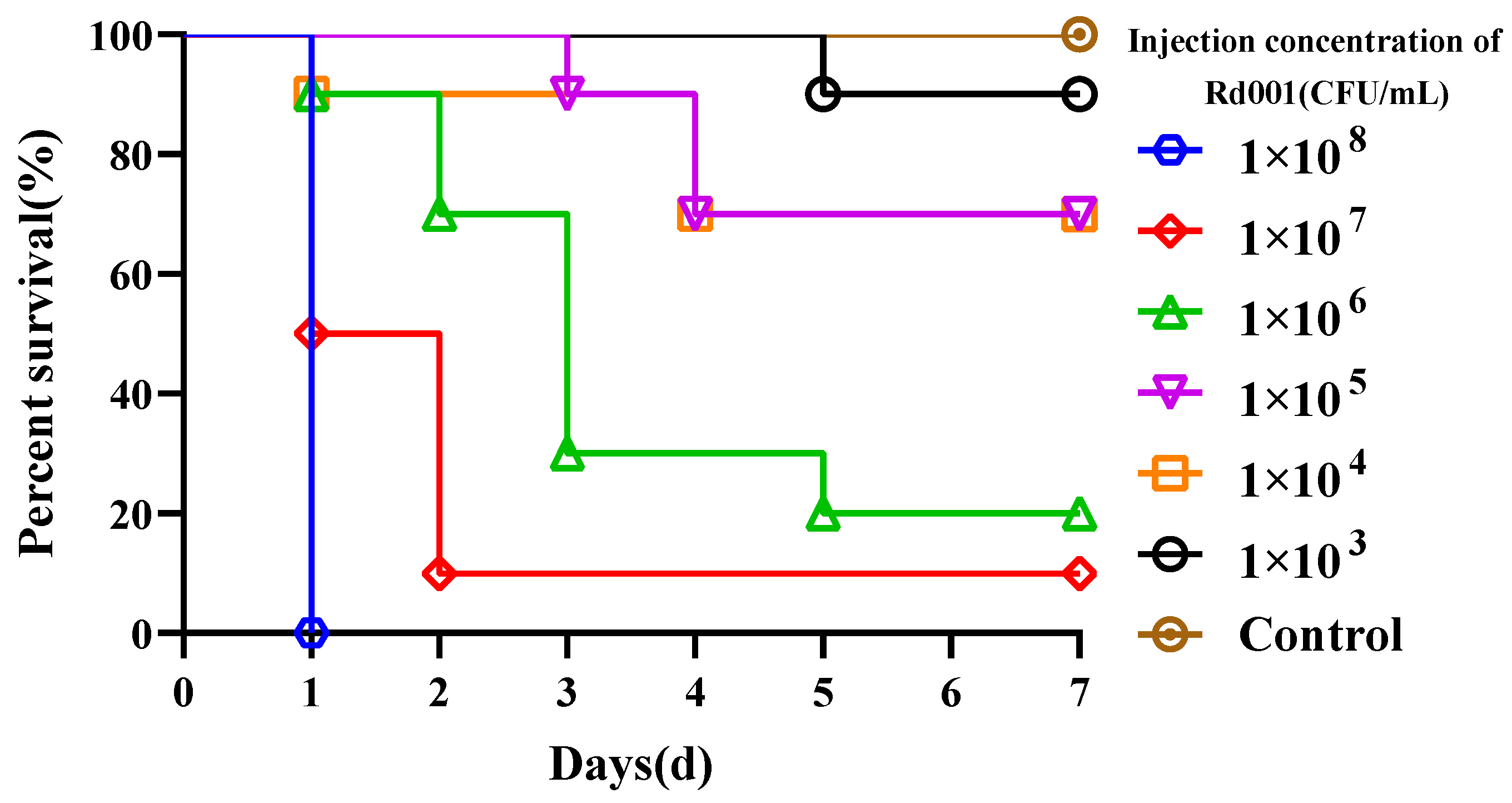
3.4.1. Determination of Median Lethal Dose
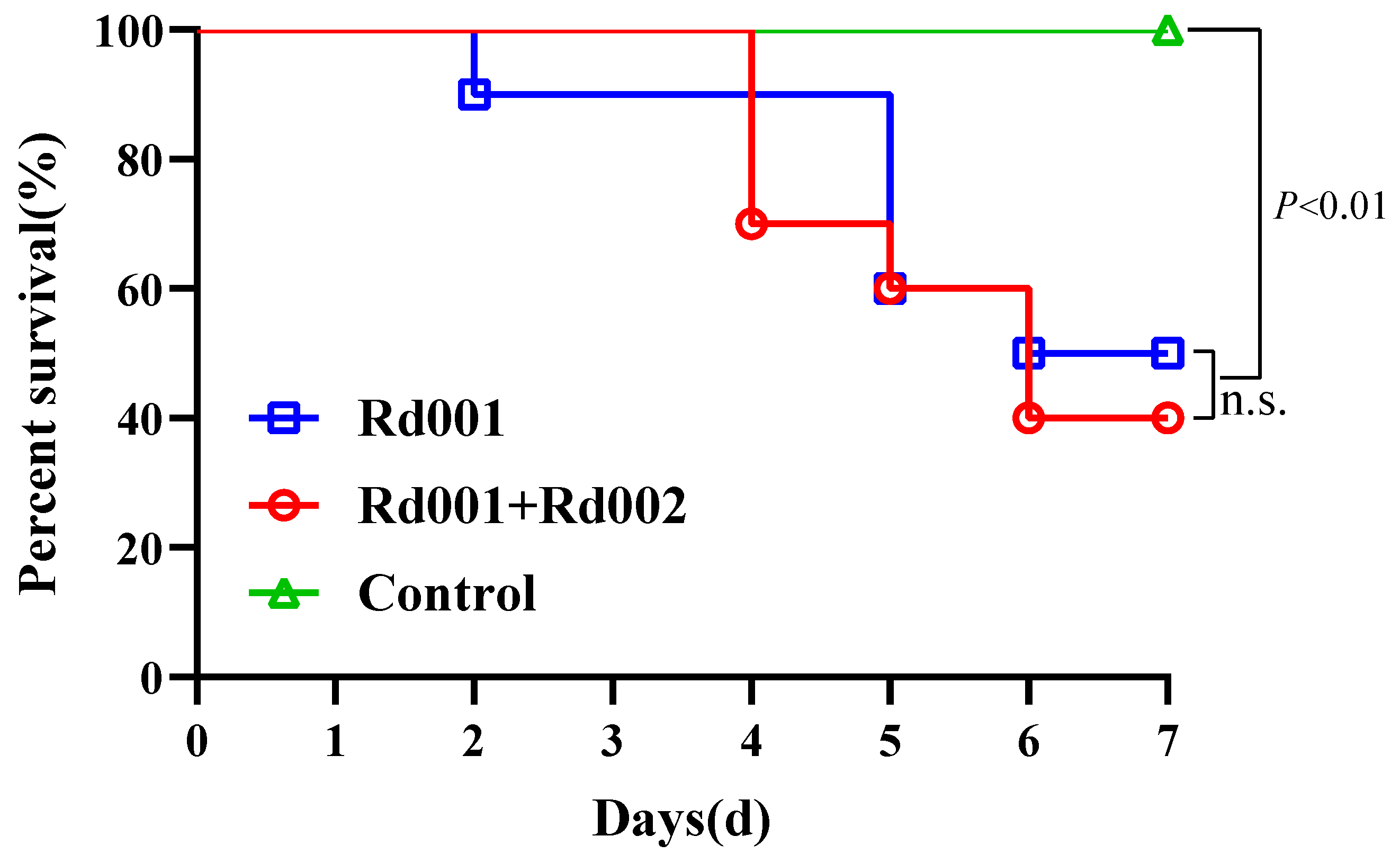
3.4.2. Single Infection and Co-Infection
3.5. Antimicrobial Susceptibility and Virulence Factors
3.6. MLST Analysis of Rd001
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, K.; Zhu, D.-Z.; Wei, Y.; Schloegel, L.M.; Chen, X.-F.; Wang, X.-L. Broad Distribution of Ranavirus in Free-Ranging Rana Dybowskii in Heilongjiang, China. EcoHealth 2010, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Hero, J. Geographic Variation in Life-history Characteristics of Amphibians: A Review. J. Anim. Ecol. 2003, 72, 270–279. [Google Scholar] [CrossRef]
- Carey, C.; Cohen, N.; Rollins-Smith, L. Amphibian Declines: An Immunological Perspective. Dev. Comp. Immunol. 1999, 23, 459–472. [Google Scholar] [CrossRef]
- Igbinosa, I.H.; Igumbor, E.U.; Aghdasi, F.; Tom, M.; Okoh, A.I. Emerging Aeromonas Species Infections and Their Significance in Public Health. Sci. World J. 2012, 2012, 625023. [Google Scholar] [CrossRef] [PubMed]
- Huys, G.; Pearson, M.; Kämpfer, P.; Denys, R.; Cnockaert, M.; Inglis, V.; Swings, J. Aeromonas hydrophila subsp. ranae subsp. nov., Isolated from Septicaemic Farmed Frogs in Thailand. Int. J. Syst. Evol. Microbiol. 2003, 53, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Bradford, D.F. Mass Mortality and Extinction in a High-Elevation Population of Rana Muscosa. J. Herpetol. 1991, 25, 174–177. [Google Scholar] [CrossRef]
- Khalifa, A.Y.Z.; AlMalki, M.A.; Bekhet, G.M. Pathological and Mortality Findings Associated with Aeromonas hydrophila from Frog Eggs in Al-Ahsa Region of Saudi Arabia. Aquac. Res. 2021, 52, 1227–1236. [Google Scholar] [CrossRef]
- Schadich, E.; Cole, A.L. Pathogenicity of Aeromonas hydrophila, Klebsiella pneumoniae, and Proteus mirabilis to Brown Tree Frogs (Litoria ewingii). Comp. Med. 2010, 60, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen-Ivey, C.R.; Figueras, M.J.; McGarey, D.; Liles, M.R. Virulence Factors of Aeromonas hydrophila: In the Wake of Reclassification. Front. Microbiol. 2016, 7, 1337. [Google Scholar] [CrossRef]
- Allan, B.J.; Stevenson, R.M.W. Extracellular Virulence Factors of Aeromonas hydrophila in Fish Infections. Can. J. Microbiol. 1981, 27, 1114–1122. [Google Scholar] [CrossRef]
- Pattanayak, S.; Priyadarsini, S.; Paul, A.; Kumar, P.R.; Sahoo, P.K. Diversity of Virulence-Associated Genes in Pathogenic Aeromonas hydrophila Isolates and Their in Vivo Modulation at Varied Water Temperatures. Microb. Pathog. 2020, 147, 104424. [Google Scholar] [CrossRef] [PubMed]
- Bakiyev, S.; Smekenov, I.; Zharkova, I.; Kobegenova, S.; Sergaliyev, N.; Absatirov, G.; Bissenbaev, A. Isolation, Identification, and Characterization of Pathogenic Aeromonas hydrophila from Critically Endangered Acipenser baerii. Aquac. Rep. 2022, 26, 101293. [Google Scholar] [CrossRef]
- CLSI. Methods for Broth Dilution Susceptibility Testing of Bacteria Isolated from Aquatic Animals; Approved Guidelinet; CLSI Document VET04-A; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2006. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Suceptibility Testing of Bacteria Isolated from Aquatic Animals; Second Informational Supplement; CLSI Document VET03/VET04-S2; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2014. [Google Scholar]
- Singh, V.; Rathore, G.; Kapoor, D.; Mishra, B.N.; Lakra, W.S. Detection of Aerolysin Gene in Aeromonas hydrophila Isolated from Fish and Pond Water. Indian J. Microbiol. 2008, 48, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Nam, I.-Y.; Joh, K. Rapid Detection of Virulence Factors of Aeromonas Isolated from a Trout Farm by Hexaplex-PCR. J. Microbiol. Seoul Korea 2007, 45, 297–304. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, S.G.; Kim, S.W.; Yun, S.; Kim, H.J.; Giri, S.S.; Han, S.J.; Oh, W.T.; Park, S.C. A Case of Mortality Caused by Aeromonas hydrophila in Wild-Caught Red-Eyed Crocodile Skinks (Tribolonotus gracilis). Vet. Sci. 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Rozi; Rahayu, K.; Daruti, D.N. Detection and Analysis of Hemolysin Genes in Aeromonas hydrophila Isolated from Gouramy (Osphronemus gouramy) by Polymerase Chain Reaction (PCR). IOP Conf. Ser. Earth Environ. Sci. 2018, 137, 012001. [Google Scholar] [CrossRef]
- Li, T.; Raza, S.H.A.; Yang, B.; Sun, Y.; Wang, G.; Sun, W.; Qian, A.; Wang, C.; Kang, Y.; Shan, X. Aeromonas veronii Infection in Commercial Freshwater Fish: A Potential Threat to Public Health. Anim. Open Access J. 2020, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Rodgers, M. Distribution of Six Virulence Factors in Aeromonas Species Isolated from US Drinking Water Utilities: A PCR Identification. J. Appl. Microbiol. 2004, 97, 1077–1086. [Google Scholar] [CrossRef]
- Martino, M.E.; Fasolato, L.; Montemurro, F.; Rosteghin, M.; Manfrin, A.; Patarnello, T.; Novelli, E.; Cardazzo, B. Determination of Microbial Diversity of Aeromonas Strains on the Basis of Multilocus Sequence Typing, Phenotype, and Presence of Putative Virulence Genes. Appl. Environ. Microbiol. 2011, 77, 4986–5000. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-Access Bacterial Population Genomics: BIGSdb Software, the PubMLST.Org Website and Their Applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A Comprehensive Update on Curation, Resources and Tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, P.J.M.; Grimont, P.A.D. Taxonomy of the Genus Acinetobacter with the Recognition of Acinetobacter baumannii Sp. Nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov. and Emended Descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int. J. Syst. Evol. Microbiol. 1986, 36, 228–240. [Google Scholar] [CrossRef]
- Lin, H.; Zeng, G.; Yu, Y.; Li, H.; Chen, K.; Qin, Z.; Jiang, B.; Li, W.; Su, Y.; Lin, L.; et al. Acute Septicemia and Diagnostic Evaluation of Aeromonas veronii Infection in American Bullfrogs (Aquarana catesbeiana). Aquaculture 2024, 580, 740349. [Google Scholar] [CrossRef]
- Wang, B.; Shao, J.; Qu, L.; Xu, Q.; Zheng, D. The Sequencing of the Key Genes and End Products in the TLR4 Signaling Pathway from the Kidney of Rana dybowskii Exposed to Aeromonas hydrophila. Open Life Sci. 2023, 18, 20220704. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.A.; Newman, S.J.; Craig, L.; Carter, C.; Czarra, J.; Brown, J.P. Diagnosis of Aeromonas hydrophila, Mycobacterium Species, and Batrachochytrium dendrobatidis in an African Clawed Frog (Xenopus laevis). J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 215–220. [Google Scholar] [PubMed]
- Miller, D.L.; Rajeev, S.; Brookins, M.; Cook, J.; Whittington, L.; Baldwin, C.A. Concurrent Infection with Ranavirus, Batrachochytrium dendrobatidis, and Aeromonas in a Captive Anuran Colony. J. Zoo Wildl. Med. 2008, 39, 445–449. [Google Scholar] [CrossRef]
- Mittal, K.R.; Lalonde, G.; Leblanc, D.; Olivier, G.; Lallier, R. Aeromonas hydrophila in Rainbow Trout: Relation between Virulence and Surface Characteristics. Can. J. Microbiol. 1980, 26, 1501–1503. [Google Scholar] [CrossRef]
- Senderovich, Y.; Ken-Dror, S.; Vainblat, I.; Blau, D.; Izhaki, I.; Halpern, M. A Molecular Study on the Prevalence and Virulence Potential of Aeromonas spp. Recovered from Patients Suffering from Diarrhea in Israel. PLoS ONE 2012, 7, e30070. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Kilgore, P.B.; Andersson, J.A.; Blears, E.; Figueras, M.J.; Hasan, N.A.; Colwell, R.R.; Sha, J.; Chopra, A.K. T6SS and ExoA of Flesh-Eating Aeromonas hydrophila in Peritonitis and Necrotizing Fasciitis during Mono- and Polymicrobial Infections. Proc. Natl. Acad. Sci. USA 2019, 116, 24084–24092. [Google Scholar] [CrossRef]
- Ayoub, H.F.; Khafagy, A.R.; Esawy, A.M.; El-moaty, N.A.; Alwutayd, K.M.; Mansour, A.T.; Ibrahim, R.A.; Abdel-moneam, D.A.; El-Tarabili, R.M. Phenotypic, Molecular Detection, and Antibiotic Resistance Profile (MDR and XDR) of Aeromonas hydrophila Isolated from Farmed Tilapia zillii and Mugil cephalus. BMC Vet. Res. 2024, 20, 84. [Google Scholar] [CrossRef]
- Gonçalves Pessoa, R.B.; de Oliveira, W.F.; Marques, D.S.C.; dos Santos Correia, M.T.; de Carvalho, E.V.M.M.; Coelho, L.C.B.B. The Genus Aeromonas: A General Approach. Microb. Pathog. 2019, 130, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Tomás, J.M. The Main Aeromonas Pathogenic Factors. ISRN Microbiol. 2012, 2012, 256261. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, J.M.; Kidd, S.P.; Schmidt, R. Secreted Enzymes of Aeromonas. FEMS Microbiol. Lett. 1997, 152, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abrami, L.; Fivaz, M.; Glauser, P.-E.; Sugimoto, N.; Zurzolo, C.; van der Goot, F.G. Sensitivity of Polarized Epithelial Cells to the Pore-Forming Toxin Aerolysin. Infect. Immun. 2003, 71, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Ozaki, H.; Fujii, Y.; Kobayashi, H.; Yamanaka, H.; Arimoto, S.; Negishi, T.; Okamoto, K. Properties of Hemolysin and Protease Produced by Aeromonas trota. PLoS ONE 2014, 9, e91149. [Google Scholar] [CrossRef] [PubMed]
- Galindo, C.; Sha, J.; Fadl, A.; Pillai, L.; Chopra, A. Host Immune Responses to Aeromonas Virulence Factors. Curr. Immunol. Rev. 2006, 2, 13–26. [Google Scholar] [CrossRef]
- Galindo, C.L.; Gutierrez, C., Jr.; Chopra, A.K. Potential Involvement of Galectin-3 and SNAP23 in Aeromonas hydrophila Cytotoxic Enterotoxin-Induced Host Cell Apoptosis. Microb. Pathog. 2006, 40, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Tekedar, H.C.; Arick, M.A.I.; Hsu, C.-Y.; Thrash, A.; Blom, J.; Lawrence, M.L.; Abdelhamed, H. Identification of Antimicrobial Resistance Determinants in Aeromonas veronii Strain MS-17-88 Recovered From Channel Catfish (Ictalurus punctatus). Front. Cell. Infect. Microbiol. 2020, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Urwin, R.; Maiden, M.C.J. Multi-Locus Sequence Typing: A Tool for Global Epidemiology. Trends Microbiol. 2003, 11, 479–487. [Google Scholar] [CrossRef]
- Liu, J.; Xie, L.; Zhao, D.; Yang, T.; Hu, Y.; Sun, Z.; Yu, X. A Fatal Diarrhoea Outbreak in Farm-Raised Deinagkistrodon Acutus in China Is Newly Linked to Potentially Zoonotic Aeromonas hydrophila. Transbound. Emerg. Dis. 2019, 66, 287–298. [Google Scholar] [CrossRef]
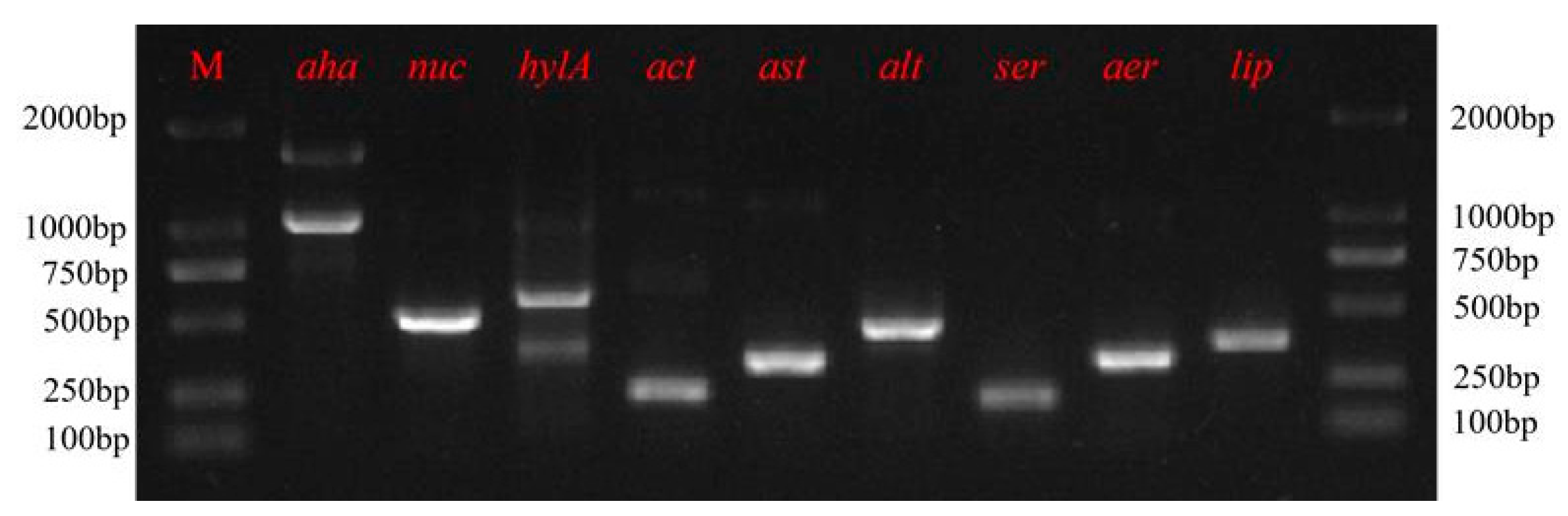

| Gene | PCR Prime Sequence (5′→3′) | Product Size (bp) | Reference |
|---|---|---|---|
| aer | F: ACAGCCAATATGTCGGTGAAG | 326 | [15] |
| R: TCACCTTCTCGCTCAGGC | |||
| ser | F: CACCGAAGTATTGGGTCAGG | 211 | [16] |
| R: GGCTCATGCGTAACTCTGGT | |||
| alt | F:TGACCCAGTCCTGGCACGGC | 442 | [17] |
| R:GGTGATCGATCACCACCAGC | |||
| ast | F:TCTCCATGCTTCCCTTCCACT | 331 | [17] |
| R:GTGTAGGGATTGAAGAGCCG | |||
| act | F:AGAAGGTGACCACCACCAAGAACA | 232 | [17] |
| R:AACTGACATCGGCCTGAACTC | |||
| hlyA | F:GGCCGGTGGCCCGAAGATACGGG | 592 | [18] |
| R:GGCGGCGCCGGACGAGACGGGG | |||
| nuc | F:CAGGATCTGAACCGCCTCTATCAGG | 504 | [15] |
| R:GTCCCAAGCTTCGAACAGTTTACGC | |||
| aha | F:GGTATTGTATCCCGGCTCTGTT | 1082 | [19] |
| R:CGGTCCATCGTCGTCCATCTTG | |||
| lip | F: TCTTCTCCGACTGGTTCGG | 382 | [20] |
| R: GTGCCAGGACTGGGTCTT |
| Characteristics | Rd001 | A. hydrophila ATCC35654 [23] | Rd002 | A. johnsonii ATCC17909 [24] |
|---|---|---|---|---|
| Gram stain | – | – | – | – |
| N-Acetyl-D-glucosamine | + | + | – | – |
| Adonitol | + | – | + | – |
| Citrate | + | + | + | – |
| Sorbitol | – | – | – | – |
| Sucrose | + | + | – | – |
| Glucose | + | + | – | – |
| D-Galactose | + | + | – | – |
| Maltulose | + | + | – | – |
| L-Rhamnose | – | – | – | – |
| D-Gluconic acid | + | + | – | – |
| D-Melibiose | – | – | – | – |
| L-Arabinose | – | + | – | – |
| Methyl-B-Glucoside | + | + | – | – |
| Urea | – | – | – | – |
| Esculin | + | + | – | – |
| Group | Antibiotic | MIC (μg/mL) | Susceptibility |
|---|---|---|---|
| 4-quinolones | Enrofloxacin | 0.25 | S |
| Flumequine | 2 | S | |
| Chloramphenicols | Thiamphenicol | 512 | R |
| Florfenicol | 512 | R | |
| Sulfonamides | Sulfamonomethoxine | 512 | R |
| Trimethoprim/Sulfamethoxazole | 4/76 | R | |
| Aminogly cosides | Neomycin | 1 | S |
| Tetracyclines | Doxycycline | 8 | I |
| Isolate | Rd001 |
|---|---|
| gyrB | 804 |
| groL | 369 |
| gltA | 1041 |
| metG | 301 |
| ppsA | 269 |
| recA | 1130 |
| Assigned ST | 2378 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Wang, J.; Wang, D.; Wang, Y.; Hu, G.; Li, S. Isolation, Identification, and Characterisation of a Novel ST2378 Aeromonas hydrophila Strain from Naturally Diseased Frogs, Rana dybowskii. Pathogens 2024, 13, 552. https://doi.org/10.3390/pathogens13070552
Zhao R, Wang J, Wang D, Wang Y, Hu G, Li S. Isolation, Identification, and Characterisation of a Novel ST2378 Aeromonas hydrophila Strain from Naturally Diseased Frogs, Rana dybowskii. Pathogens. 2024; 13(7):552. https://doi.org/10.3390/pathogens13070552
Chicago/Turabian StyleZhao, Ran, Jing Wang, Di Wang, Yanan Wang, Guo Hu, and Shaowu Li. 2024. "Isolation, Identification, and Characterisation of a Novel ST2378 Aeromonas hydrophila Strain from Naturally Diseased Frogs, Rana dybowskii" Pathogens 13, no. 7: 552. https://doi.org/10.3390/pathogens13070552






