Turkey Hemorrhagic Enteritis (THE): A Short Overview
Abstract
:1. Introduction
2. Historical Background
3. Current Status
4. Etiology
5. Pathogenesis
THEV Infection and Immune Response
6. The Impact of THEV on Immune Cells: An Analysis of the Main Effects
7. Clinical Signs
8. Macroscopic and Microscopic Lesions
8.1. Macroscopic Findings
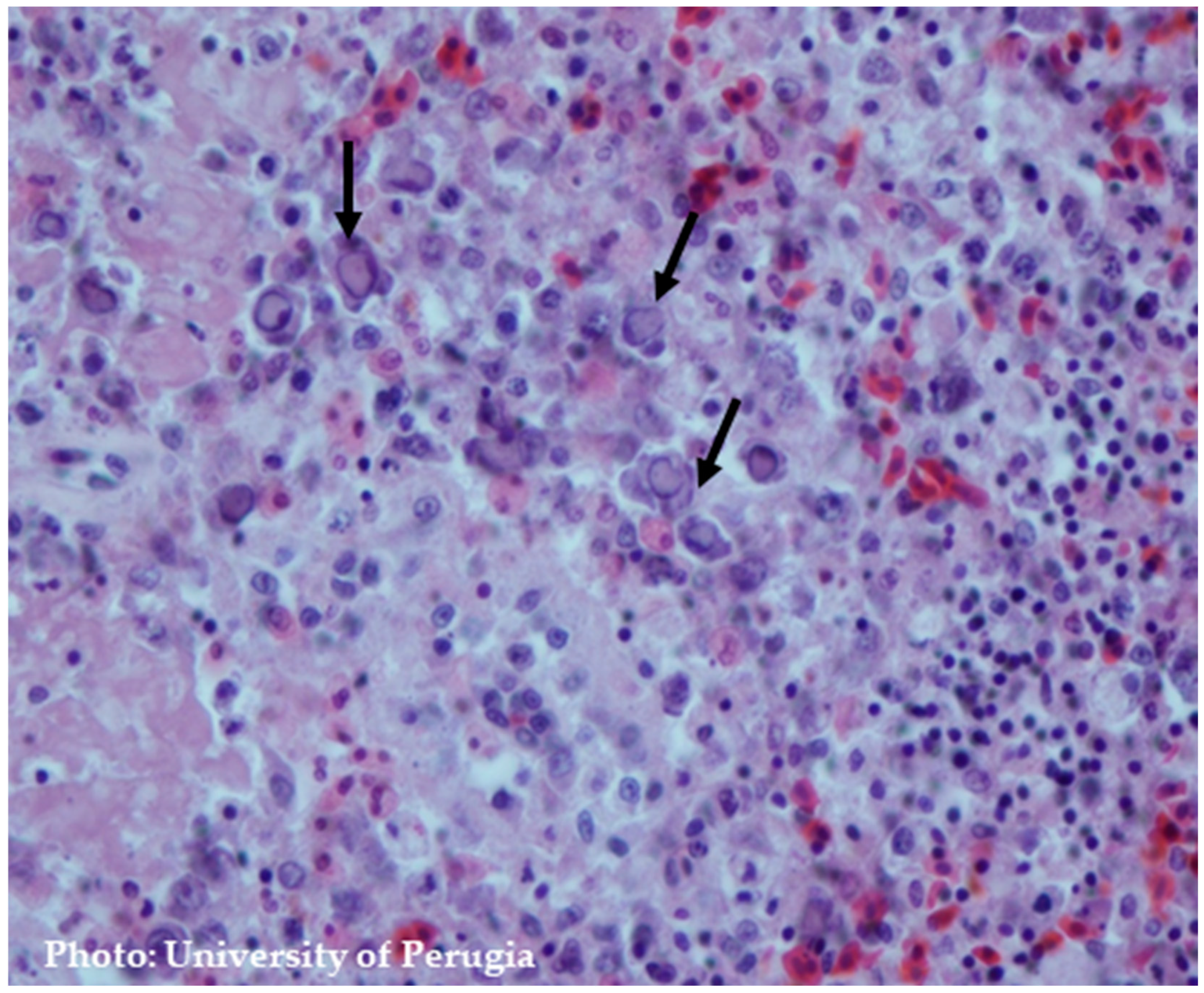
8.2. Microscopic Findings
9. Diagnosis
10. Vaccination Strategies
- I.
- Live-attenuated autogenous vaccine: This vaccine is produced by infecting 6-week-old female Specific Pathogen-Free (SPF) turkeys with the Domermuth strain (Virginia Avirulent Strain) [39]. The administration is carried out via drinking water, and the vaccination starts when the turkeys reach 4 weeks of age. Currently, these vaccines are widely used in the United States and some European countries. Vaccinated flocks demonstrating 60% or higher seroconversion with splenic homogenate indicate full protection. For flocks with low seroconversion, particularly when cell culture-based vaccines are used, a second vaccination should be administered one week after the first. The presence of immunosuppressive agents such as aMPV or residual water sanitizers in the pipeline can reduce vaccination efficacy [69].
- II.
- Inactivated vaccine: The production of this vaccine involves the infection of female turkeys in the shed and then the removal of their spleens to quantify and inactivate the virus. The inactivated vaccine is mixed with liquid paraffin and administered through a subcutaneous injection in the middle third of the neck region. The initial immunization is administered at 3–4 weeks of age, with the subsequent vaccination administered at 7–8 weeks of age.
11. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mottet, A.; Tempio, G. Global Poultry Production: Current State and Future Outlook and Challenges. World’s Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Efremova, A. Role of poultry industry in public food supply. In Proceedings of the 2018 International Scientifi c Conference ‘Economic Sciences for Agribusiness and Rural Economy’ No 2, Warsaw, Poland, 7–8 June 2018; pp. 29–36. [Google Scholar]
- European Parliament. Directorate General for Parliamentary Research Services. In The EU Poultry Meat and Egg Sector: Main Features, Challenges and Prospects: In Depth Analysis; Publications Office: Luxembourg, 2019. [Google Scholar]
- Palomino-Tapia, V.; Mitevski, D.; Inglis, T.; van der Meer, F.; Abdul-Careem, M.F. Molecular Characterization of Hemorrhagic Enteritis Virus (HEV) Obtained from Clinical Samples in Western Canada 2017–2018. Viruses 2020, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Pitcovski, J.; Mualem, M.; Rei-Koren, Z.; Krispel, S.; Shmueli, E.; Peretz, Y.; Gutter, B.; Gallili, G.E.; Michael, A.; Goldberg, D. The Complete DNA Sequence and Genome Organization of the Avian Adenovirus, Hemorrhagic Enteritis Virus. Virology 1998, 249, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Cassmann, E.; Zaffarano, B.; Chen, Q.; Li, G.; Haynes, J. Novel Siadenovirus Infection in a Cockatiel with Chronic Liver Disease. Virus Res. 2019, 263, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Ohya, K.; Kubo, M.; Murata, K.; Yanai, T.; Fukushi, H. A Novel Budgerigar-Adenovirus Belonging to Group II Avian Adenovirus of Siadenovirus. Virus Res. 2009, 144, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Rautenschlein, S.; Suresh, M.; Neumann, U.; Sharma, J.M. Comparative Pathogenesis of Haemorrhagic Enteritis Virus (HEV) Infection in Turkeys and Chickens. J. Comp. Pathol. 1998, 119, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Palya, V.; Nagy, M.; Glávits, R.; Ivanics, E.; Szalay, D.; Dán, A.; Süveges, T.; Markos, B.; Harrach, B. Investigation of Field Outbreaks of Turkey Haemorrhagic Enteritis in Hungary. Acta Vet. Hung. 2007, 55, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Gowthaman, V.; Karthik, K.; Tiwari, R.; Sachan, S.; Kumar, M.A.; Palanivelu, M.; Malik, Y.S.; Singh, R.K.; Munir, M. Haemorrhagic Enteritis of Turkeys—Current Knowledge. Vet. Q. 2017, 37, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald: Adenovirus Infections. Available online: https://scholar.google.com/scholar_lookup?title=Adenovirus+Infections&author=Fitzgerald,+S.D.&author=Rautenschlein,+S.&author=Mahsoub,+H.M.&author=Pierson,+F.W.&author=Reed,+W.M.&author=Jack,+S.W.&publication_year=2020&pages=321%E2%80%93363 (accessed on 27 November 2023).
- Koncicki, A. First report of adenoviral haemorrhagic enteritis in turkeys in Poland. Med. Weter. 1990, 46, 16–17. [Google Scholar]
- Weier, S. Improved immunoprophylaxis against Haemorrhagic Enteritis Virus (HEV) in turkeys with repeated drinking water vaccination. Prakt. Tierarzt 2013, 94, 732–739. [Google Scholar]
- Brugere Picoux, J.; Vaillancourt, J.-P.; Bouzouaia, M. Manual of Poultry Diseases; English ed.; AFAS: Paris, France, 2015; ISBN 978-2-908014-02-0. [Google Scholar]
- Diseases of Poultry, 13th Edition | Wiley. Available online: https://www.wiley.com/en-us/Diseases+of+Poultry%2C+13th+Edition-p-9781118719732 (accessed on 24 July 2024).
- Van Eck, N.J.; Waltman, L. VOSviewer Manual, version 1.6.20; University of Leiden: Leiden, The Netherlands, 2019. [Google Scholar]
- Nagaraja, K.V.; Patel, B.L.; Emery, D.A.; Pomeroy, B.S.; Newman, J.A. In Vitro Depression of the Mitogenic Response of Lymphocytes from Turkeys Infected with Hemorrhagic Enteritis Virus. Am J Vet Res 1982, 43, 134–136. [Google Scholar] [PubMed]
- Diseases of Poultry | Wiley Online Books. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781119371199 (accessed on 13 February 2024).
- Tykałowski, B.; Śmiałek, M.; Koncicki, A.; Ognik, K.; Zduńczyk, Z.; Jankowski, J. The Immune Response of Young Turkeys to Haemorrhagic Enteritis Virus Infection at Different Levels and Sources of Methionine in the Diet. BMC Vet. Res. 2019, 15, 387. [Google Scholar] [CrossRef] [PubMed]
- Darbyshire, J.H.; Peters, R.W. Studies on EDS-76 Virus Infection in Laying Chickens. Avian Pathol. 1980, 9, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Domermuth, C.H.; van der Heide, L.; Faddoul, G.P. Pulmonary Congestion and Edema (Marble Spleen Disease) of Chickens Produced by Group II Avian Adenovirus. Avian Dis. 1982, 26, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Fasina, S.O.; Fabricant, J. In Vitro Studies of Hemorrhagic Enteritis Virus with Immunofluorescent Antibody Technique. Avian Dis. 1982, 26, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, G.; Mandelli, G.; Rampin, T.; Guidobono, L.C.; Gallazzi, D. Enterite Emorragica Da Adenovirus Neltacchino. Clin. Vet. 1977, 100, 437–447. [Google Scholar]
- Ceruti, R.; Ceruti, R.; Valentina, D.M.; Gavazzi, L.; Venni, A.; Ferrazzi, V.; Grilli, G. Haemorrhagic Enteritis Seroconversion in Turkey Breeders: FIeld Observations. Ital. J. Anim. Sci. 2007, 6, 321–325. [Google Scholar] [CrossRef]
- Alessandri, E.; Della Valentina, M.; Vinco, L.J. Enterite Emorragica nel Tacchino: Esperienze di Campo. In Proceedings of the Atti della Società Italiana di Patologia Aviare 2008 In “Tavola Rotonda: Virosi Emergenti e Re-Emergenti Nell’allevamento Avicolo”, Teramo, Italy, 16 May 2008; pp. 25–34. [Google Scholar]
- Ramsubeik, S.; Jerry, C.; Uzal, F.A.; Stoute, S. Necrotic Enteritis in a Commercial Turkey Flock Coinfected with Hemorrhagic Enteritis Virus. J. Vet. Diagn. Invest. 2023, 35, 317–321. [Google Scholar] [CrossRef]
- Gerber, P.F.; Spatz, S.; Gray, P.; Alfirevich, S.; Walkden-Brown, S.W. Circulation and Molecular Characterization of Hemorrhagic Enteritis Virus in Commercial Turkey and Meat Chicken Flocks in Australia. Avian Dis. 2022, 66, 53–59. [Google Scholar] [CrossRef]
- Meteyer, C.U.; Mohammed, H.O.; Chin, R.P.; Bickford, A.A.; Trampel, D.W.; Klein, P.N. Relationship between Age of Flock Seroconversion to Hemorrhagic Enteritis Virus and Appearance of Adenoviral Inclusions in the Spleen and Renal Tubule Epithelia of Turkeys. Avian Dis. 1992, 36, 88–96. [Google Scholar] [CrossRef]
- Droual, R.; Farver, T.B.; Bickford, A.A. Relationship of Sex, Age, and Concurrent Intestinal Disease to Necrotic Enteritis in Turkeys. Avian Dis. 1995, 39, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.F.; Hossain, M.F.; Reynolds, P.; Hoang, P.; Burgess, S.K.; Renz, K.; McMillan, M.; Katz, M.E.; Walkden-Brown, S.W. Propagation of an Avirulent Turkey Hemorrhagic Enteritis Virus Isolate in Chickens. Avian Dis. 2018, 62, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Giovanardi, D.; Lupini, C.; Pesente, P.; Rossi, G.; Ortali, G.; Catelli, E. Longitudinal Field Studies of Avian Metapneumovirus and Turkey Hemorrhagic Enteritis Virus in Turkeys Suffering from Colibacillosis Associated Mortality. Vet. Res. Commun. 2014, 38, 129–137. [Google Scholar] [CrossRef] [PubMed]
- The State of Food Security and Nutrition in the World 2023; FAO: Rome, Italy; IFAD: Rome, Italy; UNICEF: New York, NY, USA; WFP: Rome, Italy; WHO: Geneva, Switzerland, 2023; ISBN 978-92-5-137226-5.
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef] [PubMed]
- Virus Taxonomy, 1st ed.; Elsevier Shop. Available online: https://shop.elsevier.com/books/virus-taxonomy/fauquet/978-0-08-057548-3 (accessed on 13 May 2024).
- Beach, N.M.; Duncan, R.B.; Larsen, C.T.; Meng, X.J.; Sriranganathan, N.; Pierson, F.W. Persistent Infection of Turkeys with an Avirulent Strain of Turkey Hemorrhagic Enteritis Virus. Avian Dis. 2009, 53, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Kleine, A.; Hafez, H.M.; Lüschow, D. Investigations on Aviadenoviruses Isolated from Turkey Flocks in Germany. Avian Pathol. 2017, 46, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Domermuth, C.H.; Gross, W.B. Effect of Disinfectants and Drying on the Virus of Hemorrhagic Enteritis of Turkeys. Avian Dis. 1971, 15, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Domermuth, C.H.; Gross, W.B. Effect of Chlorine on the Virus of Hemorrhagic Enteritis of Turkeys. Avian Dis. 1972, 16, 952–953. [Google Scholar] [CrossRef] [PubMed]
- Domermuth, C.H.; Gross, W.B.; Douglass, C.S.; DuBose, R.T.; Harris, J.R.; Davis, R.B. Vaccination for Hemorrhagic Enteritis of Turkeys. Avian Dis. 1977, 21, 557–565. [Google Scholar] [CrossRef]
- Domermuth, C.H.; Gross, W.B.; Schwartz, L.D.; Mallinson, E.T.; Britt, R. Vaccination of Ring-Necked Pheasant for Marble Spleen Disease. Avian Dis 1979, 23, 30–38. [Google Scholar] [CrossRef]
- Silim, A.; Thorsen, J.; Carlson, H.C. Experimental Infection of Chickens with Hemorrhagic Enteritis Virus. Avian Dis. 1978, 22, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Silim, A.; Thorsen, J. Hemorrhagic Enteritis: Virus Distribution and Sequential Development of Antibody in Turkeys. Avian Dis. 1981, 25, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Wiethoff, C.M.; Nemerow, G.R. Adenovirus Membrane Penetration: Tickling the Tail of a Sleeping Dragon. Virology 2015, 479–480, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Greber, U.F.; Webster, P.; Weber, J.; Helenius, A. The Role of the Adenovirus Protease on Virus Entry into Cells. EMBO J. 1996, 15, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Kremer, E.J.; Nemerow, G.R. Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex. PLoS Pathog. 2015, 11, e1004821. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.T.; Anjo, S.I.; Bhide, M.; Varela Coelho, A.; Manadas, B.; Lecchi, C.; Grilli, G.; Ceciliani, F. Changes in the Intestinal Mucosal Proteome of Turkeys (Meleagris Gallopavo) Infected with Haemorrhagic Enteritis Virus. Vet. Immunol. Immunopathol. 2019, 213, 109880. [Google Scholar] [CrossRef] [PubMed]
- Rautenschlein, S.; Sharma, J.M. Immunopathogenesis of Haemorrhagic Enteritis Virus (HEV) in Turkeys. Dev. Comp. Immunol. 2000, 24, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Kaboudi, K. Virus-Induced Immunosuppression in Turkeys (Meleagris Gallopavo): A Review. Open Vet. J. 2019, 9, 349–360. [Google Scholar] [CrossRef]
- Hossain, M.F.; McMillan, M.; Katz, M.E.; Walkden-Brown, S.W.; Gerber, P.F. Turkey Hemorrhagic Enteritis Virus Can Be Titrated but Not Propagated in Chicken Embryos. Avian Dis. 2019, 63, 84–89. [Google Scholar] [CrossRef]
- Pierson, F.W.; Fitzgerald, S.D. Hemorrhagic Enteritis Andrelated Infections. In Diseases of Poultry, 11th ed.; Saif, Y.M., Barnes, H.J., Glisson, J.R., Fadly, A.M., McDougald, L.R., Swayne, D.E., Eds.; Iowa State University Press: Ames, IA, USA, 2003; pp. 237–247. [Google Scholar]
- Aboezz, Z.; El-Bagoury, G.; Sharawi, S.; El-Nahas, E.; Mahsoub, H.; Pierson, W. Monitoring of a Virulent Turkey Hemorrhagic Enteritis Virus on MDTCRP-19 Cells by Real Time PCR. Benha Vet. Med. J. 2019, 36, 245–251. [Google Scholar] [CrossRef]
- Chandra, R.; Kumar, A. Haemorrhagic Enteritis of Turkeys and Related Infections of Pheasants and Domestic Fowl: A Review. World’s Poult. Sci. J. 1998, 54, 253–269. [Google Scholar] [CrossRef]
- Nazerian, K.; Fadly, A.M. Propagation of Virulent and Avirulent Turkey Hemorrhagic Enteritis Virus in Cell Culture. Avian Dis. 1982, 26, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Schmid, I.; Uittenbogaart, C.H.; Giorgi, J.V. Sensitive Method for Measuring Apoptosis and Cell Surface Phenotype in Human Thymocytes by Flow Cytometry. Cytometry 1994, 15, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Razin, E.; Ihle, J.N.; Seldin, D.; Mencia-Huerta, J.M.; Katz, H.R.; LeBlanc, P.A.; Hein, A.; Caulfield, J.P.; Austen, K.F.; Stevens, R.L. Interleukin 3: A Differentiation and Growth Factor for the Mouse Mast Cell That Contains Chondroitin Sulfate E Proteoglycan. J. Immunol. 1984, 132, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, K.V.; Kang, S.Y.; Newman, J.A. Immunosuppressive Effects of Virulent Strain of Hemorrhagic Enteritis Virus in Turkeys Vaccinated Against Newcastle Disease. Poult. Sci. 1985, 64, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Pertile, T.L.; Sharma, J.M.; Walser, M.M. Reovirus Infection in Chickens Primes Splenic Adherent Macrophages to Produce Nitric Oxide in Response to T Cell-Produced Factors. Cell. Immunol. 1995, 164, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Reiss, C.S.; Komatsu, T. Does Nitric Oxide Play a Critical Role in Viral Infections? J. Virol. 1998, 72, 4547–4551. [Google Scholar] [CrossRef] [PubMed]
- Gross, W.B.; Moore, W.E.C. Hemorrhagic Enteritis of Turkeys. Avian Dis. 1967, 11, 296–307. [Google Scholar] [CrossRef]
- Norton, R.A.; Skeeles, J.K.; Newberry, L.A. Evaluation of the Interaction of Eimeria Meleagrimitis with Hemorrhagic Enteritis Virus or Marble Spleen Disease Virus in Turkeys. Avian Dis. 1993, 37, 290–294. [Google Scholar] [CrossRef]
- Carlson, H.C.; Al-Sheikhly, F.; Pettit, J.R.; Seawright, G.L. Virus Particles in Spleens and Intestines of Turkeys with Hemorrhagic Enteritis. Avian Dis. 1974, 18, 67–73. [Google Scholar] [CrossRef]
- Gómez-Villamandos, J.C.; Carranza, J.; Sierra, M.A.; Carrasco, L.; Hervás, J.; Blanco, A.; Fernández, A. Hemorrhagic Enteritis by Adenovirus-Like Particles in Turkeys: A Possible Pathogenic Mechanism. Avian Dis. 1994, 38, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Itakura, C.; Carlson, H.C.; Lang, G.N. Experimental Transmission of Haemorrhagic Enteritis of Turkeys. Avian Pathol. 1974, 3, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Opengart, K.; Eyre, P.; Domermuth, C.H. Increased Numbers of Duodenal Mucosal Mast Cells in Turkeys Inoculated with Hemorrhagic Enteritis Virus. Am. J. Vet. Res. 1992, 53, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Choi, C.U.; Rings, B.S.; Shaw, D.P.; Nagaraja, K.V. Pathogenesis of Hemorrhagic Enteritis Virus Infection in Turkeys. J. Vet. Med. Ser. B 1993. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1439-0450.1993.tb00196.x (accessed on 11 January 2024).
- Suresh, M.; Sharma, J.M. Hemorrhagic Enteritis Virus Induced Changes in the Lymphocyte Subpopulations in Turkeys and the Effect of Experimental Immunodeficiency on Viral Pathogenesis. Vet. Immunol. Immunopathol. 1995, 45, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.; Gerber, P.F.; Reynolds, P.; McMillan, M.; Gray, P.; Walkden-Brown, S.W. Preliminary Testing in Turkeys of the Safety and Efficacy of a Putative Haemorrhagic Enteritis Virus Vaccine. Aust. Vet. J. 2019, 97, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Razmyar, J.; Peighambari, S.M. Isolation and Characterization of a Very Virulent Infectious Bursal Disease Virus from Turkey. Acta Virol. 2009, 53, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Chary, P.; Rautenschlein, S.; Sharma, J.M. Reduced Efficacy of Hemorrhagic Enteritis Virus Vaccine in Turkeys Exposed to Avian Pneumovirus. Avian Dis. 2002, 46, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Fadly, A.M.; Nazerian, K.; Nagaraja, K.; Below, G. Field Vaccination against Hemorrhagic Enteritis of Turkeys by a Cell-Culture Live-Virus Vaccine. Avian Dis 1985, 29, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.M.; Karaca, K.; Pertile, T. Virus-Induced Immunosuppression in Chickens. Poult. Sci. 1994, 73, 1082–1086. [Google Scholar] [CrossRef]
- Beach, N.; Duncan, R.; Larsen, C.; Meng, X.-J.; Sriranganathan, N.; Pierson, F. Comparison of 12 Turkey Hemorrhagic Enteritis Virus Isolates Allows Prediction of Genetic Factors Affecting Virulence. J. Gen. Virol. 2009, 90, 1978–1985. [Google Scholar] [CrossRef]
- Alkie, T.N.; Guenther, R.; Rautenschlein, S. Molecular Characterization of Hemorrhagic Enteritis Viruses (HEV) Detected in HEV-Vaccinated Commercial Turkey Flocks in Germany. Avian Dis. 2017, 61, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, G.; Di Francesco, A.; Catelli, E.; Mescolini, G.; Lupini, C. Turkey Adenovirus 3: ORF1 Gene Sequence Comparison between Vaccine-like and Field Strains. Vet. Res. Commun. 2023, 47, 2307–2313. [Google Scholar] [CrossRef] [PubMed]




| Genus | Species | Species of Host Affected | Disease |
|---|---|---|---|
| Aviadenovirus (Group I Adenoviruses) | Fowl adenovirus (FAdV) Goose adenovirus (GoSdV) Duck adenovirus B (DAdV 2) Pigeon adenovirus B (PiAdV 2) Turkey adenovirus B (TAdV) | Chickens, turkeys, ducks, geese, pigeons, wild species. | Inclusion body hepatitis, hydropericardium syndrome, gizzard erosions. Adenovirus infection in duck, pigeon, and turkey. |
| Siadenovirus (Group II Adenoviruses) | Turkey adenovirus A (TAdV 3) | Chickens, turkeys, pheasants | Hemorrhagic enteritis (turkey), Marble spleen disease (Pheasant), Avian adenovirus splenomegaly (chicken). |
| Atadenovirus (Group III Adenoviruses) | Duck adenovirus A (DAdV-1) | Ducks, bovine, ovine, deer, possums, snakes | Egg drop syndrome |
| Commercial Name | Company | Country | Strain | Administration Route | Age | Species |
|---|---|---|---|---|---|---|
| Dindoral | Boehringer Ingelheim (Germany) | Europe | Domermuth (Marble Spleen Disease avirulent virus) | Drinking water | From the 4th week | Turkeys, Pheasants |
| Hemorrhagic enteritis vaccine, | Hygieia (Canada) | USA | Type 2 avian adenovirus of pheasant origin | Drinking water | From the 5th week | Turkeys |
| H.E. Vac | Arko (USA) | USA; Canada | Type II avian adenovirus is propagated in a lymphoblastoid cell line (MDTC-RP19) | Drinking water | At thirty days | Turkeys |
| Oralvax-HE | MSD AH (USA& Canada) | USA; Canada | Avirulent Type II avian adenovirus of pheasant origin | Drinking water | 6 weeks or older | Turkeys |
| Pro’tect Hemorrhagic enteritis vaccine | Brinton (USA) | USA | Live cell culture-grown virus | Drinking water | 22 days or older | Turkeys |
| Adenomune ll | Ceva (Canada) | USA | Live avirulent strain of hemorrhagic enteritis virus of pheasant origin | Drinking water | 5 weeks or older | Turkeys |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, L.; Rapi, M.C.; Franciosini, M.P.; Lupini, C.; Catelli, E.; Addis, M.F.; Grilli, G. Turkey Hemorrhagic Enteritis (THE): A Short Overview. Pathogens 2024, 13, 663. https://doi.org/10.3390/pathogens13080663
Musa L, Rapi MC, Franciosini MP, Lupini C, Catelli E, Addis MF, Grilli G. Turkey Hemorrhagic Enteritis (THE): A Short Overview. Pathogens. 2024; 13(8):663. https://doi.org/10.3390/pathogens13080663
Chicago/Turabian StyleMusa, Laura, Maria Cristina Rapi, Maria Pia Franciosini, Caterina Lupini, Elena Catelli, Maria Filippa Addis, and Guido Grilli. 2024. "Turkey Hemorrhagic Enteritis (THE): A Short Overview" Pathogens 13, no. 8: 663. https://doi.org/10.3390/pathogens13080663









