Quantitative Risk Assessment of Wind-Supported Transmission of Highly Pathogenic Avian Influenza Virus to Dutch Poultry Farms via Fecal Particles from Infected Wild Birds in the Environment
Abstract
:1. Introduction
2. Materials and Methods
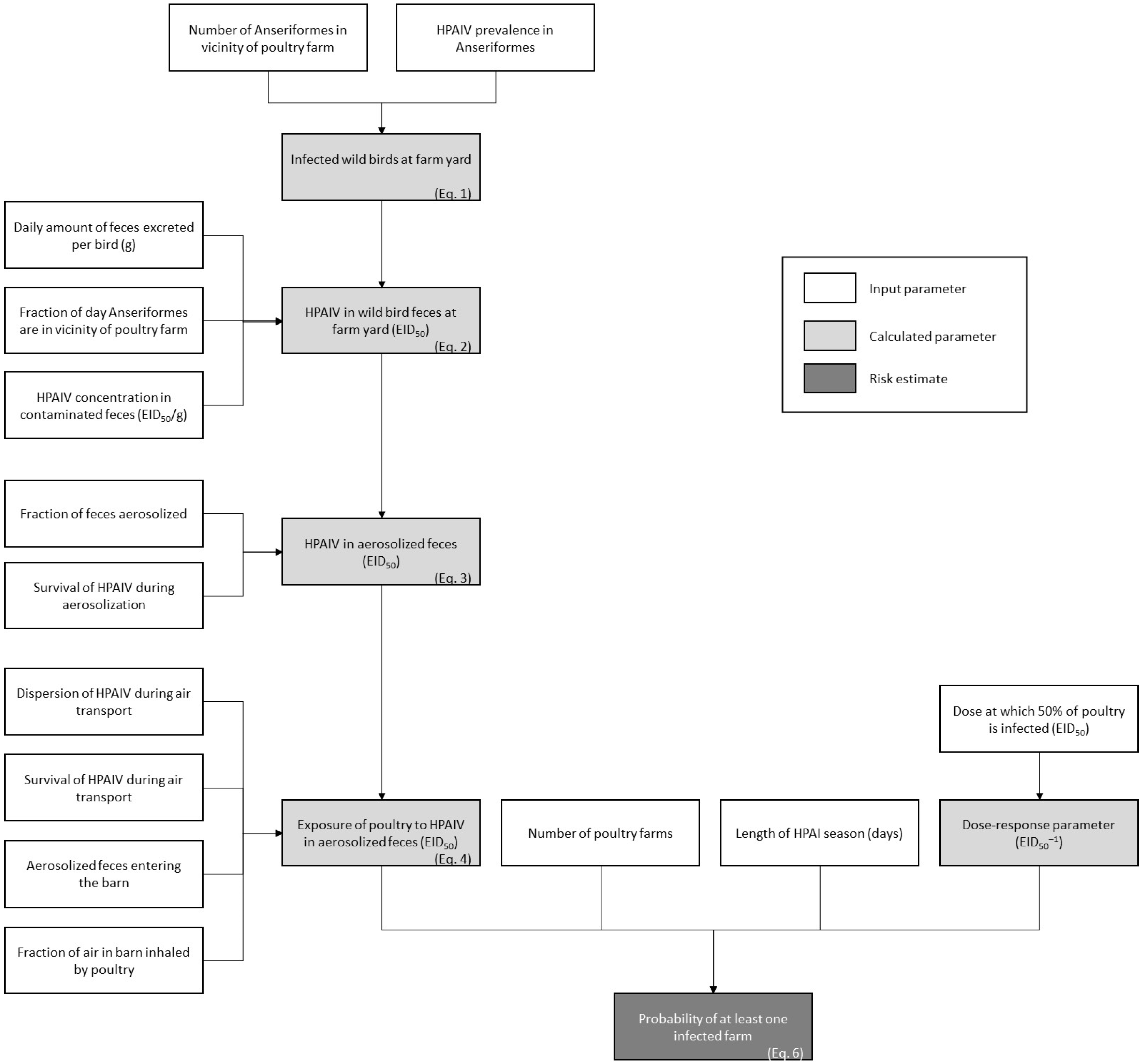
2.1. Model Calculations
2.1.1. Exposure Assessment
2.1.2. Risk Characterization
2.2. Model Input
2.2.1. Exposure Assessment
2.2.2. Risk Characterization
2.3. Uncertainty Analysis
3. Results
3.1. Baseline Scenario
3.2. Uncertainty Analysis
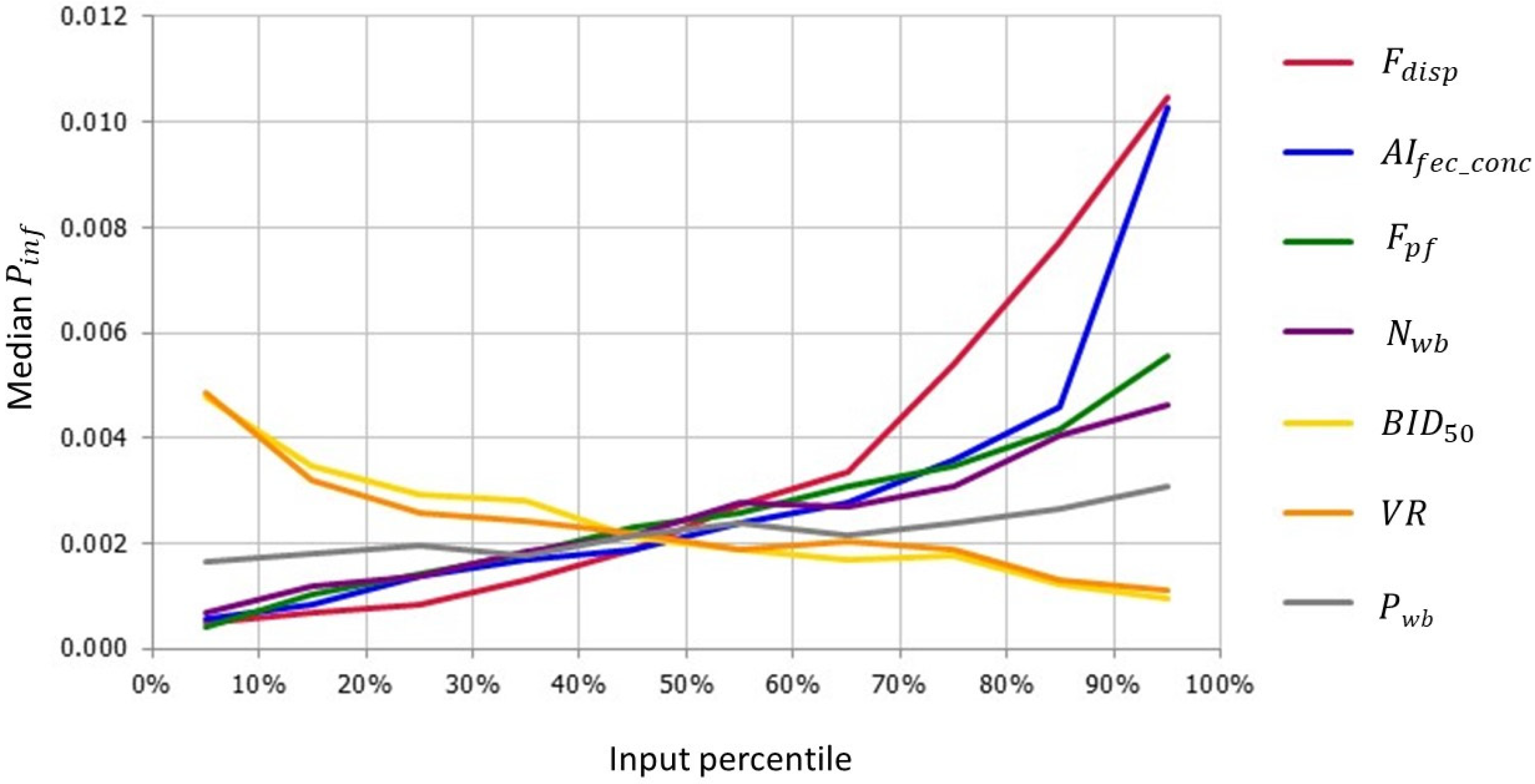
3.2.1. Sensitivity Analysis
3.2.2. What-If Analysis
4. Discussion
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stallknecht, D.E.; Brown, J.D. Tenacity of avian influenza viruses. Rev. Sci. Tech. OIE 2009, 28, 59–67. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Gonzales, J.L. Quantification of visits of wild fauna to a commercial free-range layer farm in the Netherlands located in an avian influenza hot-spot area assessed by video-camera monitoring. Transbound. Emerg. Dis. 2019, 67, 661–677. [Google Scholar] [CrossRef]
- WOAH (World Organisation for Animal Health). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Chapter 3.3.4. Avian Influenza (Including Infection with High Pathogenicity Avian Influenza Viruses). 2023. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ (accessed on 24 June 2024).
- Gonzales, J.L.; Elbers, A.R.W. Effective thresholds for reporting suspicions and improve early detection of avian influenza outbreaks in layer chickens. Sci. Rep. 2018, 8, 8533. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Swayne, D.E. Pathogenesis and pathobiology of avian influenza virus infection in birds. Rev. Sci. Tech. OIE 2009, 28, 113–136. [Google Scholar] [CrossRef]
- Bouwstra, R.; Heutink, R.; Bossers, A.; Harders, F.; Koch, G.; Elbers, A.R.W. Full-Genome Sequence of Influenza A(H5N8) Virus in Poultry Linked to Sequences of Strains from Asia, the Netherlands, 2014. Emerg. Infect. Dis. 2015, 21, 872–874. [Google Scholar] [CrossRef]
- Beerens, N.; Heutink, R.; Bergervoet, S.A.; Harders, F.; Bossers, A.; Koch, G. Multiple Reassorted Viruses as Cause of Highly Pathogenic Avian Influenza A(H5N8) Virus Epidemic, the Netherlands, 2016. Emerg. Infect. Dis. 2017, 23, 1974–1981. [Google Scholar] [CrossRef]
- Beerens, N.; Heutink, R.; Pritz-Verschuren, S.; Germeraad, E.A.; Bergervoet, S.A.; Harders, F.; Bossers, A.; Koch, G. Genetic relationship between poultry and wild bird viruses during the highly pathogenic avian influenza H5N6 epidemic in the Netherlands, 2017–2018. Transbound. Emerg. Dis. 2019, 66, 1370–1378. [Google Scholar] [CrossRef]
- Velkers, F.C.; Manders, T.T.M.; Vernooij, J.C.M.; Stahl, J.; Slaterus, R.; Stegeman, J.A. Association of wild bird densities around poultry farms with the risk of highly pathogenic avian influenza virus subtype H5N8 outbreaks in the Netherlands, 2016. Transb. Emerg. Dis. 2021, 68, 76–87. [Google Scholar] [CrossRef]
- Swayne, D.E.; Suarez, D.L.; Sims, L.D. Influenza. In Diseases of Poultry, 14th ed.; Swayne, D.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 210–256. [Google Scholar]
- Germeraad, E.A.; Sanders, P.; Hagenaars, T.J.; de Jong, M.C.M.; Beerens, N.; Gonzales, J.L. Virus Shedding of Avian Influenza in Poultry: A Systematic Review and Meta-Analysis. Viruses 2019, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Elbers, A.R.W. Outdoor ranging of poultry: A major risk factor for the introduction and development of High-Pathogenicity Avian Influenza. Neth. J. Agric. Sci. 2006, 54, 179–194. [Google Scholar] [CrossRef]
- Jonges, M.; van Leuken, J.; Wouters, I.; Koch, G.; Meijer, A.; Koopmans, M. Wind-Mediated Spread of Low-Pathogenic Avian Influenza Virus into the Environment during Outbreaks at Commercial Poultry Farms. PLoS ONE 2015, 10, e0125401. [Google Scholar] [CrossRef]
- Torremorell, M.; Alonso, C.; Davies, P.R.; Raynor, P.C.; Patnayak, D.; Torchetti, M.; McCluskey, B. Investigation into the Airborne Dissemination of H5N2 Highly Pathogenic Avian Influenza Virus During the 2015 Spring Outbreaks in the Midwestern United States. Avian Dis. 2016, 60, 637–643. [Google Scholar] [CrossRef]
- Scoizec, A.; Niqueux, E.; Thomas, R.; Daniel, P.; Schmitz, A.; Le Bouquin, S. Airborne Detection of H5N8 Highly Pathogenic Avian Influenza Virus Genome in Poultry Farms, France. Front. Vet. Sci. 2018, 5, 15. [Google Scholar] [CrossRef]
- Filaire, F.; Lebre, L.; Foret-Lucas, C.; Vergne, T.; Daniel, P.; Lelièvre, A.; de Barros, A.; Jbenyeni, A.; Bolon, P.; Paul, M.; et al. Highly Pathogenic Avian Influenza A(H5N8) Clade 2.3.4.4b Virus in Dust Samples from Poultry Farms, France, 2021. Emerg. Infect. Dis. 2021, 28, 1446–1450. [Google Scholar] [CrossRef]
- Ssematimba, A.; Hagenaars, T.J.; de Jong, M.C.M. Modelling the wind-borne spread of highly pathogenic avian influenza virus between farms. PLoS ONE 2012, 7, e31114. [Google Scholar] [CrossRef]
- Ypma, R.J.F.; Jonges, M.; Bataille, A.; Stegeman, A.; Koch, G.; van Boven, M.; Koopmans, M.; van Ballegooijen, W.M.; Wallinga, J. Genetic Data Provide Evidence for Wind-Mediated Transmission of Highly Pathogenic Avian Influenza. J. Infect. Dis. 2012, 207, 730–735. [Google Scholar] [CrossRef]
- James, J.; Warren, C.J.; De Silva, D.; Lewis, T.; Grace, K.; Reid, S.M.; Falchieri, M.; Brown, I.H.; Banyard, A.C. The Role of Airborne Particles in the Epidemiology of Clade 2.3.4.4b H5N1 High Pathogenicity Avian Influenza Virus in Commercial Poultry Production Units. Viruses 2023, 15, 1002. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Gonzales, J.L.; Koene, M.G.J.; Germeraad, E.A.; Hakze-van der Honing, R.W.; van der Most, M.; Rodenboog, H.; Velkers, F.C. Monitoring Wind-Borne Particle Matter Entering Poultry Farms Via the Air-Inlet: Highly Pathogenic Avian Influenza Virus and Other Pathogens Risk. Pathogens 2022, 11, 1534. [Google Scholar] [CrossRef]
- Lumivero. @RISK, Probabilistic Risk Analysis in Excel. 2023. Available online: https://lumivero.com/products/at-risk/ (accessed on 18 December 2023).
- Verhagen, J.H.; van der Jeugd, H.P.; Nolet, B.A.; Slaterus, R.; Kharitonov, S.P.; de Vries, P.P.; Vuong, O.; Majoor, F.; Kuiken, T.; Fouchier, R.A. Wild bird surveillance around outbreaks of highly pathogenic avian influenza A(H5N8) virus in the Netherlands, 2014, within the context of global flyways. Eurosurveillance 2015, 20, 21069. [Google Scholar] [CrossRef]
- Poen, M.J.; Verhagen, J.H.; Manvell, R.J.; Brown, I.; Bestebroer, T.M.; van der Vliet, S.; Vuong, O.; Scheuer, R.D.; van der Jeugd, H.P.; Nolet, B.A.; et al. Lack of virological and serological evidence for continued circulation of highly pathogenic avian influenza H5N8 virus in wild birds in the Netherlands, 14 November 2014 to 31 January 2016. Eurosurveillance 2016, 21, 30349. [Google Scholar] [CrossRef] [PubMed]
- Poen, M.J.; Bestebroer, T.M.; Vuong, O.; Scheuer, R.D.; van der Jeugd, H.P.; Kleyheeg, E.; Eggink, D.; Lexmond, P.; van den Brand, J.M.A.; Begeman, L.; et al. Local amplification of highly pathogenic avian influenza H5N8 viruses in wild birds in the Netherlands, 2016 to 2017. Eurosurveillance 2018, 23, 17-00449. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W. Omgevingstransmissie van Aviaire Influenza Virus Door de Lucht via Wilde Watervogels Naar Commercieel Gehouden Pluimvee. Wageningen Bioveterinary Research, Report 2128494; 2021; 82p. Available online: https://edepot.wur.nl/556247 (accessed on 25 January 2024). (In Dutch).
- Veen, J.; Brouwer, J.; Atkinson, P.; Bilgin, C.; Blew, J.; Eksioğlu, S.; Hoffmann, M.; Nardelli, R.; Spina, F.; Tendi, C.; et al. Ornithological Data Relevant to the Spread of Avian Influenza in Europe (Phase 2): Further Identification and First Field Assessment of Higher Risk Species; Wetlands International: Wageningen, The Netherlands, 2007; 60p, Available online: https://www.wetlands.org/publication/ornithological-data-relevant-to-the-spread-of-avian-influenza-in-europe-phase-2/ (accessed on 14 February 2024).
- Burns, T.E.; Ribble, C.; Stephen, C.; Kelton, D.; Toews, L.; Osterhold, J.; Wheeler, H. Use of observed wild bird activity on poultry farms and a literature review to target species as high priority for avian influenza testing in 2 regions of Canada. Can. Vet. J. 2012, 53, 158–166. [Google Scholar] [PubMed]
- Elbers, A.R.W.; Gonzales, J.L. Efficacy of an automated laser for reducing wild bird visits to the free range area of a poultry farm. Sci. Rep. 2021, 11, 12779. [Google Scholar] [CrossRef] [PubMed]
- Le Gall-Ladevèze, C.; Guinat, C.; Fievet, P.; Vollot, B.; Guérin, J.L.; Cappelle, J.; Le Loc’h, G. Quantification and characterisation of commensal wild birds and their interactions with domestic ducks on a free-range farm in southwest France. Sci. Rep. 2022, 12, 9764. [Google Scholar] [CrossRef] [PubMed]
- Martelli, L.; Fornasiero, D.; Scarton, F.; Spada, A.; Scolamacchia, F.; Manca, G.; Mulatti, P. Study of the Interface between Wild Bird Populations and Poultry and Their Potential Role in the Spread of Avian Influenza. Microorganisms 2023, 11, 2601. [Google Scholar] [CrossRef] [PubMed]
- Andrikovics, S.; Gere, G.; Juhász, J.; Lakatos, G. Mallard waste production and effects on water quality in small water bodies. In “Limnology and Aquatic Birds”. Abstracts and Selected Papers from the 4th Conference of the Societas Internationalis Limnologiae (SIL) Aquatic Birds Working Group; Canadian Wildlife Service Technical Report Series No. 474 Atlantic Region; Environment Canada: Ottowa, ON, Canada, 2006; pp. 125–130. [Google Scholar]
- Kear, J. The Agricultural Importance of Wild Goose Droppings. The Wildfowl Trust Fourteenth Annual Report, 1961–1962. 1963, pp. 72–77. Available online: https://wildfowl.wwt.org.uk/index.php/wildfowl/article/view/203 (accessed on 9 February 2024).
- KNMI. Daggegevens van Het Weer in Nederland, Station 260 De Bilt. Koninklijk Nederlands Meteorologisch Instituut, 2023. Available online: https://www.knmi.nl/nederland-nu/klimatologie/daggegevens (accessed on 19 December 2023). (In Dutch).
- Zarkov, I.S.; Urumova, V.S. Effects of humidity and temperature on avian influenza virus H6N2 persistence in faecal samples from experimentally infected ducks (Anas platyrynchos). Revue Méd. Vét. 2013, 164, 343–347. [Google Scholar]
- Lighthart, B.; Mohr, A.J. Estimating downwind concentrations of viable airborne microorganisms in dynamic atmospheric conditions. Appl. Environ. Microbiol. 1987, 53, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.J. Airborne micro-organisms: Survival tests with four viruses. J. Hyg. 1961, 59, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Winkel, A.; Mosquera, L.J.; Groot Oberkampf, P.W.G.; Ogink, N.W.M.; Aarnink, A.J.A. Emissions of particulate matter from animal houses in the Netherlands. Atmos. Environ. 2015, 111, 202–212. [Google Scholar] [CrossRef]
- Zhao, Y.; Richardson, B.; Takle, E.; Chai, L.; Schmitt, D.; Xin, H. Airborne transmission may have played a role in the spread of 2015 highly pathogenic avian influenza outbreaks in the United States. Sci. Rep. 2019, 9, 11755. [Google Scholar] [CrossRef]
- Sergeev, A.A.; Demina, O.K.; Pyankov, O.V.; Pyankova, O.G.; Agafonov, A.P.; Kiselev, S.A.; Agranovski, I.E.; Sergeev, A.A.; Shikov, A.N.; Shishkina, L.N.; et al. Infection of chickens caused by avian influenza virus A/H5N1 delivered by aerosol and other routes. Transbound. Emerg. Dis. 2013, 60, 159–165. [Google Scholar] [CrossRef]
- Agrimatie. Informatie over de Agrosector. Pluimveehouderij, 2023. Available online: https://agrimatie.nl/SectorResultaat.aspx?subpubID=2232§orID=2249 (accessed on 9 February 2024). (In Dutch).
- Gobbo, F.; Fornasiero, D.; De Marco, M.A.; Zecchin, B.; Mulatti, P.; Delogu, M.; Terregino, C. Active Surveillance for Highly Pathogenic Avian Influenza Viruses in Wintering Waterbirds in Northeast Italy, 2020–2021. Microorganisms 2021, 9, 2188. [Google Scholar] [CrossRef]
- Wade, D.; Ashton-Butt, A.; Scott, G.; Reid, S.M.; Coward, V.; Hansen, R.D.E.; Banyard, A.C.; Ward, A.I. High pathogenicity avian influenza: Targeted active surveillance of wild birds to enable early detection of emerging disease threats. Epidemiol. Infect. 2023, 151, e15. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild-Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, T.; Zhao, X.; Qian, J.; Jiang, M.; Jia, M.; Xu, Y.; Yang, W.; Feng, L. High activity levels of avian influenza upwards 2018–2022: A global epidemiological overview of fowl and human infections. One Health 2023, 16, 100511. [Google Scholar] [CrossRef]
- WAHIS. Avian Influenza. World Organization for Animal Health. 2024. Available online: https://www.woah.org/en/disease/avian-influenza/ (accessed on 9 February 2024).
- Purcell, S.L. The Significance of Waterfowl Feces as a Source of Nutrients to Algae in a Prairie Wetland. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 1999; 118p. Available online: https://www.collectionscanada.gc.ca/obj/s4/f2/dsk2/ftp01/MQ41759.pdf (accessed on 9 February 2024).
- Nnaji, J.C. Physico-Chemical Quality and Plankton Density of Water in Duck-Fish Production Systems. Am. Chem. Sci. J. 2014, 4, 975–982. [Google Scholar] [CrossRef]
- Gere, G.; Andrikovics, S.G. Feeding of ducks and their effects on water quality. Hydrobiologica 1994, 279, 157–161. [Google Scholar] [CrossRef]
- Sovon. Aantallen Ganzen. Available online: https://sovon.nl/onderzoek/onderzoeksthemas/ganzen-en-faunaschade/aantallen-ganzen (accessed on 9 February 2024).
- Beerens, N.; Germeraad, E.A.; Venema, S.; Verheij, E.; Pritz-Verschuren, S.B.E.; Gonzales, J.L. Comparative pathogenicity and environmental transmission of recent highly pathogenic avian influenza H5 viruses. Emerg. Microb. Infect. 2021, 10, 97–108. [Google Scholar] [CrossRef]
- Shortridge, K.F.; Zhou, N.N.; Guan, Y.; Gao, P.; Ito, T.; Kawaoka, Y.; Kodihalli, S.; Krauss, S.; Markwell, D.; Murti, K.G.; et al. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 1998, 252, 331–342. [Google Scholar] [CrossRef]
- Kurmi, B.; Murugkar, H.V.; Nagarajan, S.; Tosh, C.; Dubey, S.C.; Kumar, M. Survivability of Highly Pathogenic Avian Influenza H5N1 Virus in Poultry faeces at Different Temperatures. Indian. J. Virol. 2013, 24, 272–277. [Google Scholar] [CrossRef]
- Sedlmaier, N.; Hoppenheidt, K.; Krist, H.; Lehmann, S.; Lang, H.; Büttner, M. Generation of avian influenza virus (AIV) contaminated fecal fine particulate matter (PM(2.5)): Genome and infectivity detection and calculation of immission. Vet. Microbiol. 2009, 139, 156–164. [Google Scholar] [CrossRef]
- Gonzales, J.L.; Stegeman, J.A.; Koch, G.; de Wit, S.J.; Elbers, A.R.W. Rate of introduction of a low pathogenic avian influenza virus infection in different poultry production sectors in the Netherlands. Influ. Other Respir. Viruses 2013, 7, 6–10. [Google Scholar] [CrossRef]
- Gonzales, J.L.; Hennen, W.H.G.J.; Petie, R.; de Freitas Costa, E.; Beerens, N.; Slaterus, R.; Kuiken, T.; Stahl, J.; Elbers, A.R.W. Risicofactoren Voor Introductie van HPAI-virus op Nederlandse Commerciële Pluimveebedrijven, 2014–2022. Wageningen Bioveterinary Research Report 2211632. 2022. Available online: https://edepot.wur.nl/586242 (accessed on 9 February 2024). (In Dutch).
- Brown, J.D.; Stallknecht, D.E.; Valeika, S.; Swayne, D.E. Susceptibility of wood ducks to H5N1 highly pathogenic avian influenza virus. J. Wildl. Dis. 2007, 43, 660–667. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Spackman, E.; Leyson, C.; Youk, S.; Lee, S.A.; Moon, L.M.; Torchetti, M.K.; Killian, M.L.; Lenoch, J.B.; Kapczynski, D.R.; et al. Pathogenicity in Chickens and Turkeys of a 2021 United States H5N1 Highly Pathogenic Avian Influenza Clade 2.3.4.4b Wild Bird Virus Compared to Two Previous H5N8 Clade 2.3.4.4 Viruses. Viruses 2023, 15, 2273. [Google Scholar] [CrossRef]
- Spackman, E.; Pantin-Jackwood, M.J.; Lee, S.A.; Prosser, D. The pathogenesis of a 2022 North American highly pathogenic clade 2.3.4.4b H5N1 avian influenza virus in mallards (Anas platyrhynchos). Avian Path 2023, 52, 219–228. [Google Scholar] [CrossRef]
- Swayne, D.E.; Slemons, R.D. Using mean infectious dose of high- and low-pathogenicity avian influenza viruses originating from wild duck and poultry as one measure of infectivity and adaptation to poultry. Avian Dis. 2008, 52, 455–460. [Google Scholar] [CrossRef]
- Leyson, C.; Youk, S.S.; Smith, D.; Dimitrov, K.; Lee, D.H.; Larsen, L.E.; Swayne, D.E.; Pantin-Jackwood, M.J. Pathogenicity and genomic changes of a 2016 European H5N8 highly pathogenic avian influenza virus (clade 2.3.4.4) in experimentally infected mallards and chickens. Virology 2019, 537, 172–185. [Google Scholar] [CrossRef]
- Seo, I.-H.; Lee, I.-B.; Moon, O.-K.; Jung, N.-S.; Lee, H.-J.; Hong, S.-W.; Kwon, K.-S.; Bitog, J.P. Prediction of the spread of highly pathogenic avian influenza using a multifactor network: Part 1—Development and application of computational fluid dynamics simulations of airborne dispersion. Biosys Eng. 2014, 121, 160–176. [Google Scholar] [CrossRef]
- Zhao, Y.; Aarnink, A.J.A.; De Jong, M.C.M.; Groot Koerkamp, P.W.G. Airborne Microorganisms From Livestock Production Systems and Their Relation to Dust. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1071–1128. [Google Scholar] [CrossRef]
- Van Leuken, J.P.G.; Swart, A.N.; Havelaar, A.H.; Van Pul, A.; Van der Hoek, W.; Heederik, D. Atmospheric dispersion modelling of bioaerosols that are pathogenic to humans and livestock—A review to inform risk assessment studies. Microb. Risk Anal. 2016, 1, 19–39. [Google Scholar] [CrossRef]
- Hagenaars, T.; Gonzales, J.; de Vos, C.; Aarnink, A. Modelling Emission of Bio-Aerosols Carrying Zoonotic Microorganisms from Livestock Houses: Quantification Data and Knowledge Gaps; Report WBVR-1712754; Wageningen University & Research: Lelystad, The Netherlands, 2017; 34p, Available online: https://www.rivm.nl/sites/default/files/2018-12/2017-0062_bijlage8.pdf (accessed on 9 February 2024).
- Belser, J.A.; Pulit-Penaloza, J.A.; Brock, N.; Creager, H.M.; Gustin, K.M.; Tumpey, T.M.; Maines, T.R. Inherent Heterogeneity of Influenza A Virus Stability following Aerosolization. Appl. Environ. Microbiol. 2022, 88, e0227121. [Google Scholar] [CrossRef]
- Keawcharoen, J.; van den Broek, J.; Bouma, A.; Tiensin, T.; Osterhaus, A.D.; Heesterbeek, H. Wild birds and increased transmission of highly pathogenic avian influenza (H5N1) among poultry, Thailand. Emerg. Infect. Dis. 2011, 17, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; Saad, M.; Elassal, E.; Amir, E.; Plathonoff, C.; Bahgat, V.; El-Badry, M.; Ahmed, L.S.; Fouda, M.; Gamaleldin, M.; et al. Surveillance of avian influenza viruses in migratory birds in Egypt, 2003–2009. J Wildl Dis 2012, 48, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Ip, H.S.; Dusek, R.J.; Bodenstein, B.; Torchetti, M.K.; DeBruyn, P.; Mansfield, K.G.; DeLiberto, T.; Sleeman, J.M. High Rates of Detection of Clade 2.3.4.4 Highly Pathogenic Avian Influenza H5 Viruses in Wild Birds in the Pacific Northwest During the Winter of 2014-15. Avian Dis. 2016, 60 (Suppl. 1), 354–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Kang, H.M.; Song, B.M.; Lee, Y.N.; Heo, G.B.; Lee, H.S.; Lee, Y.J.; Kim, J.H. Surveillance of avian influenza viruses in South Korea between 2012 and 2014. Virol. J. 2017, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, V.; Kleyheeg, E.; Beerens, N.; Camphuysen, K.C.J.; Cazemier, R.; Elbers, A.R.W.; Fouchier, R.A.M.; Kelder, L.; Kuiken, T.; Leopold, M.; et al. Effect of 2020–21 and 2021–22 Highly Pathogenic Avian Influenza H5 Epidemics on Wild Birds, the Netherlands. Emerg. Infect. Dis. 2024, 30, 50–57. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control); EURL (European Reference Laboratory on Avian Influenza); Brown, I.; Kuiken, T.; Mulatti, P.; Smietanka, K.; Staubach, C.; Stroud, D.; Therkildsen, O.R.; et al. Scientific report: Avian influenza overview September–November 2017. EFSA J. 2017, 15, 5141. [Google Scholar] [CrossRef]
- Racicot, M.; Venne, D.; Durivage, A.; Vaillantcourt, J.-P. Description of 44 biosecurity errors while entering and exiting poultry barns based on video surveillance in Quebec, Canada. Prev. Vet. Med. 2011, 100, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Velkers, F.C.; Blokhuis, S.J.; Veldhuis Kroeze, E.J.B.; Burt, S.A. The role of rodents in avian influenza outbreaks in poultry farms: A review. Vet. Quart. 2017, 37, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Racicot, M.; Cardinal, A.-M.; Tremblay, D.; Vaillancourt, J.-P. Technologies monitoring and improving biosecurity compliance in barn anterooms. Front. Vet. Sci. 2022, 9, 1005144. [Google Scholar] [CrossRef]

| Model Parameter | Description | Value | Source |
|---|---|---|---|
| Daily probability that wild birds are present at the farm | Beta distribution with α = 43 and β = 95 | [2] | |
| Number of wild birds at the farm yard on a day that birds are present | Pert distribution with min = 1, most likely = 6 and max = 24 | [2] | |
| Expected number of wild birds at the farm yard | Calculated parameter | ||
| Fraction of the day that wild birds spend at the farm yard | Pert distribution with min = 3.4 × 10−4, most likely = 0.16 and max = 0.77 | [2] | |
| Apparent HPAI prevalence in wild birds | Beta distribution with α = 35 and β = 8521 | [22,23,24] | |
| Daily amount of feces excreted by wild birds (dry weight in grams) | Lognormal distribution with mean = 36.9 and SD = 4.37 | [31] | |
| Conversion factor from dry to wet feces | 5 | [32] | |
| Daily amount of feces excreted by wild birds (wet weight in grams) | Calculated parameter | ||
| Concentration of HPAIv in wild bird feces (log10 EID50/g) | Lognormal distribution with mean = 3.8, 2.5 percentile = 3.15 and 97.5 percentile = 4.5 | [11] | |
| Fraction of days with suitable weather conditions for the aerosolization of feces | 0.014 | [33] | |
| Survival of HPAIv during the drying of feces | 10−3.25 | [34] | |
| Fraction of virus retained after the dispersion of aerosols over a short distance | Uniform distribution with min = −2 log10 and max = −0.5 log10 | [35] | |
| Survival of HPAIv during air transport | Uniform distribution with min = 0.61 and max = 0.70 | [36] | |
| Fraction of aerosols entering the barn | 0.05 | Estimate based on surface area of ventilation openings in poultry barns | |
| Ventilation rate of poultry house (layers) (m3/animal/hour) | Pert distribution with min = 1.1, most likely = 3.5 and max = 9 | [37] | |
| Respiratory volume of chickens (layers) (m3/hour) | 0.0224 | [38] | |
| Fraction of air in the poultry house that is inhaled by the animals | Calculated parameter | ||
| Bird infectious dose (log10 EID50) | Normal distribution with mean = 1.2 and SD = 0.2 | [39] | |
| Dose–response parameter (EID50−1) | Calculated parameter | ||
| Number of poultry farms in the Netherlands | 1353 | [40] | |
| Length of bird-flu season (days) | 212.25 | October–April (number corrected for leap years) |
| Scenario | Description | Model Parameter | Baseline Value | New Value | Source |
|---|---|---|---|---|---|
| WI-1 | Higher prevalence in wild birds | Beta distribution with α = 35 and β = 8521 | Beta distribution with α = 41 and β = 930 | [41,42] | |
| WI-2 | Lower dry weight (g) of fecal droppings (data for adult ducks) | Lognormal distribution with mean = 36.9 and SD = 4.37 | Lognormal distribution with mean = 26.3 and SD = 11.5 | [48] | |
| WI-3 | Higher dry weight (g) of fecal droppings (data for greylag geese) | Lognormal distribution with mean = 36.9 and SD = 4.37 | 100 | [32] | |
| WI-4 | Lower concentration of HPAIv in feces (log10 EID50/g) (data for H5N8-2014 in Eurasian wigeon) | Lognormal distribution with mean = 3.8, 2.5 percentile = 3.15 and 97.5 percentile = 4.5 | Lognormal distribution with mean = 3.38 and SD = 0.44 | [50] | |
| WI-5 | Higher concentration of HPAIv in feces (log10 EID50/g) (data for H5N8-2016 in Eurasian wigeon) | Lognormal distribution with mean = 3.8, 2.5 percentile = 3.15 and 97.5 percentile = 4.5 | Lognormal distribution with mean = 4.96 and SD = 0.77 | [50] | |
| WI-6 | Lower survival of HPAIv during the aerosolization (drying) of feces | 10−3.25 | 10−6.5 | Estimate based on [34] | |
| WI-7 | Higher survival of HPAIv during transport of aerosols | Uniform distribution with min = 0.61 and max = 0.70 | 0.99 | [17] | |
| WI-8 | Higher fraction of virus retained after the dispersion of aerosols | Uniform distribution with min = −2 log10 and max = −0.5 log10 | Uniform distribution with min = 0.63 and max = 0.78 | [53] | |
| WI-9 | Lower ventilation rate of poultry houses based on broilers (m3/animal/hour) | Pert distribution with min = 1.1, most likely = 3.5 and max = 9 | Pert distribution with min = 0.1, most likely = 2.1 and max = 9.6 | [37] | |
| WI-10 | Higher bird infectious dose based on H5N8 virus isolated from a tufted duck (log10 EID50) | Normal distribution with mean = 1.2 and SD = 0.2 | Pert distribution with most likely = 4.85, 2.5 percentile = 4.23 and 97.5 percentile = 5.51 MINUS 1.48 to correct for the aerosol inoculation route | [25,39,60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vos, C.J.; Elbers, A.R.W. Quantitative Risk Assessment of Wind-Supported Transmission of Highly Pathogenic Avian Influenza Virus to Dutch Poultry Farms via Fecal Particles from Infected Wild Birds in the Environment. Pathogens 2024, 13, 571. https://doi.org/10.3390/pathogens13070571
de Vos CJ, Elbers ARW. Quantitative Risk Assessment of Wind-Supported Transmission of Highly Pathogenic Avian Influenza Virus to Dutch Poultry Farms via Fecal Particles from Infected Wild Birds in the Environment. Pathogens. 2024; 13(7):571. https://doi.org/10.3390/pathogens13070571
Chicago/Turabian Stylede Vos, Clazien J., and Armin R. W. Elbers. 2024. "Quantitative Risk Assessment of Wind-Supported Transmission of Highly Pathogenic Avian Influenza Virus to Dutch Poultry Farms via Fecal Particles from Infected Wild Birds in the Environment" Pathogens 13, no. 7: 571. https://doi.org/10.3390/pathogens13070571







