Abstract
Here, 12 Fusarium strains, previously described as F. oxysporum f. sp. cepae (Foc), were examined via multi-locus sequencing of calmodulin (cmdA), RNA polymerase II second largest subunit (rpb2), and translation elongation factor 1-alpha (tef1), to verify the taxonomic position of Foc in the newly established epitype of F. oxysporum. The strains in this study were divided into two clades: F. nirenbergiae and Fusarium sp. To further determine the host specifications of the strains, inoculation tests were performed on onion bulbs and Welsh onion seedlings as potential hosts. Four strains (AC145, AP117, Ru-13, and TA) isolated from diseased onions commonly possessed the secreted in xylem (SIX)-3, 5, 7, 9, 10, 12, and 14 genes and were pathogenic and highly aggressive to onion bulbs, whereas all strains except for one strain (AF97) caused significant inhibition of Welsh onion growth. The inoculation test also revealed that the strains harboring the SIX9 gene were highly aggressive to both onion and Welsh onion and the gene was expressed during infection of both onions and Welsh onions, suggesting the important role of the SIX9 gene in pathogenicity. This study provides insights into the evolutionary pathogenicity differentiation of Fusarium strains causing Fusarium basal rot and wilt diseases in Allium species.
1. Introduction
The Fusarium oxysporum species complex (FOSC) contains cosmopolitan, soil-borne, filamentous fungi infecting over 120 economically important crops [1]. F. oxysporum species are divided into “formae speciales” according to host specificity and further subdivided into “race” based on cultivar-specific pathogenicity, which renders F. oxysporum taxonomy complicated [2]. To classify the F. oxysporum species, the genetic similarity of the gene loci, including the internal transcribed spacer (ITS) region, mitochondrial small subunit, RNA polymerase 1 and 2 (rpb1 and rpb2), and translation elongation factor 1-alpha (tef1), has been investigated [3,4,5,6]. However, identification of Fusarium species using a single locus is insufficient to distinguish between different Fusarium species [7]. The epitype of F. oxysporum has been established based on multiple loci, including cmdA, rpb2, and tef1 genes, using phylogenetic analysis to correct the taxonomic classification of F. oxysporum [8]. Moreover, phylogenetic analysis using multi-locus sequences has been used to prevent the incorrect identification of F. oxysporum [3,9,10].
Among F. oxysporum strains, F. oxysporum f. sp. cepae (Foc) is the causative agent of Fusarium basal rot and wilt disease in onions (Allium cepa L.) and Welsh onions (A. fistulosum L.), also known as Japanese bunching onion, which negatively affect onion and Welsh onion production worldwide [11,12,13,14]. Therefore, many studies have focused on the pathogenicity-related factors in Foc to understand the mechanisms by which Foc infects onion and Welsh onion. Secreted in xylem (SIX) genes promote colonization and host infection in F. oxysporum [15,16,17,18]. SIX genes are frequently found on a unique chromosome, known as the pathogenicity chromosome, in F. oxysporum. Pathogenicity chromosomes are necessary for pathogenicity to determine the host range and are transferable among strains [17,19,20,21]. F. oxysporum has been reported to have evolutionally acquired the virulence factor via chromosome transfer to exert host-specific pathogenicity toward individual plant species. Likewise, it has also been reported that the pathogenicity differentiation of F. oxysporum is correlated with individual phylogenetic evolution [4,5].
To date, SIX2, 3, 5, 7, 9, 10, 12, and 14 gene homologs have been identified in the genome of onion- and Welsh onion-infecting Foc strains [22,23]. In the onion-infecting Foc, the SIX3, 5, 7, 9, 10, 12, and 14 genes are located on a single pathogenicity chromosome that is required for full pathogenicity toward onion [22]. Additionally, the SIX3, 5, and 9 genes are highly upregulated during Foc infection of onion [23]. Foc strains showing high expression levels of the SIX9 gene are relatively more aggressive toward onions than those showing low expression levels of the SIX9 gene [24]. Furthermore, the SIX2 and SIX9 genes are located on a 3 Mb chromosome and expressed in Welsh onion-infecting Foc [25]. Pathogenicity-related factors have been extensively elucidated in Foc strains; however, the reasons for their host specificity toward onions and Welsh onions remain unknown.
In this study, we aimed to clarify the taxonomic position of Fusarium strains causing basal rot and wilt disease in onions and Welsh onions and examine their host specificity to onions and Welsh onions.
2. Materials and Methods
2.1. Fungal Strains and Plant Materials
The fungal strains previously identified as F. oxysporum based on morphology and ribosomal DNA ITS region sequences used in this study are listed in Table 1. The onion cultivar Kitamomiji 2000 (Shippou Co., Ltd., Kagawa, Japan) and Welsh onion cultivar Fuyuhiko (Nakahara Seed Product Co., Ltd., Fukuoka, Japan) were used in this study.

Table 1.
Fusarium species isolated from the diseased onion bulbs and Welsh onion seedlings in this study.
2.2. DNA Isolation, Polymerase Chain Reaction (PCR), and Sequencing
Total genomic DNA was extracted from each fungal strain cultured in a potato dextrose broth (PDB) medium (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) for five days at 25 °C, and the fungal mycelia were harvested by filtering the broth through a sterile filter paper (Advantec, Tokyo, Japan). Fungal DNA was extracted from the harvested mycelia using the Dr. GenTLE (from yeast) High Recovery Kit (TaKaRa, Osaka, Japan). For PCR and sequence analysis of the SIX genes, we used a 20 µL reaction mixture containing 10 µL of Quick Taq HS Dye Mix (Toyobo, Osaka, Japan), 0.2 µM of designated primer, and 20 ng of genomic DNA. Thermal cycling conditions consisted of 2 min at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 55–68 °C, and 1 min at 68 °C. PCR products were separated via electrophoresis on a 1.2% (w/v) agarose gel (NIPPON GENE, Tokyo, Japan), stained with ethidium bromide, and visualized under UV light. For sequencing analysis, the PCR amplicon was purified via ethanol precipitation and labeled with the BigDye Terminator v3.1 Cycle sequencing Kit (Applied Biosystems, Foster City, CA, USA). The labeled DNA amplicons were sequenced using the ABI 3500xL genetic analyzer (Applied Biosystems). All primers used in this study are listed in Table S1. The sequences of calmodulin (cmdA), RNA polymerase II second largest subunit (rpb2), and translation elongation factor 1 (tef1) of each strain used in this study were deposited into the DNA Data Bank of Japan (https://www.ddbj.nig.ac.jp/index-e.html: accessed on 18 January 2024).
2.3. Phylogenetic Analysis
A multi-locus phylogenetic tree was constructed using the maximum likelihood method. The sequences of individual loci, including cmdA, rpb2, and tef1, were aligned using Clustal 2.1 [26]. The phylogenetic tree was constructed using MEGA 11 [27].
2.4. Onion Bulb Inoculation Test
Each strain was grown on potato dextrose agar (PDA) medium and incubated in a growth chamber at 25 °C for five days. Onion bulbs were surface-sterilized with 0.05% NaOCl for 3 min. Then, the central basal part of the sterilized onion bulbs was hollowed out with a 5 mm cork borer. The edge of the colony was also hollowed out with a 5 mm cork borer and embedded in the hollowed basal part of the sterilized onion bulbs. A planar PDA medium plug was embedded in the basal tissue of hollowed-out onions as a control. The inoculated onion bulbs were placed inside a plastic bag with a wet paper towel and incubated at 25 °C in a temperature-controlled room. After three weeks, symptoms in the inoculated onion bulbs were observed. Symptomatic areas, including mycelia and brown discoloration regions, were manually captured and estimated from the photographs using the Image J software (ver. 1.53) [28]. All tests were conducted with at least three samples per iteration. All experiments were repeated twice.
2.5. Welsh Onion Seedling Inoculation Test
Fungal strains were cultured in the PDB medium for seven days in a growth chamber at 25 °C, with shaking at 120 rpm, and subsequently filtered through three layers of sterilized gauze to collect the spores for inoculation. The spores were rinsed once with sterile water. The number of spores in the suspension was determined using a hemocytometer (Erma Inc., Saitama, Japan) and adjusted to a concentration of 1 × 104 spores/mL. Welsh onion seeds were surface-disinfected with 0.05% NaOCl for 10 min and rinsed with water for 10 min. The disinfected seeds were inoculated with a spore suspension (1 × 104 spores/mL) of each fungal strain or sterilized water (as a control) for 1 h. After treatment, the seven inoculated Welsh onion seeds were sown into plastic pots containing a 4:1 sand/compost mixture and incubated in a growth chamber at 25 °C under a 16 h light/8 h dark photoperiod. Finally, the lengths of the Welsh onion seedlings were measured 21 d post-inoculation, and the average length of the leaves was calculated.
2.6. RNA Extraction and Reverse Transcriptaion (RT)-PCR
For RT-PCR, RNA was extracted from the Welsh onion roots and onion bulbs inoculated with each strain at 7 or 21 d post-inoculation using Sepasol-RNA I Super G (Nacalai Tesque, Kyoto, Japan). Then, the total RNA containing genomic DNA was treated with DNase I (TaKaRa) to remove the genomic DNA. PrimeScript RT-PCR Kit (TaKaRa) was used to synthesize cDNA, following the manufacturer’s instructions. For the RT-PCR of the SIX genes, we used a 20 µL reaction mixture containing 10 µL of Quick Taq HS Dye Mix (Toyobo), 0.2 µM of designated primer, and 1 µL of cDNA. Thermal cycling conditions consisted of 2 min at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 55 °C, and 1 min at 68 °C. PCR products were separated via electrophoresis on a 1.2% (w/v) agarose gel (NIPPON GENE), stained with ethidium bromide, and visualized under UV light.
2.7. Statistical Analyses
Experimental data are represented as the standard error of the mean. Statistically significant differences were determined using one-way analysis of variance followed by Tukey’s honest significant difference post hoc test.
3. Results
3.1. Verification of the Taxonomic Position of Foc Strains
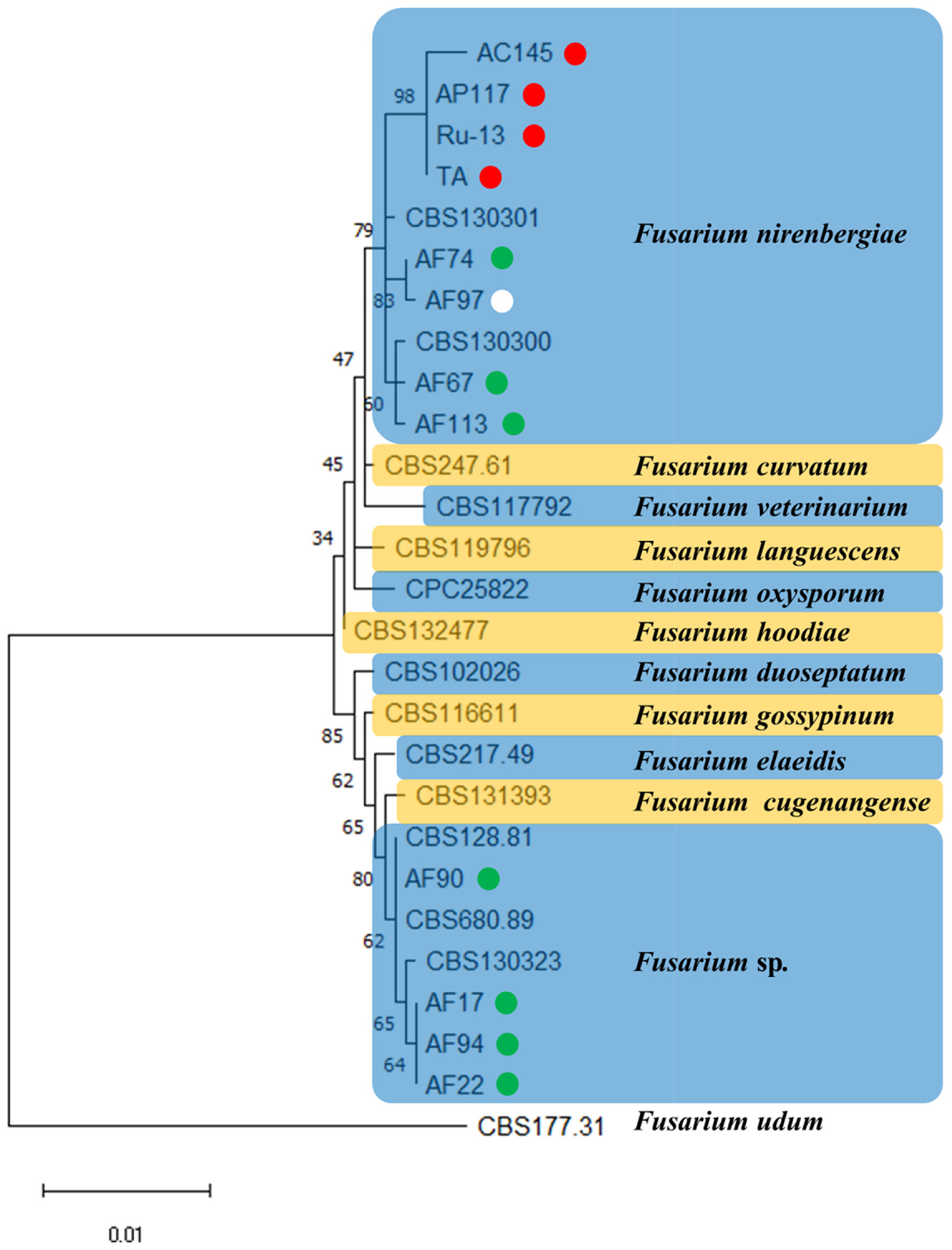
To verify the taxonomic position of strains belonging to the genus Fusarium (previously described as Foc) in the newly established epitype of F. oxysporum, we conducted phylogenetic analysis of the combined sequences of the cmdA, rpb2, and tef1 genes of each strain. Based on the results of phylogenetic analysis, eight strains (AC145, AP117, Ru-13, TA, AF67, AF74, AF97, and AF113) were grouped into a clade of F. nirenbergiae, whereas four strains (AF17, AF22, AF90, and AF94) were grouped into a clade of Fusarium sp. (Figure 1).
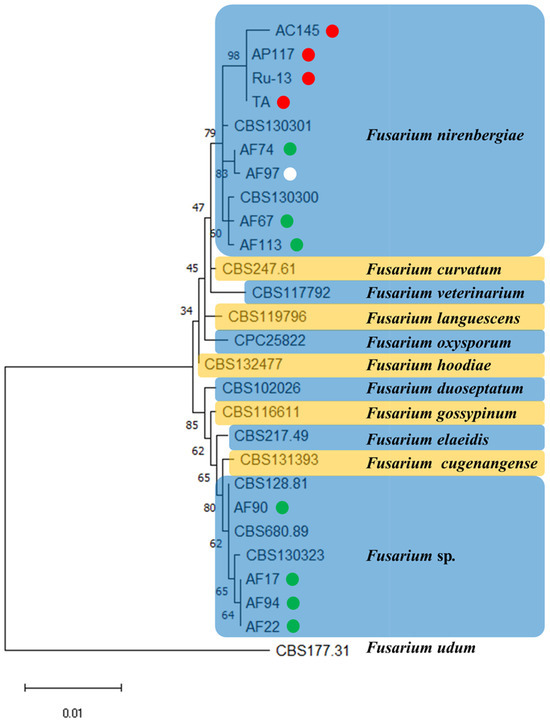
Figure 1.
A maximum likelihood (ML) phylogram inferred from the sequences of calmodulin (cmdA), RNA polymerase II second largest subunit (rpb2), and translation elongation factor 1 (tef1). The numbers on the branches indicate the percentages of congruent clusters in 1000 bootstrap trials. The different color circles next to each strain name in the phylogenetic tree show the pathogenicity and host specificity. Red, green, and white circles indicate pathogenic strain toward onion and Welsh onion (Red circle), pathogenic strain toward Welsh onion (Green circle), and non-pathogenic strain toward onion and Welsh onion (White circle), respectively.
3.2. Variation of SIX Gene Possession and Gene Expression Profiles
Pathogenicity is associated with the presence of SIX genes in F. oxysporum. Here, we verified the presence of SIX genes using PCR to assess the diversity of SIX genes in the strains isolated from onions and Welsh onions. PCR analysis revealed that the strains (AC145, AP117, Ru-13, and TA) isolated from onions commonly harbored the SIX3, 5, 7, 9, 10, 12, and 14 genes. In contrast, the strains isolated from Welsh onions (AF17, AF22, AF90, and AF94) commonly harbored the SIX2 and SIX9 genes. Notably, AF74 possessed only the SIX2 gene, and AF113 possessed only the SIX9 gene. Moreover, there were no SIX genes in the genome of AF67 or AF97 (Table 1).
SIX3, 5, and 9 levels are highly upregulated in onion-infecting Fusarium strains, whereas the SIX2 and SIX9 genes are expressed in Welsh onion-infecting Fusarium strains, suggesting these SIX genes are involved with the pathogenicity of the strains toward their respective hosts [24,26]. Here, we investigated SIX3, 5, and 9 gene expression in the Fusarium strains isolated from onions and SIX2 and 9 gene expression in the Fusarium strains isolated from Welsh onions during infection. Gene expression analysis revealed that the Fusarium strains isolated from onions expressed SIX3, 5, and 9 not only during onion infection but also during Welsh onion infection. In contrast, Fusarium strains isolated from Welsh onions only expressed SIX9 during both onion and Welsh onion infections. Notably, gene expression of SIX2 was dependent on the microbial strain and host (Figure S1).
3.3. Inoculation Tests in Onion Bulbs and Welsh Onion Seedlings
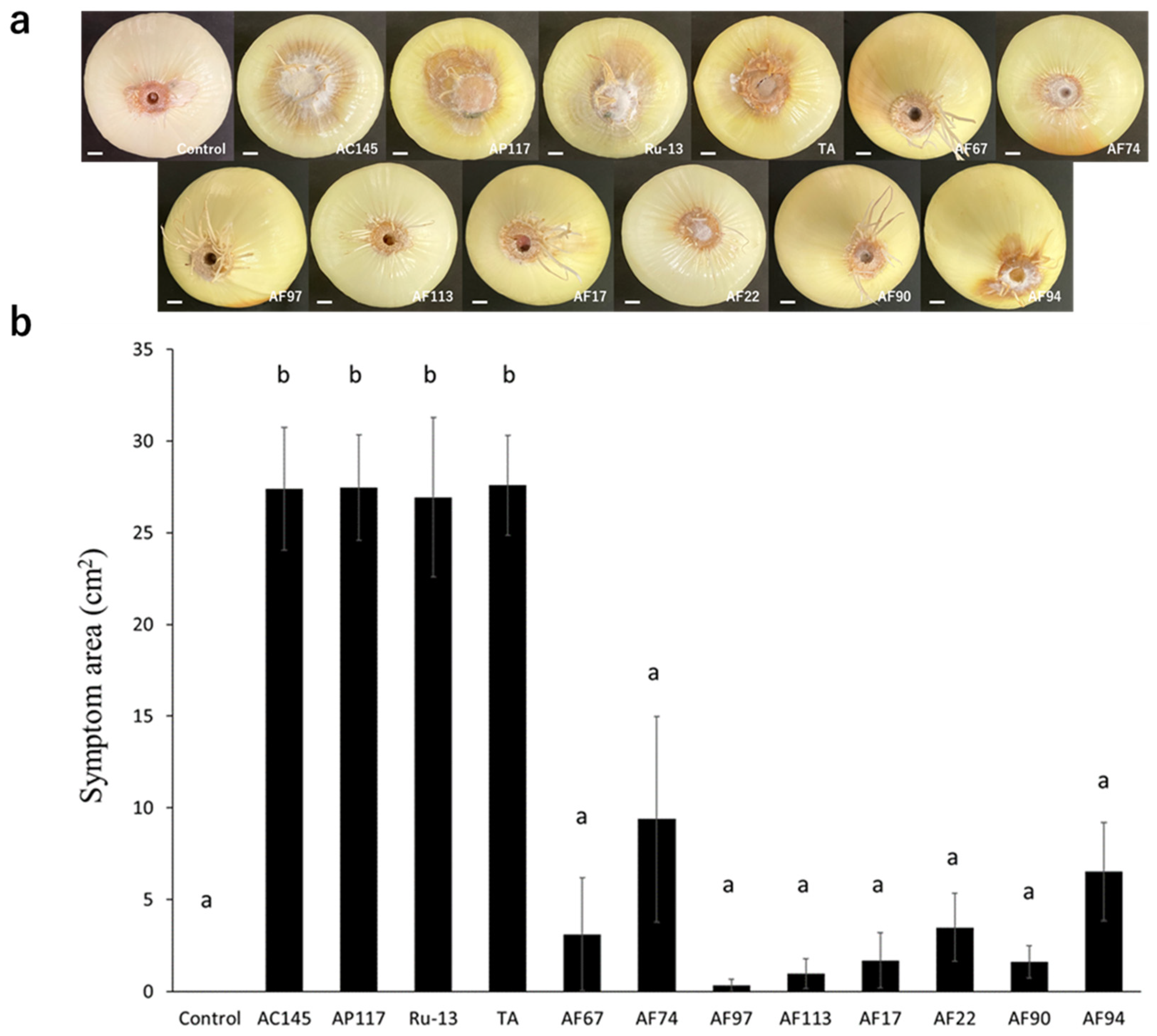
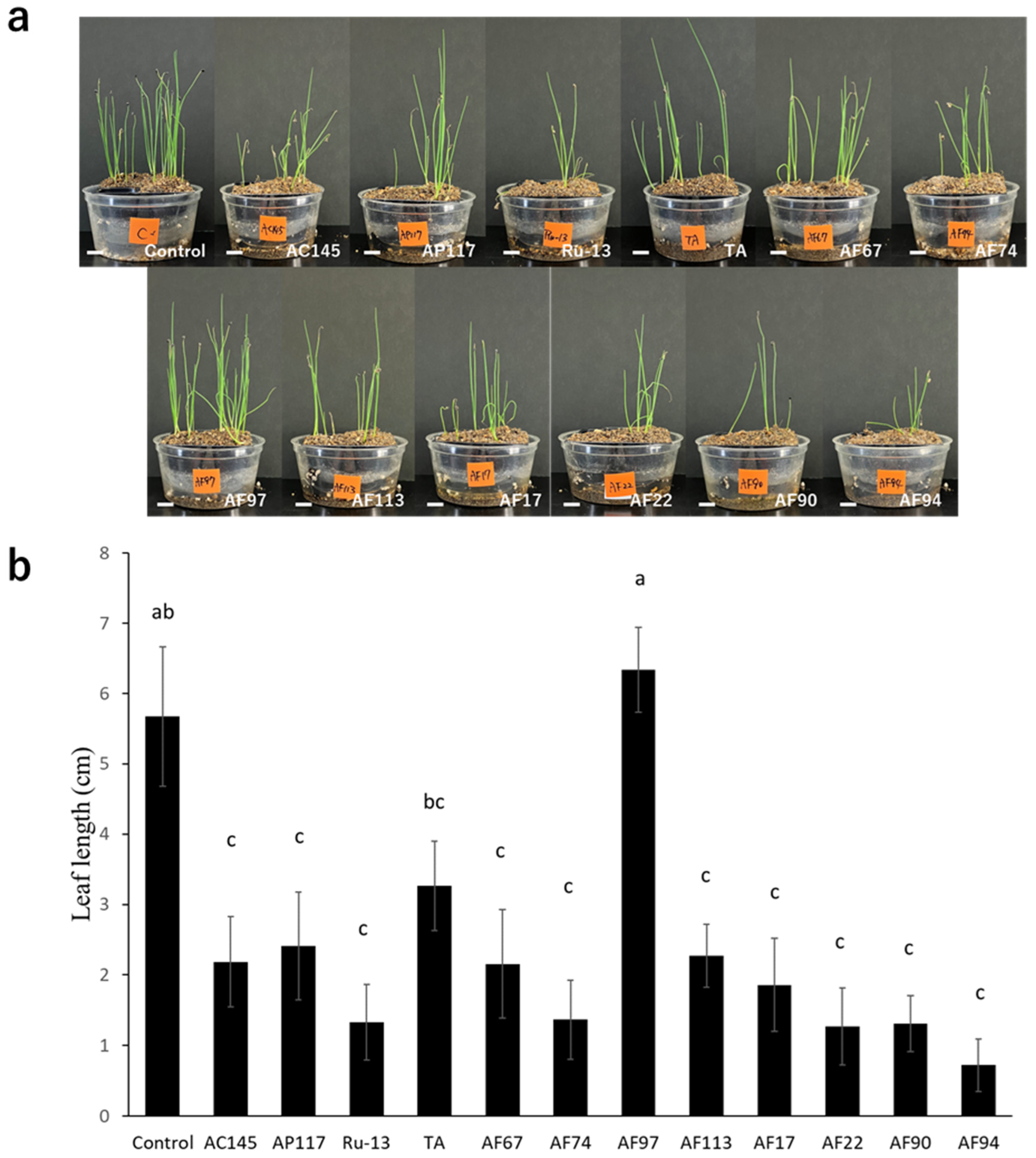
To determine the pathogenicity toward onions, inoculation tests were performed on onion bulbs. Notably, all strains (AC145, AP117, Ru-13, and TA) isolated from onions caused severe disease symptoms in the onion bulbs, whereas the strains (AF17, AF22, AF67, AF74, AF90, AF94, AF97, and AF113) isolated from Welsh onions caused significantly fewer disease symptoms in the onion bulbs (Figure 2). Moreover, the symptoms in onion bulbs caused by the strains AF17, AF22, AF67, AF74, AF90, AF94, AF97, and AF113 were comparable to those observed in the control, with no significant difference. The inoculation test of Welsh onion seedlings revealed that all strains, except AF97, caused significant inhibition of Welsh onion growth (Figure 3).
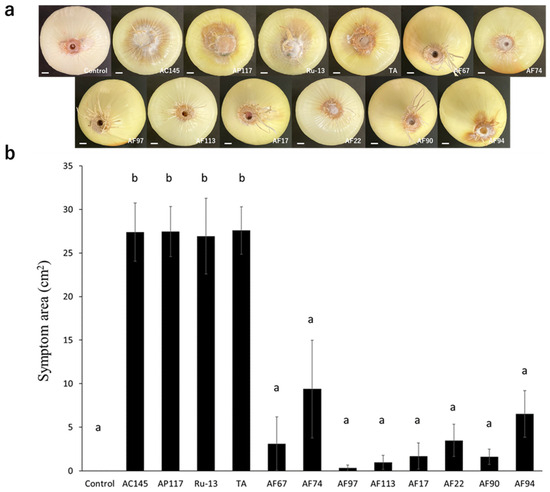
Figure 2.
Onion bulb inoculation test. (a) Representative photograph of the onion bulb inoculation test with the Fusarium species strains used in this study. The white bar indicates the 1 cm scale bar. (b) Evaluation of symptom area in inoculated onion bulbs. The results of two independent experiments are combined. Statistically significant differences at p < 0.05 were analyzed via one-way analysis of variance (ANOVA) followed by Tukey’s honest significant difference (HSD) post hoc test and different letters indicate significant differences. All data are presented as the standard error of the mean (n = 6).
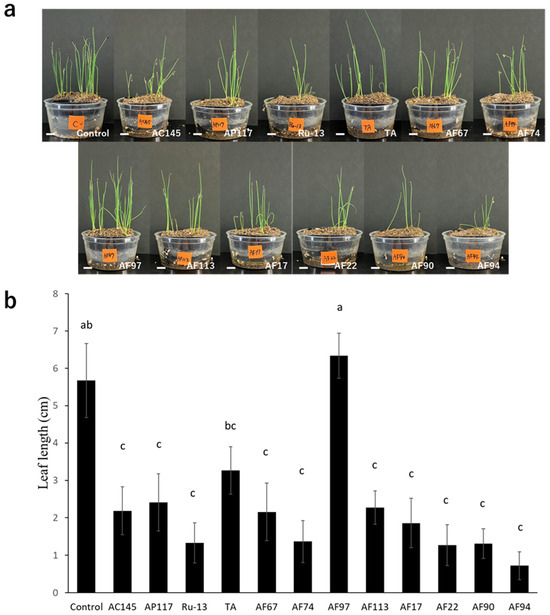
Figure 3.
Welsh onion seedling inoculation test. (a) Representative photograph of Welsh onion seedling inoculation test with the Fusarium species strains used in this study. The white bar indicates the 1 cm scale bar. (b) Evaluation of average leaf length of the Welsh onion seedling. The results of two independent experiments are combined. Statistically significant differences at p < 0.05 were analyzed via one-way ANOVA followed by Tukey’s HSD post hoc test and different letters indicate significant differences. All data are presented as the standard error of the mean (n = 49).
4. Discussion
Plants are cultivated globally to meet the human food demand. However, cultivated plants are at risk of infection by pathogenic fungi, bacteria, and viruses [29,30,31]. Furthermore, due to global climate change caused by global warming, plants and plant pathogenic organisms are shifting to more ideal places than harsh environments to continue their life cycle [32]. Therefore, precise identification of plant pathogenic organisms and understanding of their host specificity are important for sustainable crop production and disease management.
In this study, the taxonomic position of the 12 Fusarium strains causing Fusarium basal rot and wilt disease in onions and Welsh onions was clarified. Based on the phylogenetic analysis of the combined sequences of cmdA, rpb2, and tef1 loci, the strains were divided into F. nirenbergiae and Fusarium sp. clades. Notably, no one strain was identified as F. oxysporum sensu stricto, although all analyzed strains were previously identified as F. oxysporum f. sp. cepae. Presumably, the special form name can be linked to the accessory chromosome that contains genes of pathogenicity-related factors determining the host range and can be transferred via horizontal gene or chromosome transfer [8]. This leads to speculations about the transfer of pathogenicity-related factors among the Fusarium strains isolated from onions and Welsh onions.
To promote host infection, plant pathogenic organisms secrete effector proteins into the host tissue. In the Fusarium fungi, SIX proteins, known as effectors, secreted into xylem tissue have been broadly found [33,34]. Therefore, SIX gene possession analysis was conducted on the strains isolated from onions and Welsh onions. As a result of the SIX gene possession analysis using PCR, the strains (AC145, AP117, Ru-13, and TA) isolated from onions commonly possessed SIX3, 5, 7, 9, 10, 12, and 14, whereas the strains isolated from Welsh onions harbored both SIX2 and SIX9 (AF17, AF22, AF90, and AF94), SIX2 (AF74), SIX9 (AF113), or no SIX genes (AF67 and AF97). Notably, according to our results of the inoculation test for Welsh onion seedlings, all strains except AF97 were pathogenic to Welsh onions. This indicates that the AF97 strain is opportunistic toward Welsh onions. Among the strains used in this study, AF17, 22, 90, and 94, which have the SIX2 and SIX9 genes, caused relatively uniform severe disease in Welsh onion seedlings. Furthermore, the SIX2 and SIX9 genes are located on a single 3 Mb chromosome in the Welsh onion-infecting Fusarium strain [26]. It was speculated that the 3 Mb sized chromosome containing the SIX2 and SIX9 genes was evolutionarily acquired from other strains or that strains constructed the 3 Mb sized chromosome themselves, finally leading to pathogenicity toward Welsh onion. However, we could not clarify the obvious relationship between pathogenicity toward Welsh onion and the SIX genes in this study because the AF67 strains were pathogenic toward Welsh onion but did not harbor any SIX genes, and the gene expression of SIX2 was dependent on the strain and host. Thus, further investigation of various Fusarium strains isolated from diseased Welsh onions would be helpful in understanding pathogenicity differentiation and the relationship between pathogenicity toward Welsh onion and the SIX genes. Moreover, intriguingly, pathogenicity differentiation toward Welsh onion was observed between AF67 (pathogenic) and AF97 (non-pathogenic), strains which lack SIX genes, suggesting that another pivotal factor other than SIX genes exists in pathogenicity toward Welsh onion. Therefore, a comprehensive comparison between AF67 and AF97 with several aspects, including biological characteristics, genome structure, and gene expression, would be desirable to deeply understand the pathogenicity toward Welsh onion.
Inoculation tests for onion bulbs and Welsh onion seedlings revealed that only the strains (AC145, AP117, Ru-13, and TA) isolated from onions were highly aggressive to onion bulbs, whereas all isolates, except for AF97, caused wilt symptoms in Welsh onion seedlings. It is plausible that onion-infecting Fusarium strains evolutionarily acquired the SIX3, 5, 7, 9, 10, 12, and 14 genes for enhanced onion pathogenicity. Previous studies [5,35] have reported that Fusarium species strains containing SIX3 and SIX5 were relatively more aggressive to onions than those not containing these SIX genes, which is consistent with our results. All SIX genes (SIX3, 5, 7, 9, 10, 12, and 14) in the onion-infecting Japanese TA strain are located on a single pathogenicity chromosome [22]. Therefore, it was speculated that the SIX genes (SIX3, 5, 7, 9, 10, 12, and 14) possessed by strains isolated from onions are evolutionarily acquired via horizontal chromosome transfer to enhance the pathogenicity toward onions. Notably, the strains isolated from onions in this study were collected from Japan, Australia, The Netherlands, and Russia. Moreover, the strains (AC145, AP117, Ru-13, and TA) exhibited a common SIX gene profile and caused severe disease in onion bulbs, implying the global distribution of the clonal pathogenic Fusarium strains. Indeed, the Japanese TA strain used in this study exhibited high genome similarity to the pathogenic British Foc_FUS2 strain [22], further supporting our findings.
Here, inoculation tests for onion bulbs and Welsh onion seedlings revealed that the strains harboring the SIX9 gene were highly aggressive to at least either onion or Welsh onion, indicating the importance of SIX9 in pathogenicity. Gene expression of SIX9 is upregulated during infection and associated with the degree of pathogenicity toward onions [24,35,36]. Here, the SIX9 gene was expressed during infection of both onions and Welsh onions, suggesting its role in the pathogenicity toward onions and Welsh onions. Moreover, the SIX9 gene might be involved with a common invasion function in various plant species rather than host-specific pathogenicity. Indeed, in the F. oxysporum spp., F. oxysporum f. sp cubense, lycopersici, narcissi, niveum, palmarum, pisi, radicis-cucumerinum, raphani, sesame, spinaciae, Arabidopsis-infecting F. oxysporum, and non-pathogenic F. oxysporum possess the SIX9 gene [18,21,35,36,37,38,39,40,41]. Recently, gene knockout and gene interference techniques have been used to reveal the functions of genes. Therefore, further research on the SIX9 gene using gene knockout and gene interference techniques is necessary to clarify the specific roles of the SIX9 gene in determining pathogenicity. If the SIX9 gene is related to pathogenicity toward Allium spp., the SIX9 gene could be a useful molecular marker for diagnosis in infected soil to detect the highly pathogenic Fusarium strains toward Allium spp.
In this study, we clarified that the Fusarium strains previously described as Foc were placed in the clade of F. nirenbergiae and Fusarium sp. in the newly established epitype of F. oxysporum and provided insight into pathogenicity differentiation in Fusarium spp. toward Allium spp. Further comprehensive investigation would disclose the pivotal factor required for pathogenicity in Fusarium spp. toward Allium spp. and this information would be a useful tool for effectively conducting disease management and providing an understanding of the molecular mechanism of pathogen–host interaction between Fusarium spp. and Allium spp.
5. Conclusions
In summary, the strains previously identified as Foc were classified into F. nirenbergiae and Fusarium sp., but not F. oxysporum sensu stricto, via phylogenetic analysis of the combined sequences of cmdA, rpb2, and tef1. Furthermore, to verify the host specificity of the strains isolated from onions and Welsh onions, inoculation tests were performed on onions and Welsh onions as potential hosts. The results revealed the diverse host specificity of the Fusarium strains isolated from onions and Welsh onions. The inoculation tests also provided insights into the evolutionary pathogenicity differentiation of the strains causing Fusarium basal rot and wilt disease. We hypothesized that the non-pathogenic Fusarium strain might have evolved to become pathogenic in Welsh onions via the vertical or horizontal acquisition of pathogenicity-related factors. Subsequently, the strain likely evolved pathogenicity toward onions via the acquisition of pathogenicity chromosomes containing SIX genes. As only 12 strains were investigated in this study, more strains should be analyzed in future studies to verify our hypothesis. Nevertheless, this study would provide more certain proof or insight into pathogenicity differentiation in the causative agent of Fusarium basal rot and wilt disease in Allium species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13070591/s1, Figure S1: Gene expression of SIX2, SIX3, SIX5, and SIX9 during infection of onions and Welsh onions; Table S1: Primers used in this study. References [42,43,44,45,46,47] are cited in the supplementary materials.
Author Contributions
Conceptualization, K.S. (Kosei Sakane), M.S., K.S. (Kazunori Sasaki) and S.-i.I.; methodology, K.S. (Kosei Sakane) and K.S. (Kazunori Sasaki); validation, K.S. (Kosei Sakane) and K.S. (Kazunori Sasaki); formal analysis, K.S. (Kosei Sakane); investigation, K.S. (Kosei Sakane), T.U. and K.S. (Kazunori Sasaki); resources, M.S., K.S. (Kazunori Sasaki) and S.-i.I.; writing—original draft preparation, K.S. (Kosei Sakane); writing—review and editing, K.S. (Kosei Sakane), M.S., K.S. (Kazunori Sasaki) and S.-i.I.; visualization, K.S. (Kosei Sakane) and T.U.; supervision, K.S. (Kazunori Sasaki) and S.-i.I.; project administration, S.-i.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequences of calmodulin (cmdA), RNA polymerase II second largest subunit (rpb2), and translation elongation factor 1-alpha (tef1) in each strain used in this study were deposited in DNA Data Bank of Japan (DDBJ).
Acknowledgments
The authors thank Mathieu Pel (Enza Zaden, The Netherlands) for kindly providing the Fusarium oxysporum f. sp. cepae AC145 and AP117 strains.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leslie, J.F.; Summerell, B.A. Fusarium laboratory workshops–A recent history. Mycotoxin Res. 2006, 22, 73. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current status of Fusarium oxysporum formae speciales and races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Nakahara, K.; Tanaka, S.; Shigyo, M.; Ito, S.I. Genetic and pathogenic variability of Fusarium oxysporum f. sp. cepae isolated from onion and Welsh onion in Japan. Phytopathology 2015, 105, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Southwood, M.J.; Viljoen, A.; Mostert, G.; McLeod, A. Molecular identification of two vegetative compatibility groups of Fusarium oxysporum f. sp. cepae. Phytopathology 2012, 102, 204–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.C.; Crous, P.W. Epitypification of Fusarium oxysporum–clearing the taxonomic chaos. Mol. Phylogeny Evol. 2019, 43, 1–47. [Google Scholar] [CrossRef]
- Achari, S.R.; Kaur, J.; Dinh, Q.; Mann, R.; Sawbridge, T.; Summerell, B.A.; Edwards, J. Phylogenetic relationship between Australian Fusarium oxysporum isolates and resolving the species complex using the multispecies coalescent model. BMC Genom. 2020, 21, 248. [Google Scholar] [CrossRef]
- Medeiros Araujo, M.B.; Moreira, G.M.; Nascimento, L.V.; Nogueira, G.D.A.; Nascimento, S.R.D.C.; Pfenning, L.H.; Ambrósio, M.M.D.Q. Fusarium rot of melon is caused by several Fusarium species. Plant Pathol. 2021, 70, 712–721. [Google Scholar] [CrossRef]
- Dissanayake, M.L.M.C.; Kashima, R.; Tanaka, S.; Ito, S.I. Genetic diversity and pathogenicity of Fusarium oxysporum isolated from wilted Welsh onion in Japan. J. Gen. Plant Pathol. 2009, 75, 125–130. [Google Scholar] [CrossRef]
- Haapalainen, M.; Kuivainen, E.; Iivonen, S.; Niemi, M.; Latvala, S. Pathogenicity of Fusarium oxysporum and Fusarium proliferatum isolates from symptomless onions (Allium cepa) and onions with Fusarium basal rot. Plant Pathol. 2023, 72, 1122–1135. [Google Scholar] [CrossRef]
- Haapalainen, M.; Latvala, S.; Kuivainen, E.; Qiu, Y.; Segerstedt, M.; Hannukkala, A.O. Fusarium oxysporum, F. proliferatum and F. redolens associated with basal rot of onion in Finland. Plant Pathol. 2016, 65, 1310–1320. [Google Scholar] [CrossRef]
- Sasaki, K.; Nakahara, K.; Shigyo, M.; Tanaka, S.; Ito, S.I. Detection and quantification of onion isolates of Fusarium oxysporum f. sp. cepae in onion plant. J. Gen. Plant Pathol. 2015, 81, 232–236. [Google Scholar] [CrossRef]
- An, B.; Hou, X.; Guo, Y.; Zhao, S.; Luo, H.; He, C.; Wang, Q. The effector SIX8 is required for virulence of Fusarium oxysporum f. sp. cubense tropical race 4 to Cavendish banana. Fungal Biol. 2019, 123, 423–430. [Google Scholar] [PubMed]
- Gawehns, F.; Houterman, P.M.; Ichou, F.A.; Michielse, C.B.; Hijdra, M.; Cornelissen, B.J.C.; Takken, F.L.W. The Fusarium oxysporum effector Six6 contributes to virulence and suppresses I-2-mediated cell death. Mol. Plant-Microbe Interact. 2014, 27, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Van Der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Rep, M. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, L.F.; Gardiner, D.M.; Kazan, K.; Manners, J.M. A highly conserved effector in Fusarium oxysporum is required for full virulence on Arabidopsis. Mol. Plant-Microbe Interact. 2012, 25, 180–190. [Google Scholar] [CrossRef]
- Ayukawa, Y.; Asai, S.; Gan, P.; Tsushima, A.; Ichihashi, Y.; Shibata, A.; Arie, T. A pair of effectors encoded on a conditionally dispensable chromosome of Fusarium oxysporum suppress host-specific immunity. Commun. Biol. 2021, 4, 707. [Google Scholar] [CrossRef]
- Li, J.; Fokkens, L.; van Dam, P.; Rep, M. Related mobile pathogenicity chromosomes in Fusarium oxysporum determine host range on cucurbits. Mol. Plant Pathol. 2020, 21, 761–776. [Google Scholar] [CrossRef]
- Van Dam, P.; Fokkens, L.; Ayukawa, Y.; van der Gragt, M.; Ter Horst, A.; Brankovics, B.; Rep, M. A mobile pathogenicity chromosome in Fusarium oxysporum for infection of multiple cucurbit species. Sci. Rep. 2017, 7, 9042. [Google Scholar] [CrossRef]
- Sakane, K.; Akiyama, M.; Jogaiah, S.; Ito, S.I.; Sasaki, K. Pathogenicity chromosome of Fusarium oxysporum f. sp. cepae. Fungal Genet. Biol. 2024, 170, 103860. [Google Scholar] [CrossRef]
- Armitage, A.D.; Taylor, A.; Sobczyk, M.K.; Baxter, L.; Greenfield, B.P.; Bates, H.J.; Clarkson, J.P. Characterisation of pathogen-specific regions and novel effector candidates in Fusarium oxysporum f. sp. cepae. Sci. Rep. 2018, 8, 13530. [Google Scholar] [CrossRef] [PubMed]
- Haapalainen, M.; Laitala, E.; Rämö, S.; Latvala, S. Pathogenicity and toxin production of different Fusarium oxysporum isolates infecting onion (Allium cepa L.). Ann. Appl. Biol. 2022, 180, 348–360. [Google Scholar] [CrossRef]
- Sakane, K.; Akiyama, M.; Ando, A.; Shigyo, M.; Ito, S.I.; Sasaki, K. Identification of a novel effector gene and its functional tradeoff in Fusarium oxysporum f. sp. cepae that infects Welsh onion. Physiol. Mol. Plant Pathol. 2023, 123, 101939. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Foster, G.D. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Foster, G.D. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Scholthof, K.B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Foster, G.D. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S. Migrate or evolve: Options for plant pathogens under climate change. Glob. Chang. Biol. 2013, 19, 1985–2000. [Google Scholar] [CrossRef] [PubMed]
- Rep, M.; Van Der Does, H.C.; Meijer, M.; Van Wijk, R.; Houterman, P.M.; Dekker, H.L.; Cornelissen, B.J. A small, cysteine-rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I-3-mediated resistance in tomato. Mol. Microbiol. 2004, 53, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Miura, Y.; Sakane, K.; Ito, S.I.; Sasaki, K. Identification and characterization of Fusarium commune, a causal agent of lotus rhizome rot. J. Gen. Plant Pathol. 2023, 89, 170–178. [Google Scholar] [CrossRef]
- Taylor, A.; Vágány, V.; Jackson, A.C.; Harrison, R.J.; Rainoni, A.; Clarkson, J.P. Identification of pathogenicity-related genes in Fusarium oxysporum f. sp. cepae. Mol. Plant Pathol. 2016, 17, 1032–1047. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Qu, W.; Chang, S.; Li, C.; Xu, F.; Ju, M.; Miao, H. Identification of pathogenicity groups and pathogenic molecular characterization of Fusarium oxysporum f. sp. sesami in China. Phytopathology 2020, 110, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.E.; Fokkens, L.; Takken, F.L.; Rep, M. Number of candidate effector genes in accessory genomes differentiates pathogenic from endophytic Fusarium oxysporum strains. Front. Plant Sci. 2021, 12, 761740. [Google Scholar] [CrossRef] [PubMed]
- Hudson, O.; Fulton, J.C.; Dong, A.K.; Dufault, N.S.; Ali, M.E. Fusarium oxysporum f. sp. niveum molecular diagnostics past, present and future. Int. J. Mol. Sci. 2021, 22, 9735. [Google Scholar] [PubMed]
- Jenkins, S.; Taylor, A.; Jackson, A.C.; Armitage, A.D.; Mead, A.; Harrison, R.J.; Clarkson, J.P. Identification and expression of secreted in xylem pathogenicity genes in Fusarium oxysporum f. sp. pisi. Front. Microbiol. 2021, 12, 593140. [Google Scholar] [CrossRef]
- Ponukumati, S.V.; Elliott, M.L.; Des Jardin, E.A. Comparison of Secreted in Xylem (SIX) genes in two Fusarium wilt pathogens of ornamental palms. Plant Pathol. 2019, 68, 1663–1681. [Google Scholar] [CrossRef]
- Taylor, A.; Armitage, A.D.; Handy, C.; Hulin, M.T.; Harrison, R.J.; Clarkson, J.P. Basal rot of narcissus: Understanding pathogenicity in Fusarium oxysporum f. sp. narcissi. Front. Microbiol. 2019, 10, 492818. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Groenewald, J.Z.; Nakashima, C.; Nishikawa, J.; Shin, H.D.; Park, J.H.; Jama, A.N.; Crous, P.W. Species concepts in Cercospora: Spotting the weeds among the roses. Stud. Mycol. 2013, 75, 115–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Reeb, V.; Lutzoni, F.; Roux, C. Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol. Phylogenetics Evol. 2004, 32, 1036–1060. [Google Scholar] [CrossRef]
- Sasaki, K. Genetic Lineage and Pathogenicity Genes in Fusarium oxysporum f. sp. cepae. Ph.D. Thesis, Tottori University, Tottori, Japan, 2015. [Google Scholar]
- Van der Does, H.C.; Duyvesteijn, R.G.; Goltstein, P.M.; van Schie, C.C.; Manders, E.M.; Cornelissen, B.J.; Rep, M. Expression of effector gene SIX1 of Fusarium oxysporum requires living plant cells. Fungal Genet. Biol. 2008, 45, 1257–1264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).