Antiviral Activity of Angelica Tenuissima Nakai against Influenza A Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Protein Construction of ATN
2.2. Enrichment Analysis
2.3. Gene Set Construction
2.4. Preparation of Angelica Tenuissima Nakai Roots Extract
2.5. Propagation of Influenza A Virus in Madin–Darby Canine Kidney (MDCK) Cell Line
2.6. Evaluation of In Vitro Cytotoxicity of ATN
2.7. Hemagglutination Assay
2.8. Determination of Influenza A Virus Median Tissue Culture Infectious Dose (TCID50)
2.9. Hemagglutination Inhibition (HI) Assay
2.10. Microscopic Examination of Virus-Infected Cells
2.11. Time-of-Addition Assay
2.12. Viral RNA Isolation and Real-Time PCR
2.13. Statistical Analysis
3. Results
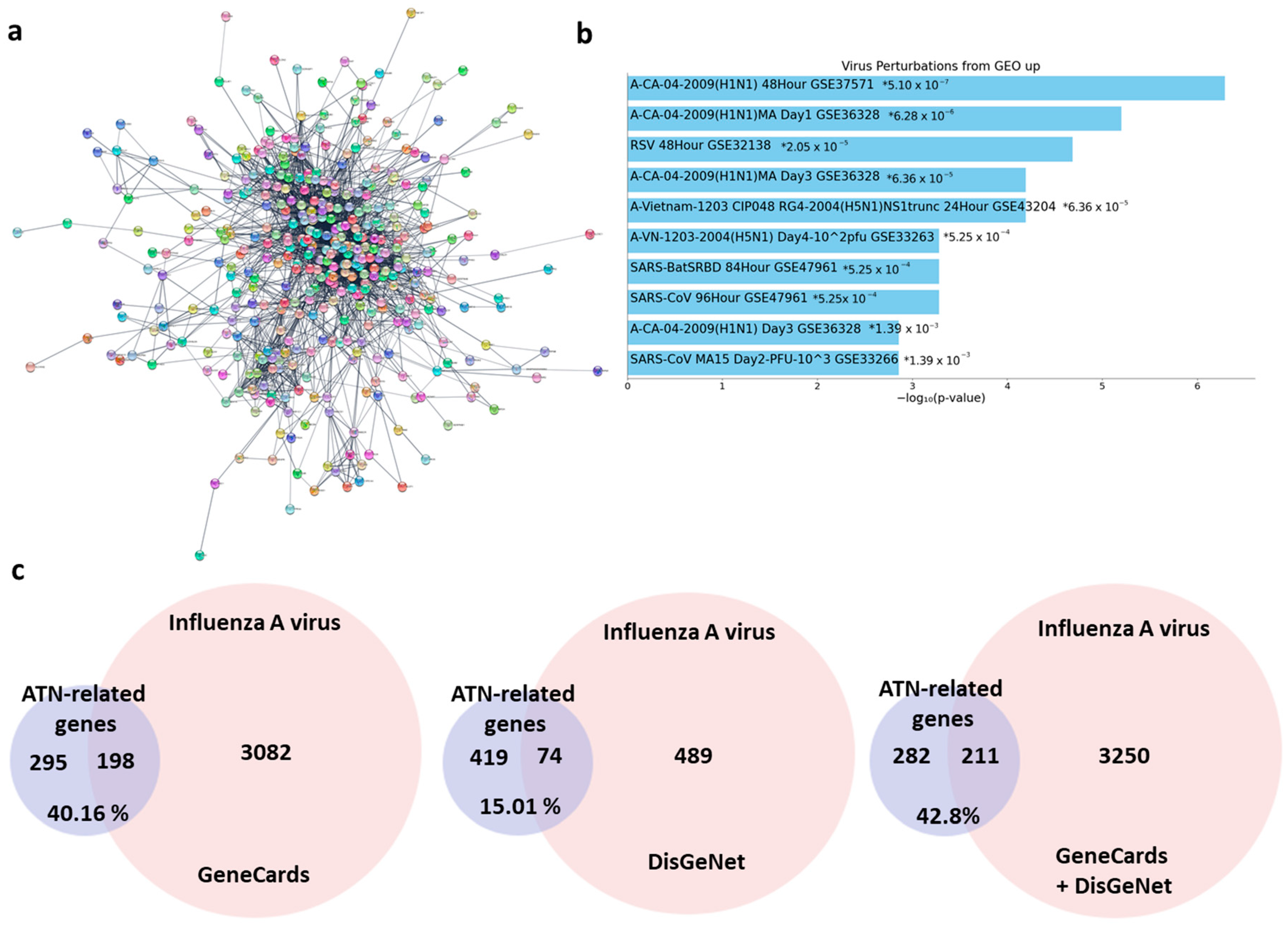
3.1. Target Proteins of ATN and Functional Enrichment Analyses of Targets
3.2. ATN Exhibits a Low Cytotoxic Effect on MDCK Cells
3.3. ATN Exhibits Antiviral Property against H1N1 and H3N2 Influenza A Viruses
3.4. ATN Enhances the Cell Survival Rate against H1N1 and H3N2 Influenza A Viruses
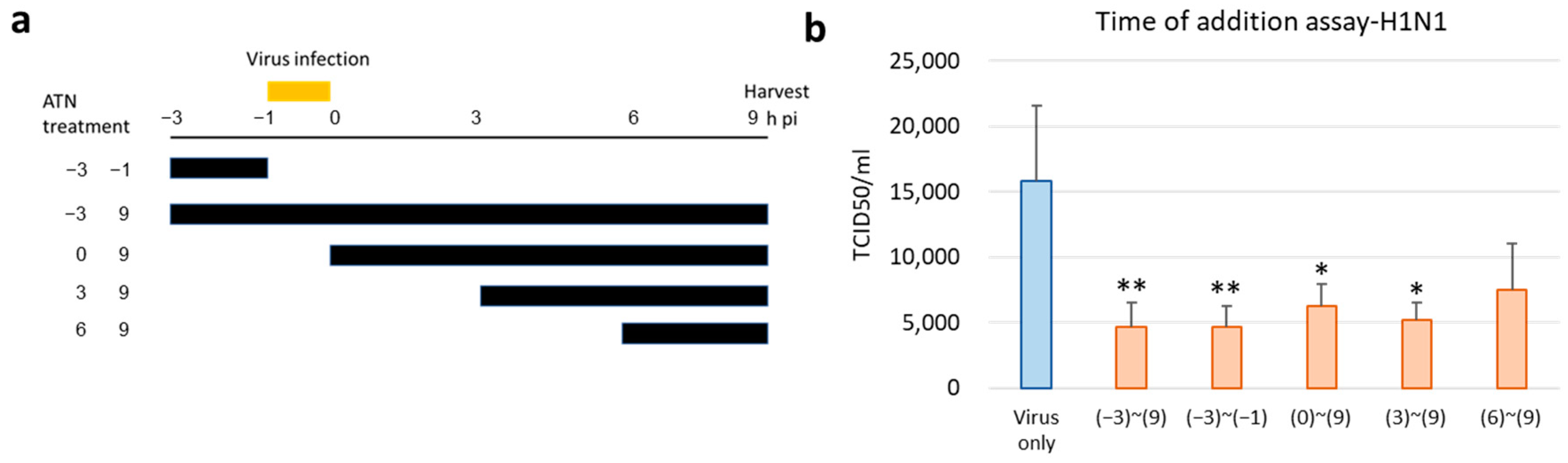
3.5. ATN Exhibits Antiviral Activity at an Early Stage of Infection
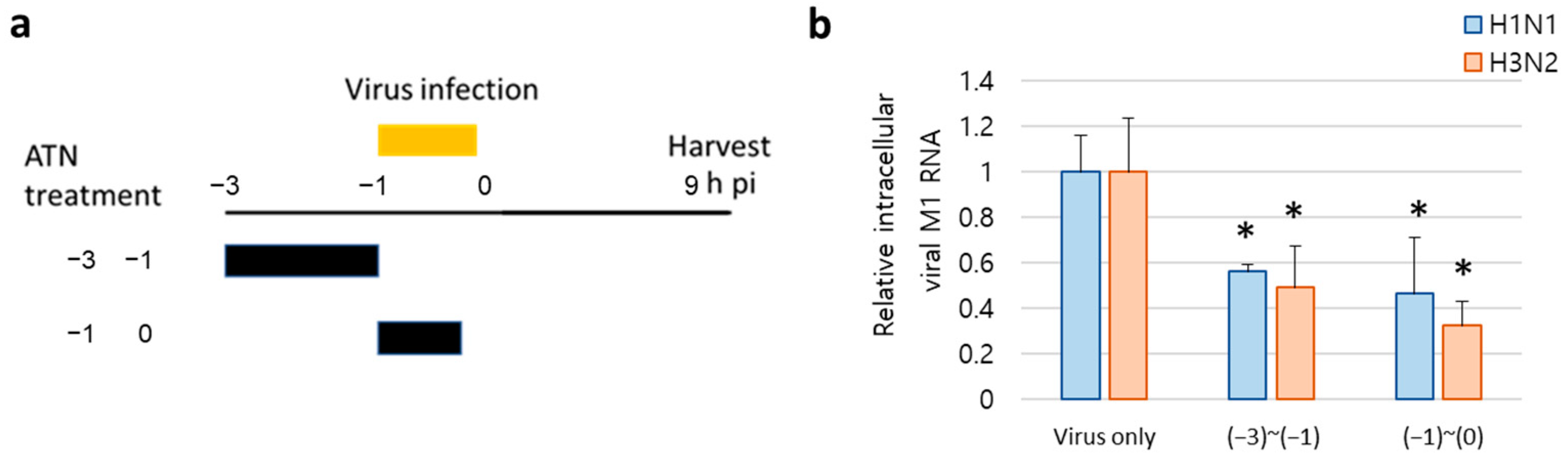
3.6. ATN Decreases Viral RNA Synthesis at Early Stages of Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooney, H.C.; Fleming, C.; Scheffer, I.E. Respiratory syncytial virus epidemic during the COVID-19 pandemic. J. Paediatr. Child Health 2022, 58, 215–216. [Google Scholar] [CrossRef]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Cold Spring Harb. Perspect. Med. 2020, 10, a038489. [Google Scholar] [CrossRef]
- Fratty, I.S.; Reznik-Balter, S.; Nemet, I.; Atari, N.; Kliker, L.; Sherbany, H.; Keller, N.; Stein, M.; Mendelson, E.; Mandelboim, M. Outbreak of Influenza and Other Respiratory Viruses in Hospitalized Patients Alongside the SARS-CoV-2 Pandemic. Front. Microbiol. 2022, 13, 902476. [Google Scholar] [CrossRef]
- Memoli, M.J.; Morens, D.M.; Taubenberger, J.K. Pandemic and seasonal influenza: Therapeutic challenges. Drug Discov. Today 2008, 13, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Hong, K.J.; Kim, H.; Nam, J.H. Influenza vaccines: Past, present, and future. Rev. Med. Virol. 2022, 32, e2243. [Google Scholar] [CrossRef] [PubMed]
- Jimbo Sotomayor, R.; Toscano, C.M.; Sanchez Choez, X.; Vilema Ortiz, M.; Rivas Condo, J.; Ghisays, G.; Haneuse, S.; Weinberger, D.M.; McGee, G.; de Oliveira, L.H. Impact of pneumococcal conjugate vaccine on pneumonia hospitalization and mortality in children and elderly in Ecuador: Time series analyses. Vaccine 2020, 38, 7033–7039. [Google Scholar] [CrossRef]
- Sugrue, R.J.; Tan, B.H.; Yeo, D.S.; Sutejo, R. Antiviral drugs for the control of pandemic influenza virus. Ann. Acad. Med. Singap. 2008, 37, 518–524. [Google Scholar] [CrossRef]
- Guan, W.; Qu, R.; Shen, L.; Mai, K.; Pan, W.; Lin, Z.; Chen, L.; Dong, J.; Zhang, J.; Feng, P.; et al. Baloxavir marboxil use for critical human infection of avian influenza A H5N6 virus. Med 2024, 5, 32–41.e5. [Google Scholar] [CrossRef] [PubMed]
- Fedson, D.S. Confronting the next influenza pandemic with anti-inflammatory and immunomodulatory agents: Why they are needed and how they might work. Influenza Other Respir. Viruses 2009, 3, 129–142. [Google Scholar] [CrossRef]
- Moss, R.B.; Davey, R.T.; Steigbigel, R.T.; Fang, F. Targeting pandemic influenza: A primer on influenza antivirals and drug resistance. J. Antimicrob. Chemother. 2010, 65, 1086–1093. [Google Scholar] [CrossRef]
- Lin, T.J.; Lin, C.F.; Chiu, C.H.; Lee, M.C.; Horng, J.T. Inhibition of endosomal fusion activity of influenza virus by Rheum tanguticum (da-huang). Sci. Rep. 2016, 6, 27768. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Je, I.G.; Kim, G.J.; Nam, J.W.; Shim, S.H.; Kim, S.H.; Choi, H. Chemical Constituents of the Root of Angelica tenuissima and their Anti-allergic Inflammatory Activity. Nat. Prod. Commun. 2017, 12, 779–780. [Google Scholar] [CrossRef]
- Choi, M.; Lee, Y.; Cho, S.H. Angelica tenuissima Nakai Ameliorates Cognitive Impairment and Promotes Neurogenesis in Mouse Model of Alzheimer’s Disease. Chin. J. Integr. Med. 2018, 24, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database J. Biol. Databases Curation 2010, 2010, baq020. [Google Scholar] [CrossRef]
- Pinero, J.; Bravo, A.; Queralt-Rosinach, N.; Gutierrez-Sacristan, A.; Deu-Pons, J.; Centeno, E.; Garcia-Garcia, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, D.; Yang, I.; Choi, B.; Lee, J.W.; Namkoong, S.; Koo, H.J.; Lee, S.R.; Park, M.R.; Lim, H.; et al. Decursin and Z-Ligustilide in Angelica tenuissima Root Extract Fermented by Aspergillus oryzae Display Anti-Pigment Activity in Melanoma Cells. J. Microbiol. Biotechnol. 2018, 28, 1061–1067. [Google Scholar] [CrossRef]
- Pasquier, C.; Souyris, C.; Moinard, N.; Bujan, L.; Izopet, J. Validation of an automated real-time PCR protocol for detection and quantitation of HIV and HCV genomes in semen. J. Virol. Methods 2006, 137, 156–159. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, M.K.; Kim, C.W.; Kim, M. TRIM Proteins and Their Roles in the Influenza Virus Life Cycle. Microorganisms 2020, 8, 1424. [Google Scholar] [CrossRef]
- Imming, P. Molecular targets of natural drug substances: Idiosyncrasies and preferences. Planta Medica 2010, 76, 1794–1801. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Jones, P.J. Safety and Health Benefits of Novel Dietary Supplements Consisting Multiple Phytochemicals, Vitamins, Minerals and Essential Fatty Acids in High Fat Diet Fed Rats. J. Diet. Suppl. 2016, 13, 353–367. [Google Scholar] [CrossRef]
- Ka, M.H.; Choi, E.H.; Chun, H.S.; Lee, K.G. Antioxidative activity of volatile extracts isolated from Angelica tenuissimae roots, peppermint leaves, pine needles, and sweet flag leaves. J. Agric. Food Chem. 2005, 53, 4124–4129. [Google Scholar] [CrossRef]
- Lee, W.; Koo, H.R.; Choi, Y.J.; Choi, J.G.; Oh, M.S.; Jin, X.; Choo, M.; Park, S.; Jeong, Y.S.; Chung, S.J.; et al. Z-ligustilide and n-Butylidenephthalide Isolated from the Aerial Parts of Angelica tenuissima Inhibit Lipid Accumulation In Vitro and In Vivo. Planta Medica 2019, 85, 719–728. [Google Scholar] [CrossRef]
- Fedson, D.S. Confronting an influenza pandemic with inexpensive generic agents: Can it be done? Lancet Infect. Dis. 2008, 8, 571–576. [Google Scholar] [CrossRef]
- Wang, W.; DeFeo, C.J.; Alvarado-Facundo, E.; Vassell, R.; Weiss, C.D. Intermonomer Interactions in Hemagglutinin Subunits HA1 and HA2 Affecting Hemagglutinin Stability and Influenza Virus Infectivity. J. Virol. 2015, 89, 10602–10611. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.L.; Akerstrom, S.; Shen, S.; Bereczky, S.; Karlberg, H.; Klingstrom, J.; Lal, S.K.; Mirazimi, A.; Tan, Y.J. An antibody against a novel and conserved epitope in the hemagglutinin 1 subunit neutralizes numerous H5N1 influenza viruses. J. Virol. 2010, 84, 8275–8286. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Wilson, I.A. Broadly neutralizing antibodies against influenza virus and prospects for universal therapies. Curr. Opin. Virol. 2012, 2, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Fleishman, S.J.; Whitehead, T.A.; Ekiert, D.C.; Dreyfus, C.; Corn, J.E.; Strauch, E.M.; Wilson, I.A.; Baker, D. Computational design of proteins targeting the conserved stem region of influenza hemagglutinin. Science 2011, 332, 816–821. [Google Scholar] [CrossRef]
- Belser, J.A.; Lu, X.; Szretter, K.J.; Jin, X.; Aschenbrenner, L.M.; Lee, A.; Hawley, S.; Kim, D.H.; Malakhov, M.P.; Yu, M.; et al. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J. Infect. Dis. 2007, 196, 1493–1499. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.-J.; Bae, G.-S.; Han, Y.-H. Antiviral Activity of Angelica Tenuissima Nakai against Influenza A Virus. Pathogens 2024, 13, 761. https://doi.org/10.3390/pathogens13090761
Park W-J, Bae G-S, Han Y-H. Antiviral Activity of Angelica Tenuissima Nakai against Influenza A Virus. Pathogens. 2024; 13(9):761. https://doi.org/10.3390/pathogens13090761
Chicago/Turabian StylePark, Won-Jong, Gi-Sang Bae, and Youn-Ho Han. 2024. "Antiviral Activity of Angelica Tenuissima Nakai against Influenza A Virus" Pathogens 13, no. 9: 761. https://doi.org/10.3390/pathogens13090761
APA StylePark, W.-J., Bae, G.-S., & Han, Y.-H. (2024). Antiviral Activity of Angelica Tenuissima Nakai against Influenza A Virus. Pathogens, 13(9), 761. https://doi.org/10.3390/pathogens13090761






