Resurgence of Clinical Malaria in Ethiopia and Its Link to Anopheles stephensi Invasion
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Malaria Epidemiology, Diagnosis, Treatment, and Prevention in Ethiopia
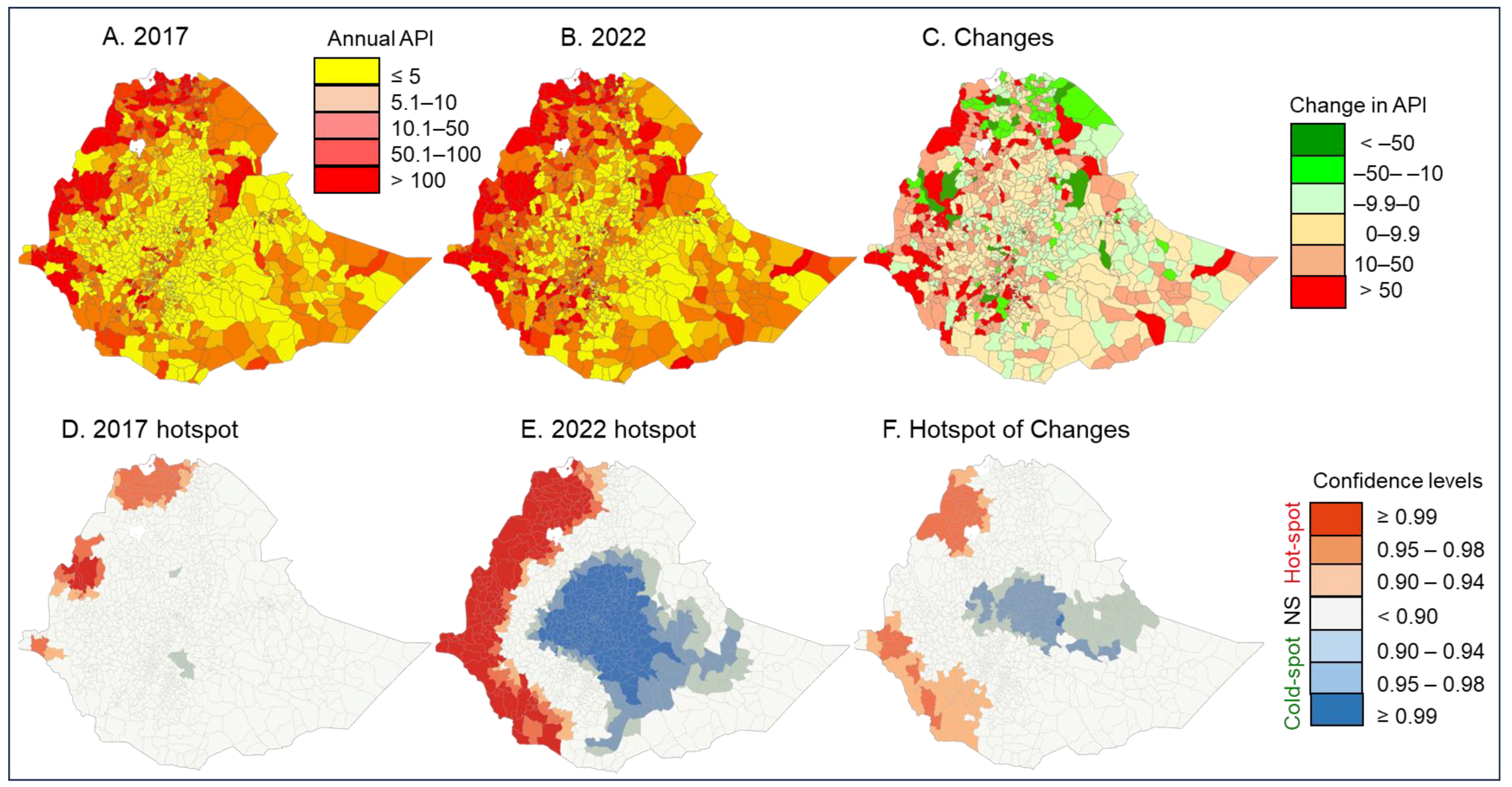
3.2. Changes in Clinical Malaria Incidence from 2017 to 2022
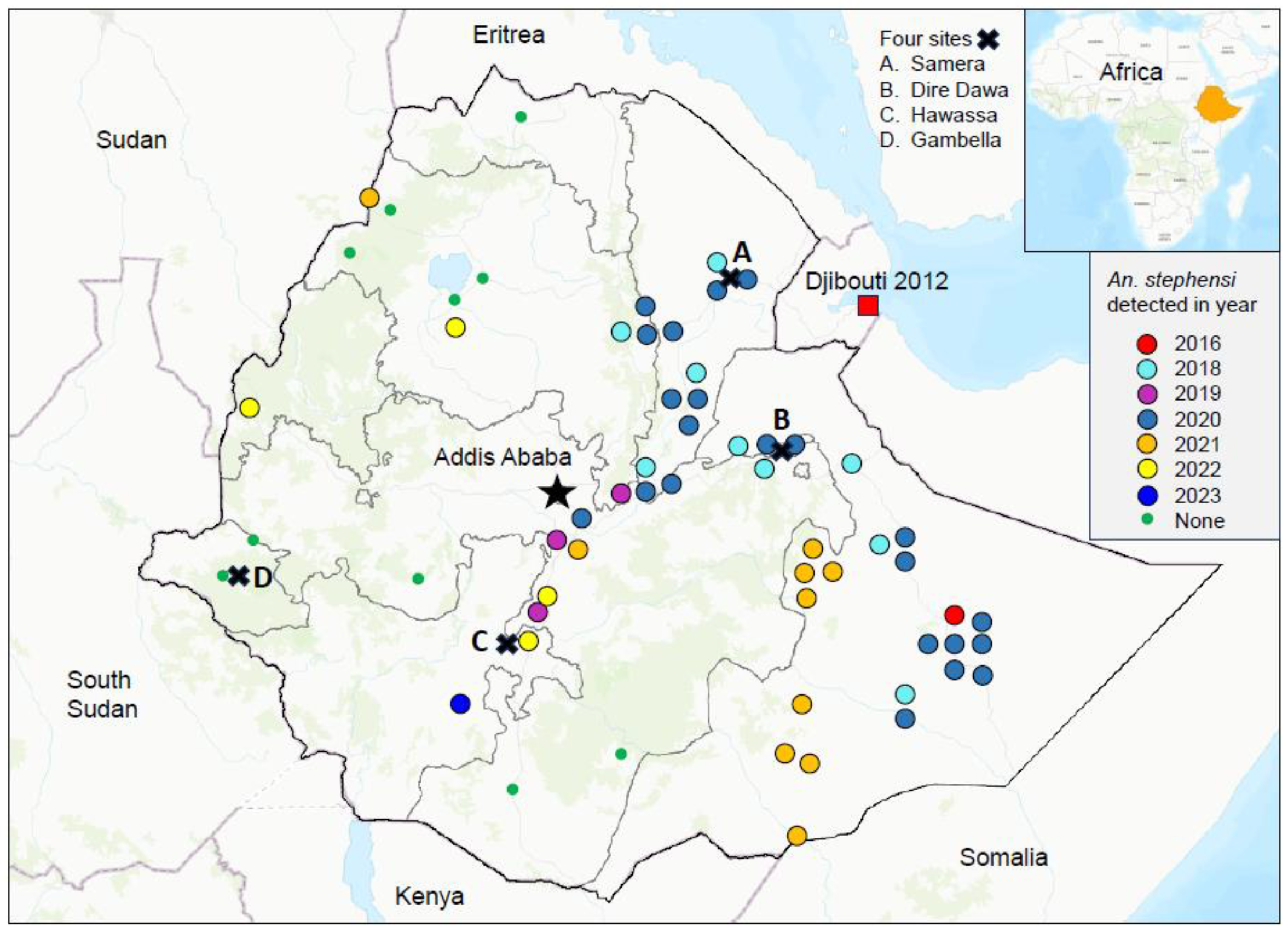
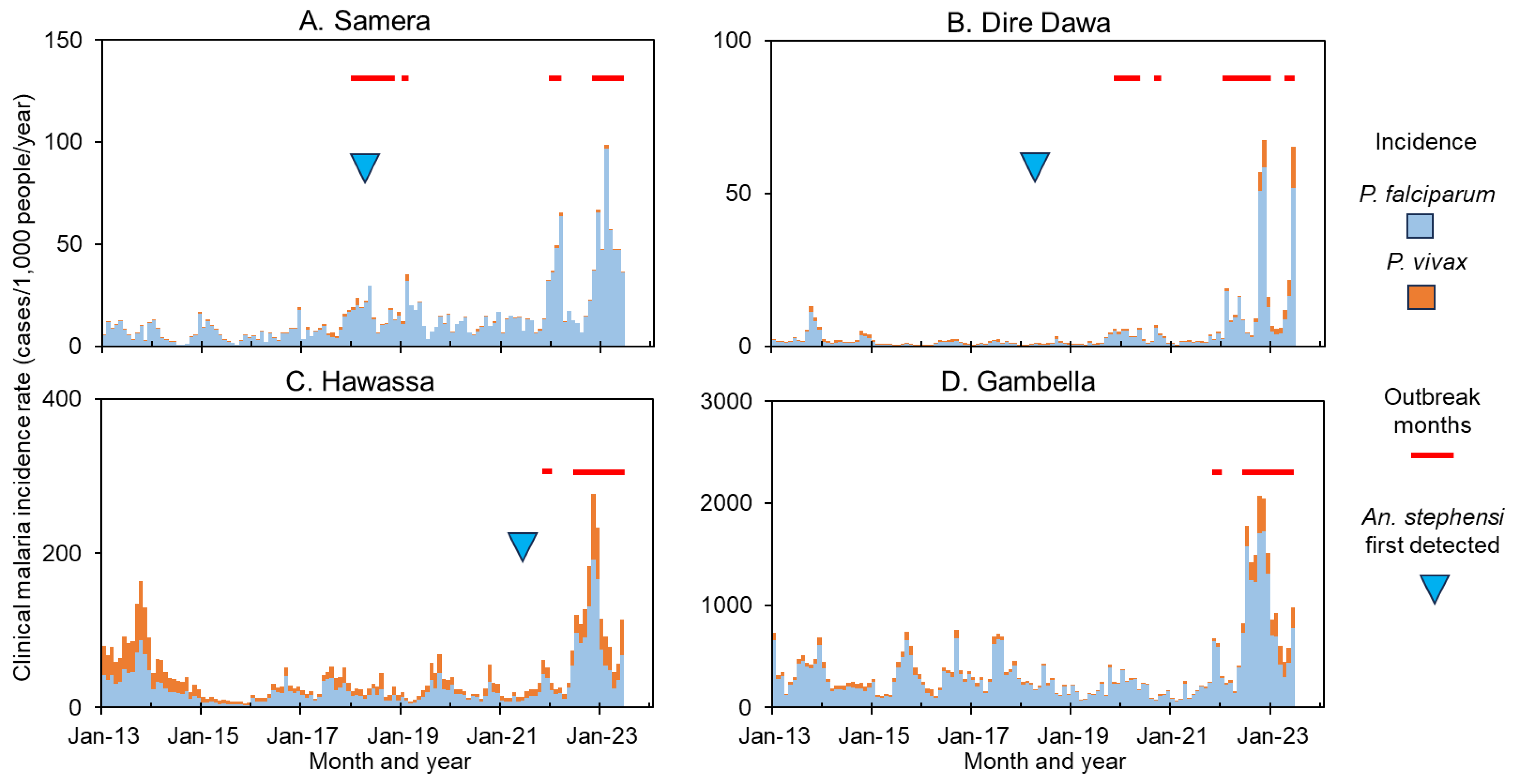
3.3. Malaria Outbreaks and the Role of An. stephensi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. World Malaria Report 2023. 2023. Available online: https://www.who.int/publications/i/item/9789240086173 (accessed on 5 October 2023).
- Taffese, H.S.; Hemming-Schroeder, E.; Koepfli, C.; Tesfaye, G.; Lee, M.-C.; Kazura, J.; Yan, G.-Y.; Zhou, G.-F. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect. Dis. Poverty 2018, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, E. National Malaria Elimination Program. Addia Ababa: Minstry of Helth, Ethiopia. 2021. Available online: http://dataverse.nipn.ephi.gov.et/bitstream/handle/123456789/1526/Ethiopia-Malaria-Elimination-Strategic-Plan-2021-2025-Agust-31.pdf?sequence=1 (accessed on 5 September 2023).
- Balkew, M.; Mumba, P.; Dengela, D.; Yohannes, G.; Getachew, D.; Yared, S.; Chibsa, S.; Murphy, M.; George, K.; Lopez, K.; et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit. Vectors. 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- The PMI VectorLink. Ethiopia Project Final Entomology Report May 2019-March 2020. Rockville, MD: Abt Associates. 2020. Available online: https://pdf.usaid.gov/pdf_docs/PA00ZC3Z.pdf (accessed on 10 July 2023).
- Balkew, M.; Mumba, P.; Yohannes, G.; Abiy, E.; Getachew, D.; Yared, S.; Worku, A.; Gebresilassie, A.; Tadesse, F.G.; Gadisa, F.; et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018-2020. Malar. J. 2021, 20, 263. [Google Scholar] [CrossRef]
- Hamlet, A.; Dengela, D.; Tongren, J.E.; Tadesse, F.; Bousema, T.; Sinka, M.; Seyoum, A.; Irish, S.R.; Armistead, J.S.; Churcher, T. The potential impact of Anopheles stephensi establishment on the transmission of Plasmodium falciparum in Ethiopia and prospective control measures. BMC Med. 2022, 20, 135. [Google Scholar] [CrossRef]
- Carter, T.E.; Yared, S.; Gebresilassie, A.; Bonnell, V.; Damodaran, L.; Lopez, K.; Ibrahim, M.; Mohammed, S.; Janies, D. First detection of Anopheles stephensi Liston, 1901 (Diptera: Culicidae) in Ethiopia using molecular and morphological approaches. Acta. Tropica 2018, 188, 180–186. [Google Scholar] [CrossRef]
- World Health Organization. WHO Initiative to Stop the Spread of Anopheles stephensi in Africa. 2023. Available online: https://www.who.int/publications/i/item/WHO-UCN-GMP-2022.06 (accessed on 2 May 2024).
- Emiru, T.; Getachew, D.; Murphy, M.; Sedda, L.; Ejigu, L.A.; Bulto, M.G.; Byrne, I.; Demisse, M.; Abdo, M.; Chali, W.; et al. Evidence for a role of Anopheles stephensi in the spread of drug- and diagnosis-resistant malaria in Africa. Nat. Med. 2023, 29, 3203–3211. [Google Scholar] [CrossRef]
- Teka, H.; Golassa, L.; Medhin, G.; Balkew, M.; Sisay, C.; Gadisa, E.; Nekorchuk, D.M.; Wimberly, M.C.; Tadesse, F.G. Trend analysis of malaria in urban settings in Ethiopia from 2014 to 2019. Malar. J. 2023, 22, 235. [Google Scholar] [CrossRef]
- de Santi, V.P.; Khaireh, B.A.; Chiniard, T.; Pradines, B.; Taudon, N.; Larréché, S.; Mohamed, A.B.; de Laval, F.; Berger, F.; Gala, F.; et al. Role of Anopheles stephensi Mosquitoes in Malaria Outbreak, Djibouti, 2019. Emerg. Infect. Dis. 2021, 27, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Moustafa, M.; Tageldin, R.; Harwood, J.F. Effects of seasonal conditions on abundance of malaria vector Anopheles stephensi mosquitoes, Djibouti, 2018–2021. Emerg. Infect. Dis. 2023, 29, 801–805. [Google Scholar] [CrossRef]
- PMI. Ethiopia Malaria Operational Plan FY 2023. US President’s Malaria Initiative. 2023. Available online: https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2023/01/FY-2023-Ethiopia-MOP.pdf (accessed on 4 May 2024).
- The PMI VectorLink Project. Ethiopia Final Entomological Report, April 2022-March 2023. Rockville, MD. Abt Associates Inc. 2023. Available online: https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2023/09/Entomological-Monitoring-Report-Ethiopia-2023.pdf (accessed on 10 May 2024).
- The PMI VectorLink Project Ethiopia. Ethiopia Final Entomological Report, APRIL 2021-MARCH 2022. In: PMI, editor. Rockville, MD: Abt Associates. 2022. Available online: https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2023/02/Entomological-Monitoring-Report-Ethiopia-2022.pdf (accessed on 10 November 2023).
- The PMI VectorLink Project Ethiopia. Ethiopia Final Entomology Report May 2020-March 2021. Rockville, MD, USA: The PMI VectorLink Project, Abt Associates Inc. 2021. Available online: https://pdf.usaid.gov/pdf_docs/PA00ZC42.pdf (accessed on 10 January 2024).
- Teshome, A.; Erko, B.; Golassa, L.; Yohannes, G.; Irish, S.R.; Zohdy, S.; Dugassa, S. Laboratory-based efficacy evaluation of Bacillus thuringiensis var. israelensis and temephos larvicides against larvae of Anopheles stephensi in Ethiopia. Malar. J. 2023, 22, 48. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992; 24, 189–206. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local spatial autocorrelation statistics: Distributional issues and an application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Cullen, J.R.; Chitprarop, U.; Doberstyn, E.B.; Sombatwattanangkul, K. An epidemiological early warning system for malaria control in northern Thailand. Bull World Health Organ 1984, 62, 107–114. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2536271/ (accessed on 10 June 2024). [PubMed]
- World Health Organization. World Malaria Report 2023. 2023. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 10 June 2024).
- Li, C.; Managi, S. Global malaria infection risk from climate change. Environ. Res. 2022, 214, 114028. [Google Scholar] [CrossRef]
- Lubinda, J.; Haque, U.; Bi, Y.; Hamainza, B.; Moore, A.J. Near-term climate change impacts on sub-national malaria transmission. Sci. Rep. 2021, 11, 751. [Google Scholar] [CrossRef]
- Leal Filho, W.; May, J.; May, M.; Nagy, G.J. Climate change and malaria: Some recent trends of malaria incidence rates and average annual temperature in selected sub-Saharan African countries from 2000 to 2018. Malar. J. 2023, 22, 248. [Google Scholar] [CrossRef]
- Ahmed, A.; Mulatu, K.; Elfu, B. Prevalence of malaria and associated factors among under-five children in Sherkole refugee camp, Benishangul-Gumuz region, Ethiopia. A cross-sectional study. PLoS ONE 2021, 16, e0246895. [Google Scholar] [CrossRef]
- Asebe, G.; Mamo, G.; Wieland, B.; Medhin, G.; Tilahun, G.; Abegaz, W.E.; Legesse, M. Community awareness and experiences of health workers concerning mosquito-borne viral diseases in selected districts of Gambella Region, Southwestern Ethiopia. Infect. Ecol. Epidemiol. 2021, 11, 1988453. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.; Weetman, D.; Sauskojus, H.; Budge, S.; Hawail, T.B.; Baheshm, Y. Confirmation of the presence of Anopheles stephensi among internally displaced people’s camps and host communities in Aden city, Yemen. Malar. J. 2023, 22, 1. [Google Scholar] [CrossRef]
- Weiss, D.J.; Bertozzi-Villa, A.; Rumisha, S.F.; Amratia, P.; Arambepola, R.; Battle, K.E.; Cameron, E.; Chestnutt, E.; Gibson, H.S.; Harris, J.; et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: A geospatial modelling analysis. Lancet Infect. Dis. 2021, 21, 59–69. [Google Scholar] [CrossRef]
- Gao, L.; Shi, Q.; Liu, Z.; Li, Z.; Dong, X. Impact of the COVID-19 pandemic on malaria control in Africa: A preliminary analysis. Trop. Med. Infect. Dis. 2023, 8, 67. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Malaria and COVID-19 in malarial endemic area: Incidence and coincidence. Turkiye Parazitolojii Dergisi. 2022, 46, 84–85. [Google Scholar] [CrossRef] [PubMed]
- Sanou, A.; Moussa Guelbéogo, W.; Nelli, L.; Hyacinth Toé, K.; Zongo, S.; Ouédraogo, P.; Cissé, F.; Mirzai, N.; Matthiopoulos, J.; Sagnon, N. Evaluation of mosquito electrocuting traps as a safe alternative to the human landing catch for measuring human exposure to malaria vectors in Burkina Faso. Malar. J. 2019, 18, 386. [Google Scholar] [CrossRef] [PubMed]
- Govella, N.J.; Maliti, D.F.; Mlwale, A.T.; Masallu, J.P.; Mirzai, N.; Johnson, P.C.; Ferguson, H.M.; Killeen, G. An improved mosquito electrocuting trap that safely reproduces epidemiologically relevant metrics of mosquito human-feeding behaviours as determined by human landing catch. Malar. J. 2016, 15, 465. [Google Scholar] [CrossRef]
- Kenea, O.; Balkew, M.; Tekie, H.; Gebre-Michael, T.; Deressa, W.; Loha, E.; Lindtjørn, B.; Overgaard, H.J. Comparison of two adult mosquito sampling methods with human landing catches in south-central Ethiopia. Malar. J. 2017, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Abubakr, M.; Ali, Y.; Siddig, E.E.; Mohamed, N.S. Vector control strategy for Anopheles stephensi in Africa. Lancet Microbe 2022, 3, e403. [Google Scholar] [CrossRef]

| Risk Level ‡ | At Risk Woredas and Population † | Risk Changes from 2017–2022 # | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2022 | No Change | Reduced | Increased | |||||||
| N Woreda | Population | N Woreda | Population | N Woreda | Population | N Woreda | Population | N Woreda | Population | ||
| API = 0 | 47 | 7,189,225 | 19 | 2,422,574 | 16 | 2,056,271 | NA | 31 | 5,132,954 | ||
| API ≤ 5 | 478 | 58,200,085 | 339 | 43,763,043 | 270 | 36,176,014 | 3 | 366,303 | 205 | 21,657,768 | |
| 5 < API < 10 | 148 | 13,092,751 | 129 | 13,451,299 | 30 | 2,884,527 | 25 | 2,028,818 | 93 | 8,179,406 | |
| 10 ≤ API < 50 | 250 | 18,536,821 | 331 | 29,899,828 | 135 | 10,343,174 | 35 | 2,959,958 | 80 | 5,233,689 | |
| API ≥ 50 | 161 | 8,330,825 | 266 | 15,812,963 | 129 | 6,636,411 | 32 | 1,694,414 | NA | ||
| Total | 1,084 | 105,349,707 | 1084 | 105,349,707 | 580 | 58,096,397 | 95 | 7,049,493 | 409 | 40,203,817 | |
| Region | Woreada/City | Outbreak Detection | Anopheles Stephensi # | |||
|---|---|---|---|---|---|---|
| Status † | Rate Ratio ‡ | p-Value § | Status | Proportion | ||
| Afar | Awash Fentale | No | 0.87 | 0.0573 | Yes | 92% |
| Semera | Yes | 1.83 | 0.0334 | Yes | 100% | |
| Amhara | Bahir Dar Town | Yes | 2.69 | 0.0088 | No | 0% |
| Debre Markos Town | Yes | 2.46 | 0.0069 | No | 0% | |
| Gondar Zuriya | Yes | 3.41 | 0.0007 | No | 0% | |
| Kemise Town | No | 0.60 | 0.0036 | No | 0% | |
| Woreta | Yes | 2.12 | 0.0297 | No | 0% | |
| Benishangul-Gumuz | Assosa | Yes | 1.47 | 0.0116 | No | 0% |
| Bambasi | Yes | 1.66 | 0.0013 | No | 0% | |
| Dire Dawa | Dire Dawa | Yes | 9.04 | 0.0093 | Yes | 96% |
| Gambella | Abobo | Yes | 4.02 | 0.0020 | No | 0% |
| Gambella | Yes | 1.60 | 0.0112 | No | 0% | |
| Oromia | Adama | Yes | 1.64 | 0.1161 | Yes | 50% |
| Batu | Yes | 2.21 | 0.0328 | Yes | 77% | |
| Jimma Town | Yes | 3.82 | 0.0030 | No | 0% | |
| SNNP * | Arba Minch | Yes | 1.18 | 0.1050 | Yes | 48.% |
| Hawassa Town | Yes | 3.01 | 0.0303 | Yes | 62% | |
| Somali | Fik | No | 0.69 | 0.0054 | Yes | 100% |
| Godey | No | 0.88 | 0.1020 | Yes | 64% | |
| Degehabur | Yes | 1.01 | 0.4818 | Yes | 58% | |
| Kebri Dehar | Yes | 1.52 | 0.0063 | Yes | 75% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Taffese, H.S.; Zhong, D.; Wang, X.; Lee, M.-C.; Degefa, T.; Getachew, D.; Haileselassie, W.; Hawaria, D.; Yewhalaw, D.; et al. Resurgence of Clinical Malaria in Ethiopia and Its Link to Anopheles stephensi Invasion. Pathogens 2024, 13, 748. https://doi.org/10.3390/pathogens13090748
Zhou G, Taffese HS, Zhong D, Wang X, Lee M-C, Degefa T, Getachew D, Haileselassie W, Hawaria D, Yewhalaw D, et al. Resurgence of Clinical Malaria in Ethiopia and Its Link to Anopheles stephensi Invasion. Pathogens. 2024; 13(9):748. https://doi.org/10.3390/pathogens13090748
Chicago/Turabian StyleZhou, Guofa, Hiwot S. Taffese, Daibin Zhong, Xiaoming Wang, Ming-Chieh Lee, Teshome Degefa, Dejene Getachew, Werissaw Haileselassie, Dawit Hawaria, Delenasaw Yewhalaw, and et al. 2024. "Resurgence of Clinical Malaria in Ethiopia and Its Link to Anopheles stephensi Invasion" Pathogens 13, no. 9: 748. https://doi.org/10.3390/pathogens13090748
APA StyleZhou, G., Taffese, H. S., Zhong, D., Wang, X., Lee, M.-C., Degefa, T., Getachew, D., Haileselassie, W., Hawaria, D., Yewhalaw, D., & Yan, G. (2024). Resurgence of Clinical Malaria in Ethiopia and Its Link to Anopheles stephensi Invasion. Pathogens, 13(9), 748. https://doi.org/10.3390/pathogens13090748






