Abstract
Lower respiratory tract infections (LRTIs) remain the leading cause of infant morbidity and mortality worldwide and affect long-term respiratory health. Identifying immunological determinants of LRTI susceptibility may help stratify disease risk and identify therapies. This study aimed to identify neonatal immunological factors predicting LRTI risk in infancy. Cord blood plasma from 191 neonates from the Boston Birth Cohort was analyzed for 28 soluble immune factors. LRTI was defined as bronchiolitis, bronchitis, or pneumonia during the first year of life. Welch’s t-test demonstrated significantly higher log10 transformed concentrations of IL-17 and IFNγ in the LRTI group compared to neonates without LRTI in the first year of life (p < 0.05). Risk associations were determined using multivariate survival models. There were 29 infants with LRTIs. High cord blood levels of IFNγ (aHR = 2.35, 95% CI 1.07–5.17), TNF-β (aHR = 2.86, 95% CI 1.27–6.47), MIP-1α (aHR = 2.82, 95% CI 1.22–6.51), and MIP-1β (aHR = 2.34, 95% CI 1.05–5.20) were associated with a higher risk of LRTIs. RANTES was associated with a lower risk (aHR = 0.43, 95% CI 0.19–0.97). Soluble immune factors linked to antiviral immunity (IFNγ) and cytokines mediating inflammatory responses (TNF-β), and cell homing (MIP-1α/b), at birth were associated with an increased risk of LRTIs during infancy.
1. Introduction
Lower respiratory tract infections (LRTIs), such as bronchiolitis and pneumonia, are the leading cause of infant morbidity and mortality worldwide [1]. They account for approximately 18% of deaths in children younger than 5 years old, with most deaths occurring during the first 2 years of life [2,3]. Moreover, acute early-life LRTIs are linked to substantial, life-long respiratory burden, including wheezing, asthma, and chronic obstructive pulmonary disease (COPD) [4].
Several immunological markers in cord blood, including IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, and IFN-γ, have been linked to LRTI in infants [5,6,7]. However, previous studies often included heterogenous populations and immunological states, and we still lack robust immunological biomarkers for LRTI risk estimation [5,6,7]. Furthermore, our understanding of the interaction between those markers and clinical factors influencing LRTI responses remains incomplete.
In this study, we sought to use a large birth cohort to understand the relationship between baseline levels of 28 soluble immune factors and LRTI during infancy. Specifically, we investigated associations with soluble immune factors that have been implicated in LRTI immune responses in early childhood [5,6,7]. In addition, we examined cytokines involved in host defense to respiratory infections but not yet associated with early-life LRTIs. For example, sentinel myeloid cells in the lungs release TNF-α and IL-1 to upregulate inflammatory responses against pneumococcal pneumonia infection [8,9]. Additionally, IL-17 signaling instructs lung epithelial cells to secrete chemokines necessary for neutrophilic defense against klebsiella pneumonia [10]. IL-12 upregulates IFN-γ signaling to aid in neutrophil defense against pneumonia [11]. TREM-1 positively amplifies TNF-α, and IL-1 and is a strong predictor of pneumonia when found in bronchoalveolar-lavage fluid [12,13].
Accordingly, we analyzed soluble immune factors in the cord blood of neonates along with their clinical characteristics, hypothesizing these would be associated with a different likelihood of early-life LRTIs. Using adjusted Cox regression models, we identified immune markers associated with an increased risk of contracting LRTIs within the first year of life.
2. Methods
2.1. Study Population
This study was conducted among 191 mother–infant dyads enrolled in the Boston Birth Cohort (BBC) between 2003 and 2006 and examined the relationship between soluble immune biomarkers in cord blood and LRTIs during infancy (0–1 years of age). The BBC is a large, predominantly inner-city birth cohort with over 8600 mother–infant pairs enrolled to date. A detailed description of the parent cohort, as well as methods for selecting participants in the BBC cord blood immune biomarker sub-study, are described in previous work by our group [14,15]. For this study, 927 newborns with available cord blood immune profiling data (28 cytokines and soluble immune factors) were screened for eligibility, and those with complete demographic and available follow-up data during the observation period (0–12 months) were included. A total of 191 infants were included in the final analyses. The Institutional Review Boards at Boston University Medical Center and Johns Hopkins Bloomberg School of Public Health approved the study protocol.
2.2. Biomarker Assays
Sample collection was previously described [15]. In brief, umbilical cord blood samples were collected at birth in tubes with EDTA and immediately stored at 4 °C. Samples underwent refrigerated centrifugation at 2500 rpm for 10 min, after which the supernatant was carefully collected to ensure it was free of platelets. Subsequently, each plasma sample was divided into three aliquots and cryopreserved at −80 °C. We assessed 28 immune biomarkers using Flowmetric Luminex xMAP immunoassays (Luminex Corp, Austin, TX, USA). Biomarker quantification was performed using a sandwich immunoassay, employing biotinylated antibodies and phycoerythrin-labeled streptavidin. Further details for biomarker quantification, as well as the limits of detection for each cytokine, were previously described [16]. Additional details on the specific biomarkers are provided in Supplementary Table S1.
2.3. Definitions of Outcomes and Covariables
The main outcome of this study was LRTI during infancy, defined as the presence of bronchiolitis, bronchitis, or pneumonia in infants aged 0–12 months. LRTI was identified by ICD-9 or ICD-10 diagnoses recorded in the BBC database. Maternal characteristics analyzed as covariates included mode of delivery (C-section or vaginal), age at delivery, self-reported race and ethnicity, education level, parity (number of previous pregnancies resulting in live births), and pre-pregnancy BMI categorized as either non-overweight (<25 kg/m2) or overweight (≥25 kg/m2). We also included maternal health conditions, such as smoking during pregnancy, diabetes (none, gestational, or diabetes mellitus), and hypertensive disorders of pregnancy (preeclampsia, eclampsia, and chronic hypertension). Relevant infant variables included gestational age (prematurity), low birth weight (under 2500 g), sex, and breastfeeding status.
2.4. Statistical Analyses
During exploratory analysis, we assessed variable distributions, identified missing data, skewness, and outliers, and subsequently removed subjects with incomplete data. For inflammatory markers, we imputed measurements below the level of detection using the lowest level of detection divided by the square root of two. Log10-transformed values were used to mitigate skewness and reduce the impact of outliers. Continuous variables were summarized using means, standard deviations, medians, and interquartile ranges. Demographic and clinical differences between neonates with and without LRTIs were assessed using Chi-square, Student’s t-test, and Wilcoxon rank-sum tests. For cord blood immune biomarkers, we employed Welch’s t-test to compare levels between infants with and without LRTIs during their first year of life.
Survival models were used to determine risk associations between cord blood biomarkers. The cumulative risk of LRTIs during infancy was estimated for neonates with high (above the 50th percentile) or low (at or below the 50th percentile) immune biomarker concentrations using univariate and multivariate Cox proportional hazards models. See Supplementary Table S2 for median immune biomarker concentrations. Covariables in the adjusted model were limited to sex and prematurity status due to insufficient power to test additional covariables. Time to incident LRTI was defined as the age at which the first LRTI was recorded. Children who did not develop an LRTI were censored at 12 months of age. Survival models were performed using STATA version 14. All other analyses were performed using R version 4.2.3.
3. Results
3.1. Clinical Characteristics of Mother–Neonate Dyads in the BBC
Our analysis included 191 births occurring between 2003 and 2006 and followed for one year. A total of 29 newborns experienced LRTIs within the first year of life. Among the affected infants, the median age at onset was 5 months (IQR: 3–6). Table 1 presents an overview of the characteristics of the two study groups. Consistent with prior literature, the LRTI group exhibited a higher likelihood of being exclusively bottle-fed (41.4% vs. 20.4%, p = 0.012) and born to mothers who smoked during pregnancy (24.1% vs. 5.6%, p = 0.001) [17,18,19]. Notably, birth weight, gestational age, race, and parity were not significantly different between the LRTI and no LRTI groups. Similarly, there were no significant differences observed in other infant characteristics (mode of delivery and birth season/year) or maternal factors (race, age at delivery, educational level, BMI, diabetes, eclampsia, and chronic hypertension).

Table 1.
Clinical Characteristics and Demographics of Mother-Child Dyads. Summary of clinical and demographic characteristics by LRTI and no LRTI status during the first year of life. There were 162 infants without an LRTI in the first year of life and 29 with an LRTI in the first year of life. There was a higher proportion of babies that were bottle-fed in the LRTI group. No other infant characteristics were significantly different between groups. A significantly greater proportion of infants in the LRTI group were born to mothers who smoked continually during pregnancy. No other maternal characteristics were significantly different between groups. Notably, there were significant missing data on childcare attendance. IQR = Interquartile Range, SD = standard deviation, AA = African American, AAPI = Asian American and Pacific Islander, HS = High School, BMI = Body Mass Index, GDM = Gestational Diabetes, DM = Diabetes Mellitus. Bolded p-values indicate statistical significance (p < 0.05).
3.2. Differentially Abundant Cord Blood Immune Biomarkers between Infants with and without Early-Life LRTI
We first explored the association between immune biomarkers in cord blood and LRTI susceptibility during infancy using Welch’s t-test. To facilitate comparison, cord blood immune biomarker concentrations were log10-transformed. Among the 28 immune biomarkers analyzed, IFNγ and IL-17 exhibited significantly higher concentrations in the LRTI group (Figure 1). The remaining immune biomarkers were not significantly different between groups. When analyzed in categories, there was a larger proportion of newborns with high cord blood levels of IFNγ, TNF-β, MIP-1α, and MIP-1β (defined as cord blood levels in the two higher quartiles, see Supplementary Table S2 for medians) compared with the no LRTI group.
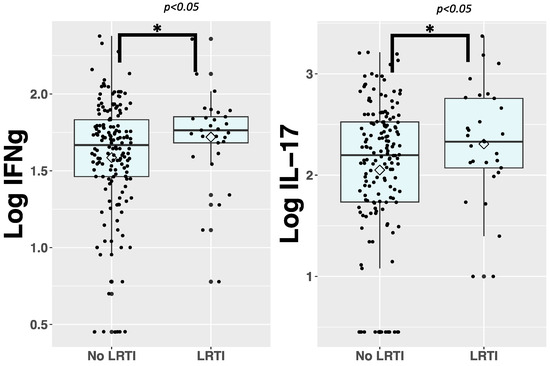
Figure 1.
Box and whisker plots presenting immune biomarker concentrations that are significantly different between LRTI and no LRTI groups. We collected 28 immune biomarkers from cord blood of neonates and quantified them using immunoassays. We then log10 transformed the data and used Welch’s t-test to compare levels of immune biomarkers in the cord blood of neonates with (n = 29) and without (n = 162) LRTI before one year of life. Biomarker concentrations that are significantly different between comparison groups are presented here. * = p ≤ 0.05. The elements of the box and whisker plots represent the median, IQR, and the whiskers are 1.5 times the IQR. Lozenges represent the mean.
3.3. IFN-γ, TNF-β, MIP-1α, and MIP-1β are Associated with a Higher LRTI Risk during Infancy
We next applied multivariate models to examine the relationship between the markers in our study, relevant covariates, and LRTI risk. Specifically, Cox proportional hazards models were employed, adjusting for sex and prematurity as covariates. We conducted univariate and multivariate analyses of the 28 markers included in this study. Infants in the top 50% of IFN-γ levels exhibited a 2.3-fold increase in LRTI risk compared to those in the bottom 50% (adjusted hazard ratio [aHR] = 2.35, 95% confidence interval [CI] 1.07–5.17) (Table 2). Similarly, TNF-β (aHR = 2.86, 95% CI 1.27–6.47), MIP-1α (aHR = 2.82, 95% CI 1.22–6.51), and MIP-1β concentration (aHR = 2.34, 95% CI 1.05–5.20) conferred significantly higher LRTI risk, while RANTES had the inverse relationship (aHR = 0.43, 95% CI 0.19–0.97). In contrast, the levels of other immune factors did not exhibit statistical significance beyond the predefined threshold.

Table 2.
Adjusted Immune Biomarker Predictors of LRTI. Adjusted Cox proportional hazards models predict LRTI occurrence by immune biomarker concentration. Cox proportional hazards analysis, adjusted by preterm status and sex, were performed for all immune biomarkers. Newborns with high concentrations of IFNγ, MIP-1α, MIP-1β, and TNF-β at birth demonstrated a significantly elevated risk of contracting LRTI in the first year of life compared to newborns with low concentrations. Newborns with high concentrations of RANTES at birth demonstrated lower risk of LRTI during infancy. IL-17, as well as the remaining immune biomarkers investigated in this study, demonstrated no associations with the risk of contracting an early-life LRTI. High cytokine levels: >50th percentile, low cytokine levels: ≤50th percentile (Supplementary Table S2). CI = Confidence Interval. Bolded p-values indicate statistical significance (p < 0.05).
4. Discussion
The major findings of our study are that increased IFNγ, TNF-β, MIP-1α, and MIP-1β levels at birth are associated with a higher risk of LRTI during infancy while RANTES is associated with a lower risk. Although previous studies have identified associations between cord-blood cytokines and LRTI in early childhood, ours investigates a wide array of immune biomarkers at baseline in an inner city, predominantly minority birth cohort, and identifies predictors of early-life LRTI risk in this population [5,6,7].
In our study, clinical factors were associated with the occurrence of future LRTIs [18,20]. The links between breastfeeding status and maternal smoking and LRTI are consistent with previous research [21]. Of note, certain variables previously implicated in early-life LRTI risk, such as parity, gestational age, and maternal weight, did not exhibit significant disparities in our analysis [19,22]. Considering that prior research in larger subsets of the BBC has identified parity, gestational age, and maternal weight as associated with early-life LRTI [23], it is possible that our current analysis is underpowered to detect differences in those variables. Additionally, regarding prematurity effects, the majority of preterm infants in our analysis (46 out of 57) were born mildly premature (between 33 and 36 weeks of gestational age). This might obscure the higher risk of lower respiratory tract infections (LRTIs) and respiratory issues commonly observed in infants born at or before 32 weeks of gestation (severely or extremely preterm infants) who often bear the brunt of respiratory diseases [24].
Our cohort and the scale of our immune biomarker panel allowed for the identification of multiple cytokines associated with LRTI. We noted elevated levels of IFN-γ, TNF-β, MIP-1α, and MIP-1β in neonates who experienced LRTIs during the first year of life. These biomarkers showed an association with an increased risk of LRTI during infancy in further analyses using adjusted survival analyses. Cox regression demonstrated neonates with high baseline IFN-γ, TNF-β, MIP-1α, and MIP-1β levels had greater than a two-fold higher risk of contracting LRTI within the first year of life, independently of gestational age and sex, which are well-known clinical risk factors for early-life LRTIs. Due to study power limitations and variable amounts of missing data, we omitted covariables such as maternal smoking, childcare, or breastfeeding.
IFN-γ is secreted by T cells and is involved in Th1 responses [25,26]. IFN-γ recruits macrophages and monocytes and plays a central role in antiviral immunity [27,28]; infants with high IFN-γ levels during RSV infection have a milder course than infants with low levels [29]. However, high IFN-γ in mice has also been shown to attenuate antibody response during RSV infection [30]. Based on previous literature, we expected IFN-γ levels to be lower in the LRTI group [6,7]. Intriguingly, our study reveals the opposite. One possible explanation is that prior studies evaluated cytokines collected from mononuclear cells stimulated with phytohemagglutinin or other immunogenic antigens, thus collecting cytokines in a stimulated state, while our study evaluated circulating cytokines at birth, absent of stimulation. As these previous investigations were functional studies, our observational study cannot be compared. It may be the case that the total change in cytokine concentration is more immunologically important in combating pathogens than is the cytokine concentrations at any one state. Thus, babies who naturally have higher levels of these circulating cytokines when not stimulated may be less able to mount a robust immune response, as immune cells may become desensitized over time. Indeed, T cell exhaustion is well documented, and overproduction of pro-inflammatory cytokines in a non-infectious state could lead to downregulation of cytokine receptors and a diminished immune response during infection. [31,32,33,34,35,36]. Excessive interferon signaling may also be associated with delayed development of adaptive, virus-specific T-cell and antibody responses [37]. Our results demonstrate that there may be a subset of infants with high cord blood IFN-γ concentrations that are more predisposed to LRTI during infancy.
High cord blood levels of macrophage inflammatory proteins 1α (MIP-1α) and 1b (MIP-1β), also known as CCL3 and CCL4, were associated with LRTIs during infancy in our study. These findings are consistent with previous observations in adults where elevated serum levels of these chemokines during respiratory viral infections, such as COVID-19, influenza, and Middle East respiratory syndrome (MERS), have been linked to increased disease severity and the presence of acute respiratory distress syndrome (ARDS) and thus, proposed as biomarkers of adverse outcomes [38,39]. However, unlike previous studies where elevated levels of MIP-1α and MIP-1β were identified as markers of severe disease during the course of a clinical illness, our discovery of higher levels of these chemokines at birth, associated with future risk, is novel. MIP-1α and MIP-1β are chemokines secreted by various immune cells, including activated lymphocytes and macrophages, contributing to the recruitment of inflammatory cells in the lungs and airways during respiratory infections, possibly exacerbating inflammation and tissue damage. We speculate that in newborns, elevated levels of these cytokines may indicate early activation of inflammatory pathways due to prenatal or perinatal environmental stimuli, which may influence later respiratory outcomes [20,40]. Further characterizing the association between MIP-1α, MIP-1β, and LRTI risk could help guide preemptive strategies to identify and mitigate risks associated with early-life LRTIs.
Transforming growth factor-beta (TGF-β) also emerged as a significant predictor associated with an increased risk of LRTIs in our study. TGF-β is crucial in regulating immune responses to respiratory infections, serving as a modulator of both innate and adaptive immunity [41]. It attenuates the antiviral IFN-β response, thereby facilitating viral replication in influenza infections [41]. In the context of respiratory syncytial virus (RSV), rhinovirus, and parainfluenza, TGF-β also impairs the innate antiviral immune response. For example, TGF-β produced by epithelial cells facilitates RSV replication by inducing cell cycle arrest. However, during the later stages of infection, TGF-β assumes a protective role by regulating adaptive immunity and promoting mucosal IgA responses [41]. In our study, elevated baseline levels of TGF-β may be linked to a more subdued antiviral response to respiratory viruses, which are the most prevalent pathogens in early life. However, further research is warranted to elucidate the mechanisms underlying this association.
Our study also showed a higher baseline level of RANTES is associated with lower early-life LRTI risk. RANTES is most closely involved with Th1 response and is pro-inflammatory [42]. It is secreted by T cells (among other cells) and is chemotactic for eosinophils, monocytes, macrophages, and other T cells [43]. Although to our knowledge, ours is the first study to show this association in human newborns at birth, previous murine models have shown RANTES knockout to cause poor cytokine production, higher inhibitory receptors associated with T cell exhaustion, and elevated levels of viral load in the setting of LCMV infection [44]. Moreover, the interaction of RANTES with its cognate receptor CCR5 is necessary for viral clearance during influenza infection through activation of resident macrophages, NK and T cell recruitment, and the establishment of immunological memory in the respiratory tract [45,46,47,48,49]. Aligned with these findings, our study suggests that baseline levels of RANTES may be important in mounting an immune response against viral respiratory infections, which are the most prevalent in infancy. Nonetheless, additional research is necessary to establish a definitive causal link in human infants.
Our study has limitations that warrant acknowledgment. Primarily, this is a large study to examine immunological profiles at birth as predictors of LRTI risk in infancy. However, our ability to evaluate the influence of immunological and environmental factors shaping immunity after birth was limited. We also were limited in accounting for certain potential confounding clinical variables due to a limited sample size. Furthermore, analyzing a non-randomized observational cohort inherently carries a risk of unmeasured confounding variables. Our study only includes physician-diagnosed cases, which may enrich our study population with neonates with the most severe cases of LRTI in infants who required medical evaluation, limiting the generalizability of our results outside of that group. However, the most severe cases are also most likely to result in adverse neonatal outcomes, making this a group of special interest. Additionally, the use of ICD9/ICD10 codes to ascertain patient outcomes may introduce inaccuracies to the data that increase the risk of bias. Our study population is also primarily an inner-city, racial minority birth cohort, so there could be some limitations in generalizing our study’s findings. However, our birth cohort provides valuable insights for populations historically neglected in research. Lastly, our dataset lacked information around the causative pathogen in LRTIs, which is an important factor when studying immunological profiles in association with LRTIs, but previous work reveals that viral respiratory infections are the most common cause of pneumonia and bronchiolitis in children under 5 years of age [50]. For instance, RSV accounted for 24% of LRTI hospitalizations in children younger than 5 years of age between 1997 and 2006 [51,52].
Nonetheless, our study advances the broader understanding of neonatal LRTI susceptibility. Moreover, the identification of potential predictive immune biomarkers holds promise for refining risk stratification methodologies. This work underscores the imperative for continued research in validation studies and the exploration of novel variables to better understand the multifactorial contributions to early-life LRTIs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13090765/s1, Table S1: Table Showcasing the Immune Biomarkers Investigated in this Study. Table S2: Immune Biomarker Concentration Medians of Cytokines Found to be Significant in the Study.
Author Contributions
Conceptualization, E.M., X.W., G.N. and M.J.G.; data curation, X.H. and M.J.G.; formal analysis, E.M. and M.J.G.; funding acquisition, X.W.; investigation, E.M. and M.J.G.; methodology, E.M. and M.J.G.; supervision, X.H., X.W. and M.J.G.; visualization, E.M.; writing—original draft, E.M.; writing—review and editing, G.N., X.H., X.W. and M.J.G. All authors have read and agreed to the published version of the manuscript.
Funding
The Boston Birth Cohort (the parent study) was supported in part by the National Institutes of Health (NIH) grants (2R01HD041702, R01HD098232, R01ES031272, R21AI154233, R01ES031521, and U01 ES034983) and the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) (UT7MC45949). MJG is supported by NIH grant K23HD104933. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by any funding agencies.
Institutional Review Board Statement
Study protocols for the BBC are approved by The Institutional Review Boards (IRBs) at Boston Medical Center and The Johns Hopkins Bloomberg School of Public Health (IRB Approval No.: 3966/CR1489).
Informed Consent Statement
Patient consent for enrollment into the BBC was obtained according to the study’s protocols.
Data Availability Statement
The data presented in this study are available from Xiaobin Wang (xwang82@jhu.edu), Principal Investigator of the Boston Birth Cohort, upon reasonable request and after review and approval of the Institutional Review Board.
Acknowledgments
The authors would like to thank the participants of the Boston Birth Cohort. We are also grateful for the field team at the Boston Medical Center.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Black, R.E.; Cousens, S.; Johnson, H.L.; Lawn, J.E.; Rudan, I.; Bassani, D.G.; Jha, P.; Campbell, H.; Walker, C.F.; Cibulskis, R.; et al. Global, Regional, and National Causes of Child Mortality in 2008: A Systematic Analysis. Lancet 2010, 375, 1969–1987. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, Regional, and National Causes of Child Mortality: An Updated Systematic Analysis for 2010 with Time Trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.A.; Medici, M.C.; Arcangeletti, M.C.; Lanari, M.; Merolla, R.; Paparatti, U.D.L.; Silvestri, M.; Pistorio, A.; Chezzi, C. Risk Factors for Severe RSV-Induced Lower Respiratory Tract Infection over Four Consecutive Epidemics. Eur. J. Pediatr. 2007, 166, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Verwey, C.; Nunes, M.C.; Dangor, Z.; Madhi, S.A. Pulmonary Function Sequelae after Respiratory Syncytial Virus Lower Respiratory Tract Infection in Children: A Systematic Review. Pediatr. Pulmonol. 2020, 55, 1567–1583. [Google Scholar] [CrossRef]
- Gern, J.E.; Brooks, G.D.; Meyer, P.; Chang, A.; Shen, K.; Evans, M.D.; Tisler, C.; DaSilva, D.; Roberg, K.A.; Mikus, L.D.; et al. Bidirectional Interactions between Viral Respiratory Illnesses and Cytokine Responses in the First Year of Life. J. Allergy Clin. Immunol. 2006, 117, 72–78. [Google Scholar] [CrossRef]
- Copenhaver, C.C.; Gern, J.E.; Li, Z.; Shult, P.A.; Rosenthal, L.A.; Mikus, L.D.; Kirk, C.J.; Roberg, K.A.; Anderson, E.L.; Tisler, C.J.; et al. Cytokine Response Patterns, Exposure to Viruses, and Respiratory Infections in the First Year of Life. Am. J. Respir. Crit. Care Med. 2004, 170, 175–180. [Google Scholar] [CrossRef]
- Ly, N.P.; Rifas-Shiman, S.L.; Litonjua, A.A.; Tzianabos, A.O.; Schaub, B.; Ruiz-Pérez, B.; Tantisira, K.G.; Finn, P.W.; Gillman, M.W.; Weiss, S.T.; et al. Cord Blood Cytokines and Acute Lower Respiratory Illnesses in the First Year of Life. Pediatrics 2007, 119, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Alcamo, E.; Mizgerd, J.P.; Horwitz, B.H.; Bronson, R.; Beg, A.A.; Scott, M.; Doerschuk, C.M.; Hynes, R.O.; Baltimore, D. Targeted Mutation of TNF Receptor I Rescues the RelA-Deficient Mouse and Reveals a Critical Role for NF-ΚB in Leukocyte Recruitment. J. Immunol. 2001, 167, 1592–1600. [Google Scholar] [CrossRef]
- Quinton, L.J.; Jones, M.R.; Simms, B.T.; Kogan, M.S.; Robson, B.E.; Skerrett, S.J.; Mizgerd, J.P. Functions and Regulation of NF-κB RelA during Pneumococcal Pneumonia. J. Immunol. 2007, 178, 1896–1903. [Google Scholar] [CrossRef]
- Ye, P.; Rodriguez, F.H.; Kanaly, S.; Stocking, K.L.; Schurr, J.; Schwarzenberger, P.; Oliver, P.; Huang, W.; Zhang, P.; Zhang, J.; et al. Requirement of Interleukin 17 Receptor Signaling for Lung CXC Chemokine and Granulocyte Colony-Stimulating Factor Expression, Neutrophil Recruitment, and Host Defense. J. Exp. Med. 2001, 194, 519–528. [Google Scholar] [CrossRef]
- Sun, K.; Salmon, S.L.; Lotz, S.A.; Metzger, D.W. Interleukin-12 Promotes Gamma Interferon-Dependent Neutrophil Recruitment in the Lung and Improves Protection against Respiratory Streptococcus Pneumoniae Infection. Infect. Immun. 2007, 75, 1196–1202. [Google Scholar] [CrossRef]
- Bartlett, J.G. Soluble Triggering Receptor Expressed on Myeloid Cells and the Diagnosis of Pneumonia. Infect. Dis. Clin. Pract. 2004, 12, 268–269. [Google Scholar] [CrossRef]
- Klesney-Tait, J.; Turnbull, I.R.; Colonna, M. The TREM Receptor Family and Signal Integration. Nat. Immunol. 2006, 7, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.; Bartell, T.; Wang, G.; Hong, X.; Rusk, S.A.; Fu, L.; Cerda, S.; Bustamante-Helfrich, B.; Kuohung, W.; Yarrington, C.; et al. Boston Birth Cohort Profile: Rationale and Study Design. Precis. Nutr. 2022, 1, e00011. [Google Scholar] [CrossRef]
- Matoba, N.; Yu, N.; Mestan, K.; Pearson, C.; Ortiz, K.; Porta, N.; Thorsen, P.; Skogstrand, K.; Hougaard, D.M.; Zuckerman, B.; et al. Differential Patterns of 27 Cord Blood Immune Biomarkers across Gestational Age. Pediatrics 2009, 123, 1320–1328. [Google Scholar] [CrossRef]
- Skogstrand, K.; Thorsen, P.; Nørgaard-Pedersen, B.; Schendel, D.E.; Sørensen, L.C.; Hougaard, D.M. Simultaneous Measurement of 25 Inflammatory Markers and Neurotrophins in Neonatal Dried Blood Spots by Immunoassay with XMAP Technology. Clin. Chem. 2005, 51, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Vanker, A.; Barnett, W.; Workman, L.; Nduru, P.M.; Sly, P.D.; Gie, R.P.; Zar, H.J. Early-Life Exposure to Indoor Air Pollution or Tobacco Smoke and Lower Respiratory Tract Illness and Wheezing in African Infants: A Longitudinal Birth Cohort Study. Lancet Planet. Health 2017, 1, e328–e336. [Google Scholar] [CrossRef]
- Koopman, L.P.; Smit, H.A.; Heijnen, M.L.A.; Wijga, A.; Van Strien, R.T.; Kerkhof, M.; Gerritsen, J.; Brunekreef, B.; De Jongste, J.C.; Neijens, H.J. Respiratory Infections in Infants: Interaction of Parental Allergy, Child Care, and Siblings—The PIAMA Study. Pediatrics 2001, 108, 943–948. [Google Scholar] [CrossRef]
- Gutierrez, M. Prenatal and Perinatal Risk Factors for Lower Respiratory Tract Infections in Inner-City Minority Infants. J. Allergy Clin. Immunol. 2021, 147, AB78. [Google Scholar] [CrossRef]
- Fuentes-Leonarte, V.; Estarlich, M.; Ballester, F.; Murcia, M.; Esplugues, A.; Aurrekoetxea, J.J.; Basterrechea, M.; Fernández-Somoano, A.; Morales, E.; Gascón, M.; et al. Pre- and Postnatal Exposure to Tobacco Smoke and Respiratory Outcomes during the First Year. Indoor Air 2015, 25, 4–12. [Google Scholar] [CrossRef]
- Nafstad, P.; Jaakkola, J.J.K.; Hagen, J.A.; Botten, G.; Kongerud, J. Breastfeeding, Maternal Smoking and Lower Respiratory Tract Infections. Eur. Respir. J. 1996, 9, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Singleton, R.J.; Wirsing, E.A.; Haberling, D.L.; Christensen, K.Y.; Paddock, C.D.; Hilinski, J.A.; Stoll, B.J.; Holman, R.C. Risk Factors for Lower Respiratory Tract Infection Death among Infants in the United States, 1999–2004. Pediatrics 2009, 124, 768–776. [Google Scholar] [CrossRef]
- Gutierrez, M.J.; Nino, G.; Hong, X.; Wang, X. Maternal Pre-Pregnancy Weight and Early Life Lower Respiratory Tract Infections in a Low-Income Urban Minority Birth Cohort. Sci. Rep. 2021, 11, 9790. [Google Scholar] [CrossRef] [PubMed]
- Haataja, P.; Korhonen, P.; Ojala, R.; Hirvonen, M.; Korppi, M.; Gissler, M.; Luukkaala, T.; Tammela, O. Hospital Admissions for Lower Respiratory Tract Infections in Children Born Moderately/Late Preterm. Pediatr. Pulmonol. 2018, 53, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 Cells: Different Patterns of Lymphokine Secretion Lead to Different Functional Properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef] [PubMed]
- Bradley, L.M.; Dalton, D.K.; Croft, M. A Direct Role for IFN-Gamma in Regulation of Th1 Cell Development. J. Immunol. 1996, 157, 1350–1358. [Google Scholar] [CrossRef]
- Nathan, C.F.; Murray, H.W.; Wlebe, I.E.; Rubin, B.Y. Identification of Interferon-γ, as the Lymphokine That Activates Human Macrophage Oxidative Metabolism and Antimicrobial Activity. J. Exp. Med. 1983, 158, 670–689. [Google Scholar] [CrossRef]
- Prestwood, T.R.; Morar, M.M.; Zellweger, R.M.; Miller, R.; May, M.M.; Yauch, L.E.; Lada, S.M.; Shresta, S. Gamma Interferon (IFN-γ) Receptor Restricts Systemic Dengue Virus Replication and Prevents Paralysis in IFN-α/β Receptor-Deficient Mice. J. Virol. 2012, 86, 12561–12570. [Google Scholar] [CrossRef]
- Aberle, J.H.; Aberle, S.W.; Rebhandl, W.; Pracher, E.; Kundi, M.; Popow-Kraupp, T. Decreased Interferon-Gamma Response in Respiratory Syncytial Virus Compared to Other Respiratory Viral Infections in Infants. Clin. Exp. Immunol. 2004, 137, 146–150. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Wang, B.L.; McDonald, J.U.; Yamaguchi, Y.; Harker, J.A.; Goritzka, M.; Johansson, C.; Bukreyev, A.; Collins, P.L.; Openshaw, P.J. Neonatal Antibody Responses Are Attenuated by Interferon-γ Produced by NK and T Cells during RSV Infection. Proc. Natl. Acad. Sci. USA 2013, 110, 5576–5581. [Google Scholar] [CrossRef]
- Yan, L.; Chen, Y.; Han, Y.; Tong, C. Role of CD8+ T Cell Exhaustion in the Progression and Prognosis of Acute Respiratory Distress Syndrome Induced by Sepsis: A Prospective Observational Study. BMC Emerg. Med. 2022, 22, 182. [Google Scholar] [CrossRef]
- Kim, B.S.; Kuen, D.S.; Koh, C.H.; Kim, H.D.; Chang, S.H.; Kim, S.; Jeon, Y.K.; Park, Y.J.; Choi, G.; Kim, J.; et al. Type 17 Immunity Promotes the Exhaustion of CD8+ T Cells in Cancer. J. Immunother. Cancer 2021, 9, e002603. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Feng, Y.; Xu, J.; Liang, J. T-Cell Exhaustion in Immune-Mediated Inflammatory Diseases: New Implications for Immunotherapy. Front. Immunol. 2022, 13, 977394. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Rogers, M.C.; Williams, J.V. Reining in the CD8+ T Cell: Respiratory Virus Infection and PD-1-Mediated T-Cell Impairment. PLoS Pathog. 2019, 15, e1007387. [Google Scholar] [CrossRef] [PubMed]
- Parks, O.B.; Eddens, T.; Sojati, J.; Lan, J.; Zhang, Y.; Oury, T.D.; Ramsey, M.; Erickson, J.J.; Byersdorfer, C.A.; Williams, J.V. Terminally Exhausted CD8+ T Cells Contribute to Age-Dependent Severity of Respiratory Virus Infection. Immun. Ageing 2023, 20, 40. [Google Scholar] [CrossRef]
- Brunet-Ratnasingham, E.; Morin, S.; Randolph, H.E.; Labrecque, M.; Bélair, J.; Lima-Barbosa, R.; Pagliuzza, A.; Marchitto, L.; Hultström, M.; Niessl, J.; et al. Sustained IFN Signaling Is Associated with Delayed Development of SARS-CoV-2-Specific Immunity. Nat. Commun. 2024, 15, 4177. [Google Scholar] [CrossRef]
- Forbester, J.L. Genetic in Fl Uences on Viral-Induced Cytokine Responses in the Lung. Mucosal Immunol. 2021, 14, 14–25. [Google Scholar] [CrossRef]
- Alhetheel, A.; Albarrag, A.; Shakoor, Z.; Somily, A.; Barry, M.; Altalhi, H.; Bakhrebah, M.; Nassar, M.; Alfageeh, M.; Assiri, A.; et al. Chemokine Levels among Patients with Middle East Respiratory Syndrome Coronavirus Infection. Vaccines 2023, 11, 1048. [Google Scholar] [CrossRef]
- Bisgaard, H.; Hermansen, M.N.; Buchvald, F.; Loland, L.; Halkjaer, L.B.; Bonnelykke, K.; Brasholt, M.; Heltberg, A.; Vissing, N.H.; Thorsen, S.V.; et al. Childhood Asthma after Bacterial Colonization of the Airway in Neonates. N. Engl. J. Med. 2007, 357, 1487–1495. [Google Scholar] [CrossRef]
- Branchett, W.J.; Lloyd, C.M. Regulatory Cytokine Function in the Respiratory Tract. Mucosal Immunol. 2019, 12, 589–600. [Google Scholar] [CrossRef]
- Kawai, T.; Seki, M.; Hiromatsu, K.; Eastcott, J.W.; Watts, G.F.M.; Sugai, M.; Smith, D.J.; Porcelli, S.A.; Taubman, M.A. Selective Diapedesis of Th1 Cells Induced by Endothelial Cell RANTES. J. Immunol. 1999, 163, 3269–3278. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.A. The Unexpected Pleiotropic Activities of RANTES. J. Immunol. 2009, 182, 3945–3946. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.; Angelosanto, J.M.; Nadwodny, K.L.; Blackburn, S.D.; Wherry, E.J. A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection. PLoS Pathog. 2011, 7, e1002098. [Google Scholar] [CrossRef]
- Damdinsuren, B.; Zhang, Y.; Khalil, A.; Wood William, H.W., III; Becker, K.G.; Shlomchik, M.J.; Sen, R. Single Round of Antigen Receptor Signaling Programs Naive B Cells to Receive T Cell Help. Immunity 2010, 32, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, J.E.; Miller, S.C.; Smith, J.; Lu, B.; Gerard, C.; Cookenham, T.; Roberts, A.D.; Woodland, D.L. The Chemokine Receptor CCR5 Plays a Key Role in the Early Memory CD8+ T Cell Response to Respiratory Virus Infections. Immunity 2008, 29, 101–113. [Google Scholar] [CrossRef]
- Silva, T.; Temerozo, J.R.; do Vale, G.; Ferreira, A.C.; Soares, V.C.; Dias, S.S.G.; Sardella, G.; Bou-Habib, D.C.; Siqueira, M.; Souza, T.M.L.; et al. The Chemokine CCL5 Inhibits the Replication of Influenza A Virus Through SAMHD1 Modulation. Front. Cell. Infect. Microbiol. 2021, 11, 549020. [Google Scholar] [CrossRef]
- Tyner, J.W.; Uchida, O.; Kajiwara, N.; Kim, E.Y.; Patel, A.C.; O’Sullivan, M.P.; Walter, M.J.; Schwendener, R.A.; Cook, D.N.; Danoff, T.M.; et al. CCL5-CCR5 Interaction Provides Antiapoptotic Signals for Macrophage Survival during Viral Infection. Nat. Med. 2005, 11, 1180–1187. [Google Scholar] [CrossRef]
- Tavares, L.P.; Garcia, C.C.; Gonçalves, A.P.F.; Kraemer, L.R.; Melo, E.M.; Oliveira, F.M.S.; Freitas, C.S.; Lopes, G.A.O.; Reis, D.C.; Cassali, G.D.; et al. ACKR2 Contributes to Pulmonary Dysfunction by Shaping CCL5:CCR5-Dependent Recruitment of Lymphocytes during Influenza a Infection in Mice. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2020, 318, L655–L670. [Google Scholar] [CrossRef]
- Jain, S.; Williams, D.J.; Arnold, S.R.; Ampofo, K.; Bramley, A.M.; Reed, C.; Stockmann, C.; Anderson, E.J.; Grijalva, C.G.; Self, W.H.; et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Children. N. Engl. J. Med. 2015, 372, 835–845. [Google Scholar] [CrossRef]
- Stockman, L.J.; Curns, A.T.; Anderson, L.J.; Fischer-Langley, G. Respiratory Syncytial Virus-Associated Hospitalizations among Infants and Young Children in the United States, 1997–2006. Pediatr. Infect. Dis. J. 2012, 31, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Doucette, A.; Jiang, X.; Fryzek, J.; Coalson, J.; McLaurin, K.; Ambrose, C.S. Trends in Respiratory Syncytial Virus and Bronchiolitis Hospitalization Rates in High-Risk Infants in a United States Nationally Representative Database, 1997–2012. PLoS ONE 2016, 11, e0152208. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).