Abstract
Human T cell lymphotropic virus type 1 (HTLV-1) is a retrovirus that infects lymphocytes and causes severe diseases. HTLV-1 proviral load (PVL), i.e., the number of host cells that carry HTLV-1 proviral DNA integrated into their genome, can be measured in peripheral blood mononuclear cells (PBMCs) using quantitative polymerase chain reaction. In this narrative review, we discuss the usefulness of HTLV-1 PVL quantification and share our experience acquired during more than 30 years of follow-up of people living with HTLV-1 in the UK. Patients with HTLV-1-associated myelopathy have higher PVL than those with asymptomatic infection. This is consistent across studies in different countries. High PVL predates symptom onset for both inflammatory and proliferative diseases. High PVL is essential but not sufficient for the development of HTLV-1-associated diseases. Therefore, PVL quantification can be used to support the care of people living with HTLV-1 by identifying those most at risk of HTLV-1-associated diseases.
1. Introduction
Human T cell lymphotropic virus type 1 (HTLV-1) is a retrovirus with global distribution. HTLV-1 is transmitted by sex, vertically, and by contact with infected blood or tissues. There is a strong body of evidence that links HTLV-1 with a range of diseases and shows that, at the time of diagnosis of disease, HTLV-1 proviral load (PVL) in peripheral blood mononuclear cells (PBMCs) is high (HTLV-associated myelopathy (HAM) [1,2,3,4,5,6], Adult T cell leukaemia/lymphoma (ATL) [3], HTLV-1-associated uveitis [7], kerato-conjunctivitis sicca [8] and infective dermatitis [9,10,11]). Despite differences in methods, standards and patients, the results for proviral load in patients with HAM compared to asymptomatic carriers are strikingly similar (see Table 1 and Figure 1) [1,3,4,12,13,14,15,16,17,18,19,20,21], with a proviral load on average 7 times higher in patients with HAM than in asymptomatic carriers across studies in different populations spanning 25 years. The question therefore is whether the disease caused the high proviral load to occur or whether the high proviral load predated and thus predicts the disease. In this narrative review, we will discuss the current knowledge on HTLV-1 proviral load, focusing on its association with disease, and share our experience from a National HTLV Clinical Service in London.

Table 1.
Comparison of median HTLV-1 proviral load in patients with HTLV-1-associated myelopathy (HAM) compared with asymptomatic carriers (ACs) from published studies.
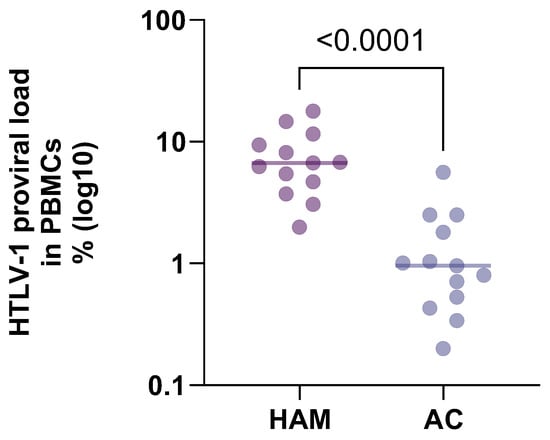
Figure 1.
Comparison of HTLV-1 proviral load by disease state from Table 1. Proviral load in patients with HTLV-associated myelopathy is compared to patients with asymptomatic HTLV-1 infection.
2. The London Experience
In 1992, we established a clinic in London to provide care for patients diagnosed with HTLV-1 and to better understand the natural history of this infection. We were aware of the already established associations of HTLV-1 with ATL and with a number of conditions characterised by inflammation, such as HAM, uveitis, myopathy and thyroiditis. We are still learning regarding the overarching question, namely, what is the full impact and spectrum of diseases caused by HTLV-1 infection?
A second and more focused question was the following: What is the importance of the viral burden? To this end, we established HTLV-1 proviral load quantification, first by limiting dilution and nested PCR and from 2000 by real-time quantitative PCR. Since 2000, as a matter of clinical routine, proviral load is measured in all patients at all visits. HTLV-1 proviral load results are reported as HTLV-1 DNA copies/100 PBMCs, which we abbreviate to %.
In 1999, we reported on our first 20 initially asymptomatic carriers [22]. The range of proviral loads was wide but stable, and high proviral load predated the onset of HTLV-1-associated myelopathy and uveitis, seen in one patient each. By 2013, we had accumulated data on more than 400 patients attending the clinic. Patients who were asymptomatic (n = 211) had a median proviral load (using our assay) of 1.8%, whilst patients with HAM (n = 84) had a median proviral load of 14.7%, and none had a proviral load less than 1.7% [3]. As expected, patients with leukaemia due to ATL had even higher proviral loads (50%), whilst even patients with ATL-lymphoma and no evidence of involvement of the peripheral blood had high median proviral load (7.9%). Through examining sequential, prospectively acquired data, we observed that in asymptomatic carriers with a minimum of 4 years (median 6.5 years) follow-up, proviral load decreased from 1.7% to 1%. In 2013, we reported on incident ATL within the cohort. Of 153 initially asymptomatic carriers followed up for a median of 4.5 years, 4 developed incident ATL and all had a pre-onset of disease proviral load > 10% [23]. By 2020, out of a total cohort of 658 people living with HTLV-1, 6 had developed ATL, with documented high proviral load predating the onset of ATL 2–10 years earlier [24]. On the basis of these results, which indicated that HTLV-1-associated disease was not occurring in asymptomatic carriers with proviral load < 1% and that proviral load was stable over many years (and tending to decrease rather than increase), we reduced the frequency of routine clinic reviews for these patients with low (<1%) proviral load to annual. Furthermore, anecdotal data from two blood donors who had become infected between donations suggested that proviral load plateaued in the first months following primary infection. This was confirmed in a prospective study of three transplant recipients who inadvertently received organs from a single donor prior to diagnosis of HTLV-1 infection in the donor (who was perceived to be HTLV low risk) [25]. Proviral load increased rapidly during early infection, at its maximum doubling every day before plateauing by day 45 from infection and thereafter remaining stable.
Having identified high proviral load as a risk for HTLV-1-associated disease, we have more recently identified additional markers that predict the development of HAM and ATL, namely activated T cells [24] and the clonal expansion of HTLV-1 infected T cells [25], respectively. In both cases, having a proviral load > 1.8% (i.e., the median proviral load among asymptomatic carriers) is the baseline requirement. Thus, in our clinic, we have only observed incident HAM in patients with a “HAM-like” viral-immune phenotype, which comprises having a proviral load > 2.1% in addition to a high expression of T cell activation markers (p = 0.004 comparing incidence to non-HAM-like asymptomatic carriers) [26]. Likewise, incident ATL has only been observed in asymptomatic carriers with both a proviral load > 4% and a high oligoclonality score on flow cytometry [27].
Thus, our data altogether confirmed that low proviral load has a good predictive value for asymptomatic infection, whilst high proviral load identifies those for additional risk evaluation. These observations have enabled much more targeted use of clinical resources, reduced the attendances of low-risk (low proviral load) asymptomatic carriers and opened the door to early interventions.
4. Final Considerations
High HTLV-1 proviral load has been found to be associated with a broad range of HTLV-1-associated diseases found across the world. Data from six cohorts totalling 19 incident cases of HAM among initially asymptomatic carriers and 20 incident cases of ATL among people living with HTLV further demonstrate that high proviral load predates both inflammatory (HAM) and malignant (ATL) HTLV-1-associated disease. In the London clinic, using qPCR the median proviral load among asymptomatic carriers is 1.8%, and HTLV-1-associated disease is only observed in PLWH above 1% (and above 4% for ATL). Although there is broad agreement that HTLV-1 proviral load is significantly (approaching 10-fold) higher in those who have or who will develop HTLV-1-associated disease, each centre should determine the appropriate cut-off according to their methods and assay. However, it is likely that this will be in the region of a proviral load of 1%. This will allow PLWH at a lower proviral load to be reassured and followed up less frequently. In our practice, we continue to offer annual review of PLWH and low viral load, as our data are still only based on a maximum of three decades of follow-up. Although there have been reports of proviral load increasing prior to the onset of disease, we have not observed this amongst >500 asymptomatic carriers, but offering annual or even biennial review addresses this possibility.
Although the predominant impact of HTLV-1 among Indigenous Australians appears to differ from other geographic regions (noting the potential impact of multiple differences in virus strain, host, environment and time of infection), there is a clear overlap with pulmonary disease associations, particularly bronchiectasis, which in addition to Australia has been reported in association with HTLV-1 in South America, Japan and Europe. Closing the data gap by demonstrating that high proviral load also predates the onset of HTLV-1-associated non-communicable diseases [45] would be useful to better understand this relationship but requires adequate follow-up of initially asymptomatic carriers and should include other measures such as markers of inflammation. More research on the impact of HTLV-1 proviral load on the risk of transmission would also be beneficial. Meanwhile, since high proviral load is essential but not sufficient for the development of HTLV-1-associated diseases, proviral load monitoring can be used to support the care of people living with HTLV-1 by identifying those most at risk of HTLV-1-associated diseases. The use of the quantification of infection burden to determine management is increasingly common in virology, and in HTLV infection a single measurement might allow the targeted utilisation of resources.
Author Contributions
G.P.T.: conceptualization, writing—original draft preparation. C.R.: writing—review and editing. W.E.: data curation and analysis. All authors have read and agreed to the published version of the manuscript.
Funding
G.P.T. is supported by the Imperial NIHR Biomedical Research Centre. This work was supported by the Medical Research Council (grant number MR/X022358/1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nagai, M.; Usuku, K.; Matsumoto, W.; Kodama, D.; Takenouchi, N.; Moritoyo, T.; Hashiguchi, S.; Ichinose, M.; Bangham, C.R.; Izumo, S.; et al. Analysis of HTLV-I Proviral Load in 202 HAM/TSP Patients and 243 Asymptomatic HTLV-I Carriers: High Proviral Load Strongly Predisposes to HAM/TSP. J. Neurovirol. 1998, 4, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lezin, A.; Olindo, S.; Oliere, S.; Varrin-Doyer, M.; Marlin, R.; Cabre, P.; Smadja, D.; Cesaire, R. Human T Lymphotropic Virus Type I (HTLV-I) Proviral Load in Cerebrospinal Fluid: A New Criterion for the Diagnosis of HTLV-I-Associated Myelopathy/Tropical Spastic Paraparesis? J. Infect. Dis. 2005, 191, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Demontis, M.A.; Hilburn, S.; Taylor, G.P. Human T Cell Lymphotropic Virus Type 1 Viral Load Variability and Long-Term Trends in Asymptomatic Carriers and in Patients with Human T Cell Lymphotropic Virus Type 1-Related Diseases. AIDS Res. Hum. Retroviruses 2012, 28, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Dehée, A.; Césaire, R.; Désiré, N.; Lézin, A.; Bourdonné, O.; Béra, O.; Plumelle, Y.; Smadja, D.; Nicolas, J.C. Quantitation of HTLV-I Proviral Load by a TaqMan Real-Time PCR Assay. J. Virol. Methods 2002, 102, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.S.; Salustiano, S.; Santos, E.V.; Slavov, S.N.; Picanço-Castro, V.; Maçonetto, J.M.; de Haes, T.M.; Takayanagui, O.M.; Covas, D.T.; Kashima, S. Monitoring of HTLV-1-Associated Diseases by Proviral Load Quantification Using Multiplex Real-Time PCR. J. NeuroVirol. 2022, 28, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kira, J.; Koyanagi, Y.; Yamada, T.; Itoyama, Y.; Goto, I.; Yamamoto, N.; Sasaki, H.; Sakaki, Y. Increased HTLV-I Proviral DNA in HTLV-I–Associated Myelopathy: A Quantitative Polymerase Chain Reaction Study. Ann. Neurol. 1991, 29, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Mochizuki, M.; Yamaguchi, K.; Miyata, N.; Watanabe, T. Increased Number of Circulating HTLV-1 Infected Cells in Peripheral Blood Mononuclear Cells of HTLV-1 Uveitis Patients: A Quantitative Polymerase Chain Reaction Study. Br. J. Ophthalmol. 1995, 79, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Castro-Lima Vargens, C.; Grassi, M.F.R.; Boa-Sorte, N.; Rathsam-Pinheiro, R.H.; Olavarria, V.N.; de Almeida Kruschewsky, R.; Galvão-Castro, B. Keratoconjunctivitis Sicca of Human T Cell Lymphotropic Virus Type 1 (HTLV-1) Infected Individuals Is Associated with High Levels of HTLV-1 Proviral Load. J. Clin. Virol. 2011, 52, 177–180. [Google Scholar] [CrossRef]
- Gillet, N.A.; Cook, L.; Laydon, D.J.; Hlela, C.; Verdonck, K.; Alvarez, C.; Gotuzzo, E.; Clark, D.; Farré, L.; Bittencourt, A.; et al. Strongyloidiasis and Infective Dermatitis Alter Human T Lymphotropic Virus-1 Clonality In Vivo. PLoS Pathog. 2013, 9, e1003263. [Google Scholar] [CrossRef] [PubMed]
- Primo, J.; Siqueira, I.; Nascimento, M.C.F.; Oliveira, M.F.; Farre, L.; Carvalho, E.M.; Bittencourt, A.L. High HTLV-1 Proviral Load, a Marker for HTLV-1 Associated Myelopathy/Tropical Spastic Paraparesis, Is Also Detected in Patients with Infective Dermatitis Associated with HTLV-1. Braz. J. Med. Biol. Res. 2009, 42, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Batista, E.S.; Oliveira, P.D.; Primo, J.; Varandas, C.M.N.; Nunes, A.P.; Bittencourt, A.L.; Farre, L. HTLV-1 Proviral Load in Infective Dermatitis Associated with HTLV-1 Does Not Increase after the Development of HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis and Does Not Decrease after IDH Remission. PLoS Negl. Trop. Dis. 2019, 13, e0007705. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.F.R.; Olavarria, V.N.; Kruschewsky, R.D.A.; Correia, L.C.L.; Maurı, C.; Castro-costa, D.; Mascarenhas, E.; Galvao, B. Human T Cell Lymphotropic Virus Type 1 (HTLV-1) Proviral Load of HTLV-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) Patients According to New Diagnostic Criteria of HAM/TSP. J. Med. Virol. 2011, 1274, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- de-Mendoza, C.; Pérez, L.; Rando, A.; Reina, G.; Aguilera, A.; Benito, R.; Eirós, J.M.; Rodríguez-Avial, I.; Ortega, D.; Pozuelo, M.J.; et al. HTLV-1-Associated Myelopathy in Spain. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2023, 169, 105619. [Google Scholar] [CrossRef] [PubMed]
- Manzarinejad, M.; Vahidi, Z.; Boostani, R.; Khadem-Rezaiyan, M.; Rafatpanah, H.; Zemorshidi, F. Pentraxin 3, a Serum Biomarker in Human T-Cell Lymphotropic Virus Type-1-Associated Myelopathy Patients and Asymptomatic Carriers. Med. Microbiol. Immunol. 2023, 212, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Tarokhian, H.; Taghadosi, M.; Rafatpanah, H.; Rajaei, T.; Azarpazhooh, M.R.; Valizadeh, N.; Rezaee, S.A.R. The Effect of HTLV-1 Virulence Factors (HBZ, Tax, Proviral Load), HLA Class I and Plasma Neopterin on Manifestation of HTLV-1 Associated Myelopathy Tropical Spastic Paraparesis. Virus Res. 2017, 228, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Best, I.; Adaui, V.; Verdonck, K.; González, E.; Tipismana, M.; Clark, D.; Gotuzzo, E.; Vanham, G. Proviral Load and Immune Markers Associated with Human T-Lymphotropic Virus Type 1 (HTLV-1)-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) in Peru. Clin. Exp. Immunol. 2006, 146, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Yakova, M.; Lézin, A.; Dantin, F.; Lagathu, G.; Olindo, S.; Jean-Baptiste, G.; Arfi, S.; Césaire, R. Increased Proviral Load in HTLV-1-Infected Patients with Rheumatoid Arthritis or Connective Tissue Disease. Retrovirology 2005, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Domingos, J.A.; Soares, L.S.; Bandeira, L.M.; Bonin, C.M.; Vicente, A.C.P.; Zanella, L.; Puga, M.A.M.; Tozetti, I.A.; Motta-Castro, A.R.C.; da Cunha, R.V. Cytokine Profile and Proviral Load among Japanese Immigrants and Non-Japanese Infected with HTLV-1 in a Non-Endemic Area of Brazil. PLoS ONE 2017, 12, e0174869. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.L.; Guimarães, J.C.; Ribas, J.G.; Romanelli, L.C.F.; de Freitas Carneiro-Proietti, A.B. Long-Term Follow-up of HTLV-1 Proviral Load in Asymptomatic Carriers and in Incident Cases of HAM/TSP: What Is Its Relevance as a Prognostic Marker for Neurologic Disease? J. NeuroVirol. 2017, 23, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, T.; Farajifard, H.; Rezaee, S.A.; Azarpazhooh, M.R.; Mahmoudi, M.; Valizadeh, N.; Rafatpanah, H. Different Roles of CXCR1 and CXCR2 in HTLV-1 Carriers and HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) Patients. Med. Microbiol. Immunol. 2019, 208, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Olindo, S.; Lézin, A.; Cabre, P.; Merle, H.; Saint-Vil, M.; Edimonana Kaptue, M.; Signate, A.; Césaire, R.; Smadja, D. HTLV-1 Proviral Load in Peripheral Blood Mononuclear Cells Quantified in 100 HAM/TSP Patients: A Marker of Disease Progression. J. Neurol. Sci. 2005, 237, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.P.; Tosswill, J.H.; Matutes, E.; Daenke, S.; Hall, S.; Bain, B.J.; Davis, R.; Thomas, D.; Rossor, M.; Bangham, C.R.; et al. Prospective Study of HTLV-I Infection in an Initially Asymptomatic Cohort. J. Acquir. Immune Defic. Syndr. 1999, 22, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Hodson, A.; Laydon, D.J.; Bain, B.J.; Fields, P.A.; Taylor, G.P. Pre-Morbid Human T-Lymphotropic Virus Type I Proviral Load, Rather than Percentage of Abnormal Lymphocytes, Is Associated with an Increased Risk of Aggressive Adult T-Cell Leukemia/Lymphoma. Haematologica 2013, 98, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.G.; Dillon, R.; Witkover, A.; Melamed, A.; Demontis, M.-A.; Gillet, N.A.; Mun, L.J.; Bangham, C.R.M.; Cook, L.B.; Fields, P.A.; et al. Evolution of Retrovirus-Infected Premalignant T-Cell Clones Prior to Adult T-Cell Leukemia/Lymphoma Diagnosis. Blood 2020, 135, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.B.M.; Melamed, A.; Demontis, M.A.; Laydon, D.J.; Fox, J.M.; Tosswill, J.H.C.; de Freitas, D.; Price, A.D.; Medcalf, J.F.; Martin, F.; et al. Rapid Dissemination of Human T-Lymphotropic Virus Type 1 during Primary Infection in Transplant Recipients. Retrovirology 2016, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Harding, D.; Rosadas, C.; Tsoti, S.M.; Heslegrave, A.; Stewart, M.; Kelleher, P.; Zetterberg, H.; Taylor, G.P.; Dhasmana, D. Refining the Risk of HTLV-1-Associated Myelopathy in People Living with HTLV-1: Identification of a HAM-like Phenotype in a Proportion of Asymptomatic Carriers. J. NeuroVirol. 2022, 28, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.N.; Haddow, J.; Greiller, C.; Taylor, G.P.; Cook, L.B.M.; Rowan, A.G. Quantification of T Cell Clonality in Human T Cell Leukaemia Virus Type-1 Carriers Can Detect the Development of Adult T Cell Leukaemia Early. Blood Cancer J. 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.S.B.S.; Andrade, R.G.; Romanelli, L.C.F.; Ribeiro, M.A.; Ribas, G.; Torres, E.B.; Barbosa-Stancioli, E.F.; Proietti, A.B.F.C.; Martins, M.L. Monitoring the HTLV-1 Proviral Load in the Peripheral Blood of Asymptomatic Carriers and Patients With HTLV-Associated Myelopathy/Tropical Spastic Paraparesis from a Brazilian Cohort: ROC Curve Analysis to Establish the Threshold for Risk Disease. J. Med. Virol. 2012, 671, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Tanajura, D.; Castro, N.; Oliveira, P.; Neto, A.; Muniz, A.; Carvalho, N.B.; Orge, G.; Santos, S.; Glesby, M.J.; Carvalho, E.M. Neurological Manifestations in Human T-Cell Lymphotropic Virus Type 1 (HTLV-1)-Infected Individuals Without HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis: A Longitudinal Cohort Study. Clin. Infect. Dis. 2015, 61, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Haziot, M.E.; Gascon, M.R.; Assone, T.; Fonseca, L.A.M.; Luiz, O.d.C.; Smid, J.; Paiva, A.M.; Marcusso, R.M.d.N.; de Oliveira, A.C.P.; Casseb, J. Detection of Clinical and Neurological Signs in Apparently Asymptomatic HTLV-1 Infected Carriers: Association with High Proviral Load. PLoS Negl. Trop. Dis. 2019, 13, e0006967. [Google Scholar] [CrossRef] [PubMed]
- Orland, J.R.; Engstrom, J.; Fridey, J.; Sacher, R.A.; Smith, J.W.; Nass, C.; Garratty, G.; Newman, B.; Smith, D.; Wang, B.; et al. Prevalence and Clinical Features of HTLV Neurologic Disease in the HTLV Outcomes Study. Neurology 2003, 61, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Okayama, A.; Stuver, S.; Matsuoka, M.; Ishizaki, J.; Tanaka, G.; Kubuki, Y.; Mueller, N.; Hsieh, C.; Tachibana, N.; Tsubouchi, H. Role of HTLV-1 Proviral DNA Load and Clonality in the Development of Adult T-cell Leukemia/Lymphoma in Asymptomatic Carriers. Int. J. Cancer 2004, 110, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Watanabe, T.; Utsunomiya, A.; Okayama, A.; Uchimaru, K.; Koh, K.-R.; Ogata, M.; Kikuchi, H.; Sagara, Y.; Uozumi, K.; et al. Human T-Cell Leukemia Virus Type I (HTLV-1) Proviral Load and Disease Progression in Asymptomatic HTLV-1 Carriers: A Nationwide Prospective Study in Japan. Blood 2010, 116, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Nakashima, H.; Matsumoto, M.; Uyama, E.; Ando, M.; Araki, S. Pulmonary Involvement in Patients with HTLV-I-Associated Myelopathy: Increased Soluble IL-2 Receptors in Bronchoalveolar Lavage Fluid. Am. Rev. Respir. Dis. 1989, 139, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Mita, S.; Tokunaga, M.; Yamaguchi, K.; Cho, I.; Matsumoto, M.; Mochizuki, M.; Araki, S.; Takatsuki, K.; Ando, M. Pulmonary Involvement in Human T-Cell Lymphotropic Virus Type-I Uveitis: T-Lymphocytosis and High Proviral DNA Load in Bronchoalveolar Lavage Fluid. Eur. Respir. J. 1993, 6, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Okada, F.; Ando, Y.; Yoshitake, S.; Yotsumoto, S.; Matsumoto, S.; Wakisaka, M.; Maeda, T.; Mori, H. Pulmonary CT Findings in 320 Carriers of Human T-Lymphotropic Virus Type 1. Radiology 2006, 240, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Steinfort, D.P.; Brady, S.; Weisinger, H.S.; Einsiedel, L. Bronchiectasis in Central Australia: A Young Face to an Old Disease. Respir. Med. 2008, 102, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Einsiedel, L.; Fernandes, L.; Spelman, T.; Steinfort, D.; Gotuzzo, E. Bronchiectasis Is Associated with Human T-Lymphotropic Virus 1 Infection in an Indigenous Australian Population. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Einsiedel, L.; Cassar, O.; Goeman, E.; Spelman, T.; Au, V.; Hatami, S.; Joseph, S.; Gessain, A. Higher Human T-Lymphotropic Virus Type 1 Subtype C Proviral Loads Are Associated with Bronchiectasis in Indigenous Australians: Results of a Case-Control Study. Open Forum Infect. Dis. 2014, 1, ofu023. [Google Scholar] [CrossRef] [PubMed]
- Honarbakhsh, S.; Taylor, G.P. High Prevalence of Bronchiectasis Is Linked to HTLV-1-Associated Inflammatory Disease. BMC Infect. Dis. 2015, 15, 258. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Elborn, S.J.; Floto, A.R.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.; et al. British Thoracic Society Guideline for Bronchiectasis in Adults. Thorax 2019, 74, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Magno Falcão, L.F.; Falcão, A.S.C.; Medeiros Sousa, R.C.; Vieira, W.d.B.; de Oliveira, R.T.M.; Normando, V.M.F.; Dias, G.A.d.S.; Santos, M.C.d.S.; Rocha, R.S.B.; Yoshikawa, G.T.; et al. CT Chest and Pulmonary Functional Changes in Patients with HTLV-Associated Myelopathy in the Eastern Brazilian Amazon. PLoS ONE 2017, 12, e0186055. [Google Scholar] [CrossRef]
- Einsiedel, L.; Cassar, O.; Spelman, T.; Joseph, S.; Gessain, A. Higher HTLV-1c Proviral Loads Are Associated with Blood Stream Infections in an Indigenous Australian Population. J. Clin. Virol. 2016, 78, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.R.; Clauss, C.S.; Cherian, S.; Woodman, R.; Einsiedel, L. Risk Factors for HTLV-1, Acute Kidney Injury, and Urinary Tract Infection among Aboriginal Adults with End Stage Kidney Disease in Central Australia. J. Med. Virol. 2021, 93, 6362–6370. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.R.; Woodman, R.; Pham, H.; Wilson, K.; Gessain, A.; Kaldor, J.; Einsiedel, L. High Human T Cell Leukaemia Virus Type 1c Proviral Loads Are Associated with Diabetes and Chronic Kidney Disease: Results of a Cross-Sectional Community Survey in Central Australia. Clin. Infect. Dis. 2022, 76, e820–e826. [Google Scholar] [CrossRef] [PubMed]
- Mozayeni, F.; Rezaee, S.A.; Jabbari Azad, F.; Shabestari, M.; Faridhosseini, R.; Rafatpanah, H.; Yousefzadeh, H.; Garivani, Y.A.; Jarahi, L.; Valizadeh, N.; et al. High Proviral Load of Human T Cell Lymphotropic Virus Type-1 Facilitates Coronary Artery Diseases. Iran. J. Basic Med. Sci. 2020, 23, 500–506. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).