Abstract
Five chromosomally encoded proteins, BB0108, BB0126, BB0298, BB0323, and BB0689, from Borrelia burgdorferi sensu lato (s.l.), were obtained in three variants each, representing the most common genospecies found in Europe (Borrelia afzelii, Borrelia burgdorferi sensu stricto (s.s.), and Borrelia garinii). The reactivity of these recombinant proteins with the IgM and IgG antibodies present in human serum was assessed using Western blot (WB) and the ELISA. In IgG-WB, the proteins exhibited varying reactivity, peaking at approximately 40–50% for BB0108 and BB0689. However, none of these proteins were recognized by specific antibodies in the IgM-WB. The sensitivity of IgG-ELISA based on three variants of BB0108 and BB0323 ranged from 71% to 82% and from 62% to 72%, respectively. Conversely, the specificity of both tested proteins was consistently above 82%. Tests utilizing single variants of BB0323 did not yield any diagnostic value in detecting IgM antibodies. However, BB0108 demonstrated recognition by antibodies present in 52% to 63% of the tested sera. These antigens appear advantageous due to the consistent reactivity observed across their variants. This observation suggests that appropriate selection of antigens conserved within B. burgdorferi s.l. could offer a solution to the issue of variable sensitivity encountered in serodiagnostic tests across Europe.
1. Introduction
Borrelia burgdorferi sensu lato (s.l.) is a group of bacteria that are an etiological factor of Lyme disease, the most prevalent tick-borne illness in the northern hemisphere. This bacterial group exhibits notable diversity, comprising 23 distinct representatives, of which 7 have been conclusively identified as pathogenic to humans (Borrelia afzelii, Borrelia burgdorferi sensu stricto (s.s.), Borrelia garinii, Borrelia bavariensis, Borrelia spielmanii, Borrelia mayonii, Borrelia lusitaniae) [1,2]. In Europe, B. afzelii, B. burgdorferi s.s., and B. garinii are the predominant genospecies responsible for human infections, although instances of B. bavariensis and B. spielmanii infections have also been recorded [3]. Conversely, in North America, B. burgdorferi s.s. stands as the primary causative agent of Lyme disease, with occasional reports of infections caused by B. mayonii [3,4].
Symptoms of Lyme disease, apart from erythema migrans, which occurs only in 60–90% of patients, are not specific [5,6]. Therefore, the diagnosis is based mainly on laboratory methods. The cultural examination of B. burgdorferi s.l. in Barbour–Stönner–Kelly–H (BSK-H) and Kelly–Pettenkofer (MKP), although it is the standard goal, can only be applied in some particular situations, such as Acrodermatitis chronica atrophicans (ACA), and is not commonly used as it requires very experienced staff [7]. PCR, while less effective, is recommended primarily in the early stages of infection or in individuals with compromised immune systems. Its sensitivity is highest when involved tissues, such as the skin, cerebrospinal fluid, and synovial fluid, are used as input material. Blood samples are not suitable clinical specimens for diagnosing Lyme disease, as except for B. mayonii, B. burgdorferi s.l. is present in low concentrations in the blood. Additionally, the detection of DNA does not confirm whether an active infection is occurring [6,8,9]. The primary diagnostic approach for Lyme disease is a two-tiered serology assay, recommended by the CDC in 1995. Initially, a highly sensitive enzyme-linked immunosorbent assay (ELISA) is conducted, followed by confirmatory Western blot (WB) testing if the ELISA result is positive or equivocal [8,10].
The genome of B. burgdorferi s.l. is unique among bacteria, consisting of a linear chromosome and at least 21 plasmids, both linear and circular in nature. It is worth noting that not every isolate harbors a complete set of plasmids; their presence ranges from 7 to 21, with only the cp26, cp32, and lp54 plasmids universally present across all members of B. burgdorferi s.l. Consequently, individual isolates can exhibit significant variations in the genetic information encoded by plasmids [11,12,13].
Moreover, the antigenic profile of Borrelia varies depending on the host. Given the disparate physiological environments within ticks and mammals, B. burgdorferi s.l. has evolved a repertoire of genes that are selectively transcribed in response to specific hosts. This process initiates in response to the changing conditions during the tick’s blood meal, signaled by a rise in temperature and a decrease in pH. These environmental cues trigger the migration of spirochetes to the tick’s salivary glands, prompting the production of new proteins crucial for successful colonization [14].
Genes located on plasmids mainly encode proteins crucial for mediating bacterial interaction with host tissues, thus determining the pathogenicity and virulence of B. burgdorferi s.l. Isolates of B. burgdorferi s.l. not only exhibit antigenic diversity due to carrying different plasmid sets, but also demonstrate high variation in the amino acid sequences of proteins associated with a low degree of conservation of plasmid sequences between genospecies [13,15]. Therefore, although plasmid-encoded antigens are highly immunogenic, their diagnostic utility is often limited by their low reactivity with antibodies directed against different variants of the same antigens from other genospecies. This issue poses a particular challenge in Europe, where numerous genospecies of B. burgdorferi s.l. are pathogenic to humans [16,17,18].
A relatively well-conserved chromosome carries mainly the genes associated with fundamental metabolic processes. These include genes responsible for the synthesis of the cell wall, DNA, RNA and proteins, biosynthesis of membrane lipids and phospholipids, DNA repair, and nucleotide metabolism [15,19]. While these antigens exhibit lower immunogenicity when compared with plasmid-encoded proteins, they have not been subjected to extensive study by researchers. However, it is possible to identify among them those that induce the production of specific antibodies [16,20,21]. It is therefore possible that the use of these proteins in diagnostic tests may at least partly contribute to solving the problems related to the diversity of B. burgdorferi s.l. genospecies.
BB0108, BB0126, BB0298, BB0323, and BB0689 are chromosomally encoded proteins whose production increases during the establishment of mammalian infections. Additionally, BB0126, BB0323, and BB0689 are localized in the outer membrane of B. burgdorferi s.l., while BB0108 and BB0298 are located in the cell envelope. Therefore, there is a high probability that they play a role in pathogenesis and are presented to the immune system cells at the initial stage of infection, which may indicate their potential diagnostic utility [22].
In this study, for the first time, BB0108, BB0126, BB0298, BB0323, and BB0689 antigens from three genospecies of B. burgdorferi s.l. (B. afzelii, B. burgdorferi s.s., B. garinii) were biotechnologically produced with the use of the prokaryotic expression system of Escherichia coli and assessed for their reactivity with specific anti-B. burgdorferi s.l. antibodies.
2. Materials and Methods
2.1. Serum Samples
The study used 148 positive sera (100 for IgG and 48 for IgM) and 100 negative sera. Lyme disease serum samples were obtained from the National Institute of Public Health NIH (Warsaw, Poland). All were collected during a routine Lyme disease diagnosis. Anonymized information about each sample included only the date of collection and the titer of anti-Borrelia antibodies. The IgG and IgM levels were re-measured using Borrelia plus VlsE and Borrelia Select: recombinant antigens with OspC (Euroimmun, Lübeck, Germany), respectively. The presence of specific anti-Borrelia antibodies was further confirmed using a EUROLINE Borrelia-RN-AT (EUROLINE WB Borrelia, Euroimmun, Lübeck, Germany).
2.2. Construction of Recombinant Plasmids
Fragments of the BB0108, BB0126, BB0208, BB0323, and BB0689 genes were PCR-amplified from genome DNA of three B. burgdorferi s.l. genospecies (B. burgdorferi s.s. strain B31, B. afzeli strain PKo, B. garinii strain 20047 (DSMZ, Braunschweig, Germany)). When identical amino acid sequences were present at both ends of the amplified fragments, the same primers were used to amplify the gene from different genospecies (Supplementary Materials Table S1).
The PCR products were inserted into the BglII restriction site of pUET1 expression plasmid using In-Fusion® HD Cloning Kit (Takara Bio USA, Inc., Mountain View, CA, USA) (Supplementary Materials Figures S1–S5) [23]. All the antigen nucleotide sequences were embedded in a frame between the His6-tag domains for the purification of the recombinant proteins using metal affinity chromatography. The nucleotide sequences of the recombinant plasmids were confirmed with DNA sequencing (Genomed, Poland).
2.3. Expression and Purification of Recombinant Proteins
All constructed recombinant plasmids were transformed into Escherichia coli BL21(DE3)pLysS. Cells transformed with recombinant plasmids were grown in LB broth supplemented with 100 μg/mL ampicillin and 34 μg/mL chloramphenicol to an optical density at λ = 600 nm of 0.4. Protein production was induced with isopropyl-β-D-thiogalactopyranoside (IPTG) at a final concentration of 1 mM, and cells were incubated with vigorous shaking at 37 °C for 18 h. Subsequently, cells were harvested via centrifugation. Proteins were purified in a one-step metal affinity chromatography in accordance with the manufacturer’s instructions (Novagen, Madison, WI, USA). The purification was carried out under native and denaturing conditions depending on whether the proteins were deposited in the dissolved cytoplasmic fraction or as inclusion bodies. Purity of the recombinant proteins was verified by 12% SDS-PAGE and quantified using Image Lab software 5.2.1 (Bio-Rad, Hercules, CA, USA). After purification, the His6-tags were not cleaved prior to serological analysis. Protein concentration was measured with a Bradford Assay Kit according to the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA) using bovine serum albumin (BSA) as a standard.
2.4. Western Blot
Due to the similar masses of recombinant proteins, it was not feasible to test five antigens on a single test strip. Therefore, three different test strips were constructed, each containing protein preparations that distinctly varied in mass according to Table 1. SDS-PAGE was conducted on gels prepared using modified combs to have a single large well (Supplementary Materials Figure S21). Each enlarged well was loaded with 40 µg of recombinant protein. After electrophoretic separation, the proteins were transferred on a nitrocellulose membrane. The membrane was blocked with 5% non-fat skim milk in Tris-buffered saline with 0.1% Tween 20 (TBS-T) for 1 h at room temperature with constant shaking. The membrane was then washed three times with TBS-T and incubated with human serum samples, diluted at 1:200 for 1 h and 1.5 h for IgG and IgM detection, respectively. After the membrane was washed, it was treated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-human IgG and IgM antibodies (Jackson ImmunoResearch, Ely, UK), diluted at 1:75,000 and 1:50,000, respectively. After further washes, the reaction was developed by the addition of chemiluminescence peroxidase substrate (Immobilon Crescendo Western HRP substrate, Merck, Darmstadt, Germany), and the results were visualized using Image Lab software 5.2.1 (Bio-Rad, Hercules, CA, USA).

Table 1.
Composition of recombinant protein preparation used for the Western blot.
2.5. ELISA
MaxiSorp microtiter plates (Nunc, Waltham, MA, USA) were coated with individual variants of BB0108 and BB0323 antigens with a total of 1 μg per well in 50 mM carbonate buffer (pH 9.6). For the ELISA utilizing three antigen variants, microtiter plates were also coated with a total of 1 μg per well, with each antigen variant applied at an amount of 0.33 µg. After overnight incubation at 4 °C, the plates were washed four times with washing buffer (50 mM Tris; 0.88% NaCl; 0.1% Tween 20, pH 7.4). Plates were blocked for 1 h at 37 °C in blocking buffer (3% non-fat milk, 0.1 % Tween 20 in PBS). The plates were washed again and incubated for 1 h at 37 °C with the human serum diluted 1:100 in blocking buffer. After washing, peroxidase-labeled antibodies directed against human IgG and IgM (Jackson ImmunoResearch, Ely, UK) were added, diluted 1:32,000 and 1:16,000, respectively. O-phenylenediamine dihydrochloride (Substrate SIGMAFAST™ OPD, Sigma, Saint Louis, MO, USA) was used as a substrate for the detection of the formed immune complexes, and absorbances were measured at λ = 492 nm.
2.6. Statistical Analysis
For all data manipulation, analysis was conducted using GraphPad Prism software (Version 9, GraphPad Software Inc., San Diego, CA, USA). Fisher’s exact test was employed to assess the statistical significance of recombinant protein reactivity with antibodies present in two groups of sera in Western blots. The distinction in absorbance between negative and positive sera was evaluated using Student’s t-test. Statistical significance was established at a p-value below 0.05 for both tests. Receiver operating characteristic (ROC) analysis was performed to determine the area under the curve (AUC), optimal cut-off, sensitivity, and specificity of assays based on recombinant proteins. The optimal cut-off was identified as the absorbance value corresponding to the point on the ROC curve nearest to the (0, 1) corner [24].
3. Results
3.1. Construction of Recombinant Plasmids
In order to produce B. burgdorferi s.l. proteins, 15 recombinant plasmids carrying gene encoding selected antigens were successfully constructed. The characteristics of obtained plasmids and the proteins they encode are presented in Table 2. The full sequences of recombinant proteins are presented in the Supplementary Materials Figures S6–S20.

Table 2.
List of constructed plasmids and characterization of recombinant proteins.
3.2. Expression and Purification of Recombinant Proteins
All the variants, BB0108, BB0126, BB0298, BB0323, and BB0689 proteins, were produced in E. coli BL21(DE3)pLysS. In this way, recombinant proteins with a molecular weight in the 23–50 kDa range were obtained (Table 2) (Supplementary Materials Figure S22). All proteins contained His6-tag domains at the N- and C-terminus which allowed for the purification of proteins using metal affinity chromatography. BB0108, BB0126, and BB0298 were produced in soluble form and purified under native conditions, while BB0323 and BB0689 were produced as inclusion bodies, thus requiring buffers containing 5 M urea for their purification. Proteins preparations were obtained with an electrophoretic purity greater than 98% (Supplementary Materials Figure S23). These prokaryotic expression systems produced 39–76 mg proteins per liter of culture (Table 2).
3.3. Western Blot
The initial assessment of the reactivity of recombinant proteins with specific anti-B. burgdorferi IgG and IgM was conducted using three groups of serum samples, each comprising 25 individual samples. These groups included negative serum samples (IgG−, IgM−), IgG-positive samples (IgG+, IgM−), and IgM-positive samples (IgG−, IgM+). Figure 1, Figure 2 and Figure 3 depict representative results from Western blot analysis determining the reactivity of the tested antigens obtained from B. afzelii with class G antibodies (negative and IgG-positive sera).
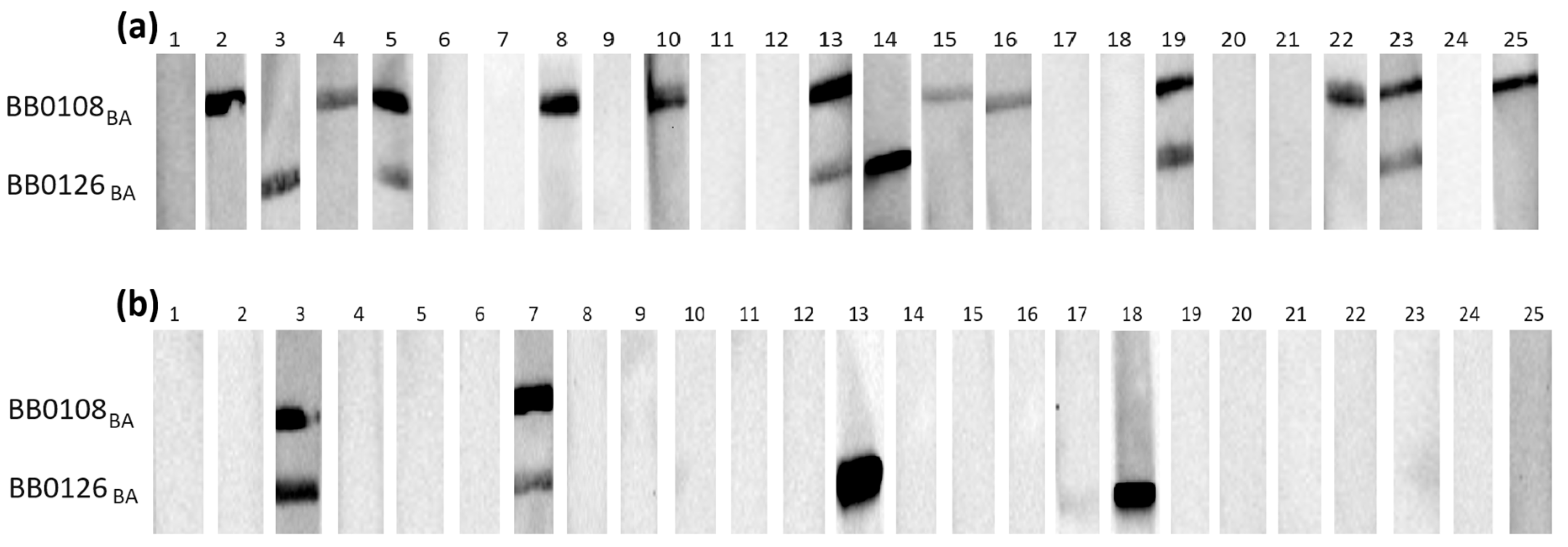
Figure 1.
IgG Western blot results for BB0108BA and BB0126BA: (a) incubation with 25 IgG-positive sera; (b) incubation with 25 negative sera.
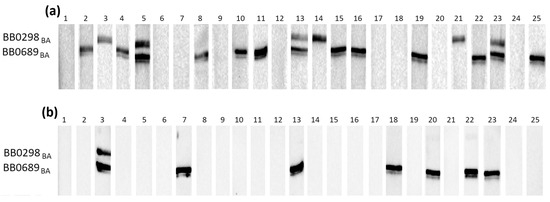
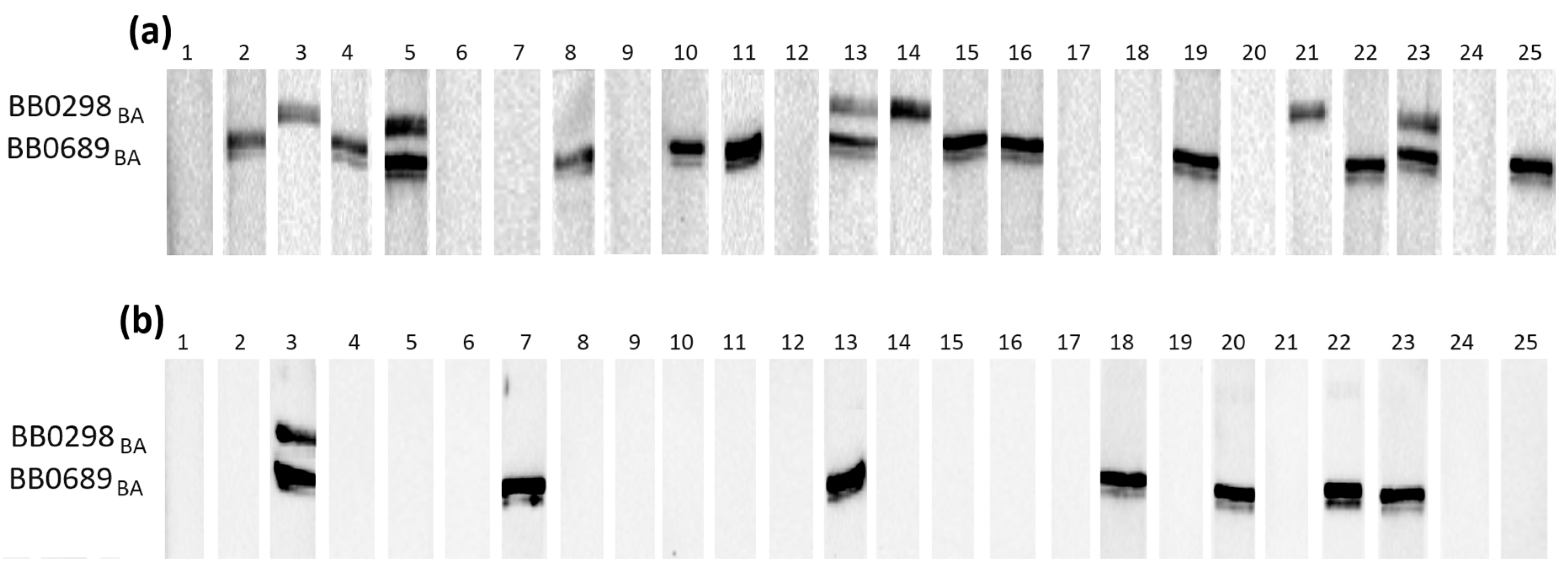
Figure 2.
IgG Western blot results for BB0298BA and BB0689BA: (a) incubation with 25 IgG-positive sera; (b) incubation with 25 negative sera.
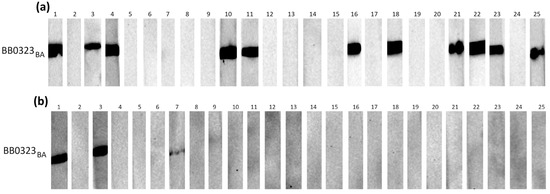
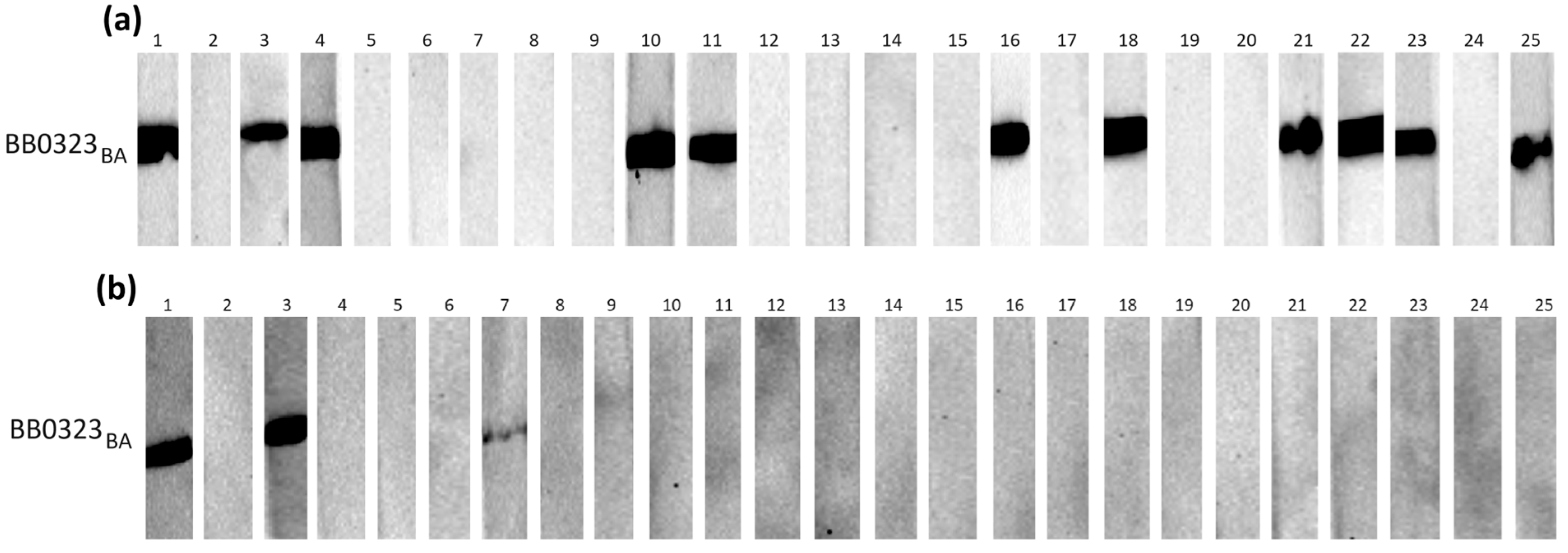
Figure 3.
IgG Western blot results for BB0323BA: (a) incubation with 25 IgG-positive sera; (b) incubation with 25 negative sera.
Individual proteins exhibited varying reactivity with antibodies from human sera, with slight differences observed among protein variants originating from distinct genospecies.
It appears that in most cases, the B. burgdorferi s.s.-derived protein was recognized by specific IgG less frequently than their B. afzelii and B. garinii equivalents (Table 3).

Table 3.
IgG-WB results.
The most frequently recognized recombinant proteins by IgG in the positive sera were BB0108 and BB0689, with minimum reactivity levels of 44%, reaching a maximum of 52% for BB0689BA. All variants of BB0126 and BB0298 were very weakly reactive, recognized only by antibodies contained in approximately 20% of the tested sera. The specificity of antigen–antibody interactions was relatively high, at least 88%, except for BB0689, where the specificity dropped to 72%. After analysis using Fisher’s exact test, it was shown that a statistically significant difference in the reactivity of recombinant proteins with IgG contained in positive and negative sera occurs only for BB0108 (all variants), BB0323 (all variants), and BB0298BG.
All tested proteins were poorly recognized by the IgM contained in serum samples (Table 4). The highest reactivity achieved for BB0108 from B. afzelii and B. garinii was 24%. The specificity of the IgM-WB was over 90% in all cases. Fisher’s exact test showed no statistically significant differences in the reactivity of all tested antigens with the IgM contained in the negative and positive sera (Table 4).

Table 4.
IgM Western blot results.
The overall sensitivity and specificity of the Western blot in detecting IgG and IgM when summing the results for the three variants of a given antigen remained unchanged relative to the most reactive one, except for IgG-WB-BB0689, where the sensitivity increased to 60% (15/25), while the specificity decreased to 64% (9/25).
Since solely WB based on BB0108 and BB0323 showed statistically significant differences in reactivity between the serum groups, only these proteins were selected for evaluating their diagnostic potential on a larger serum pool using the ELISA.
3.4. ELISA
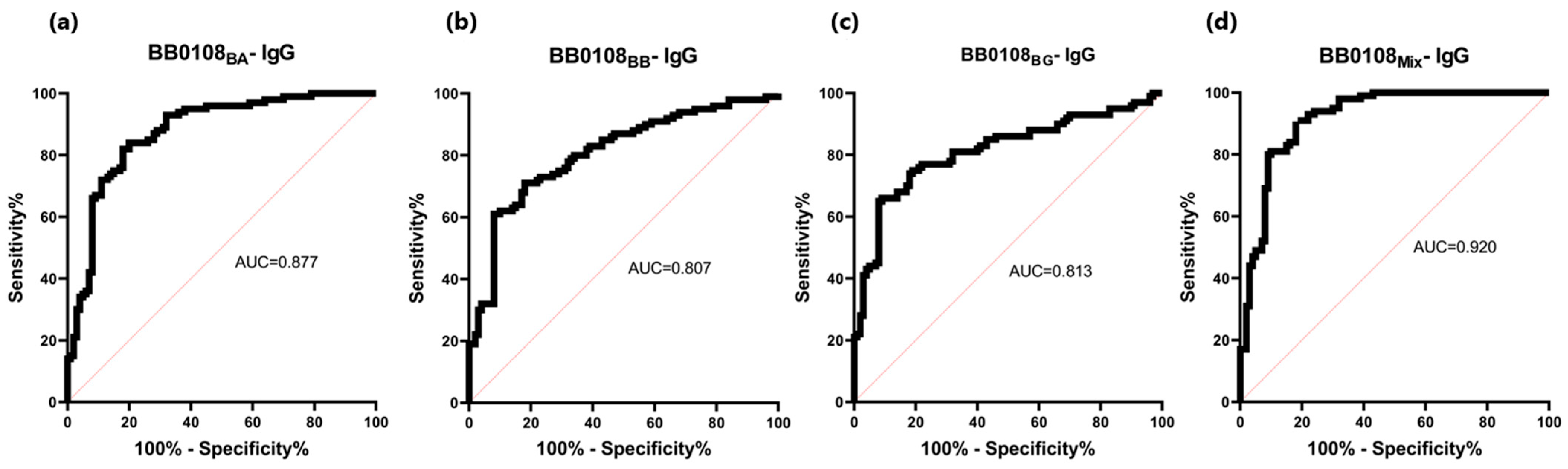
The BB0108 and BB0323 antigens showed differentiated reactivity with antibodies contained in human sera depending on the antigen variant (Table 5, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11). The sensitivity of the IgG-ELISA based on different variants of BB0108 ranged from 74% to 82%, with the highest value obtained for BB0108BA (Table 5). The specificity of all IgG-ELISA-BB0108 was 82%. The area under curve (AUC) value ranged from 0.807–0.877 and again the highest value was achieved with BB0108BA (Figure 5, Table 5). The use of a mixture of three variants of BB0108 recombinant proteins in the IgG-ELISA significantly increased the specificity of the assay and led to an increase in AUC (Figure 4 and Figure 5, Table 5). However, this did not affect the sensitivity of the assay.

Table 5.
IgG-ELISA results.
Table 5.
IgG-ELISA results.
| Recombinant Protein | Optimal Cut-Off | Sensitivity [%] | Specificity [%] | AUC | Mean Absorbance | Median Absorbance |
|---|---|---|---|---|---|---|
| BB0108BA | 0.193 | 82% (82/100) * | 82% (18/100) * | 0.877 | P 1: 0.340 N 2: 0.153 | P 1: 0.317 N 2: 0.128 |
| BB0108BB | 0.196 | 71% (71/100) * | 82% (18/100) * | 0.807 | P 1: 0.323 N 2: 0.155 | P 1: 0.297 N 2: 0.130 |
| BB0108BG | 0.199 | 74% (74/100) * | 82% (18/100) * | 0.813 | P 1: 0.355 N 2: 0.157 | P 1: 0.314 N 2: 0.132 |
| BB0108Mix | 0.254 | 80% (80/100) * | 91% (9/100) * | 0.920 | P 1: 0.391 N 2: 0.166 | P 1: 0.355 N 2: 0.141 |
| BB0323BA | 0.363 | 72% (72/100) * | 88% (12/100) * | 0.840 | P 1: 0.473 N 2: 0.326 | P 1: 0.433 N 2: 0.309 |
| BB0323BB | 0.372 | 62% (62/100) * | 90% (10/100) * | 0.805 | P 1: 0.436 N 2: 0.321 | P 1: 0.416 N 2: 0.299 |
| BB0323BG | 0.362 | 64% (64/100) * | 90% (10/100) * | 0.832 | P 1: 0.450 N 2: 0.307 | P 1: 0.413 N 2: 0.285 |
| BB0323Mix | 0.401 | 67% (67/100) * | 90% (10/100) * | 0.826 | P 1: 0.492 N 2: 0.350 | P 1: 0.452 N 2: 0.331 |
*—number of sera reactive with antigen/number of tested sera; AUC—area under the curve; 1—positive sera; 2—negative sera; Mix—a mixture of three variants of recombinant protein.
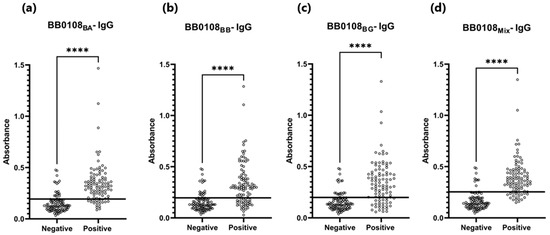
Figure 4.
Absorbance for positive and negative sera: (a) IgG-ELISA-BB0108BA; (b) IgG- ELISA- BB0108BB; (c) IgG-ELISA- BB0108BG; (d) IgG-ELISA-BB0108Mix. The horizontal line marks the cut- off. ****—p ≤ 0.0001.
Figure 4.
Absorbance for positive and negative sera: (a) IgG-ELISA-BB0108BA; (b) IgG- ELISA- BB0108BB; (c) IgG-ELISA- BB0108BG; (d) IgG-ELISA-BB0108Mix. The horizontal line marks the cut- off. ****—p ≤ 0.0001.
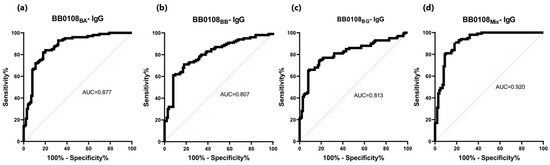
Figure 5.
ROC analysis and AUC: (a) IgG-ELISA-BB0108BA; (b) IgG-ELISA-BB0108BB; (c) IgG- ELISA- BB0108BG; (d) IgG-ELISA-BB0108Mix.
Figure 5.
ROC analysis and AUC: (a) IgG-ELISA-BB0108BA; (b) IgG-ELISA-BB0108BB; (c) IgG- ELISA- BB0108BG; (d) IgG-ELISA-BB0108Mix.
The sensitivity of the IgG-ELISA-BB0323 was the highest for BB0323BA and the lowest for BB0323BB, at 72% and 62%, respectively. The specificity for all variants of the antigen oscillated around 90%, while the AUC reached the range of 0.805–0.840 (Figure 6 and Figure 7, Table 5).
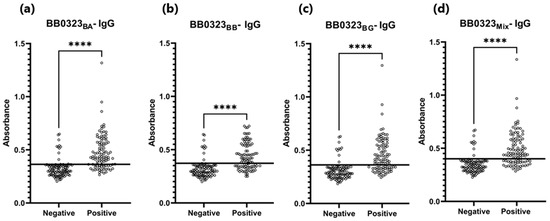
Figure 6.
Absorbance for positive and negative sera: (a) IgG-ELISA-BB0323BA; (b) IgG- ELISA- BB0323BB; (c) IgG-ELISA- BB0323BG; (d) IgG-ELISA-BB0323Mix. The horizontal line marks the cut-off. ****—p ≤ 0.0001.
Figure 6.
Absorbance for positive and negative sera: (a) IgG-ELISA-BB0323BA; (b) IgG- ELISA- BB0323BB; (c) IgG-ELISA- BB0323BG; (d) IgG-ELISA-BB0323Mix. The horizontal line marks the cut-off. ****—p ≤ 0.0001.
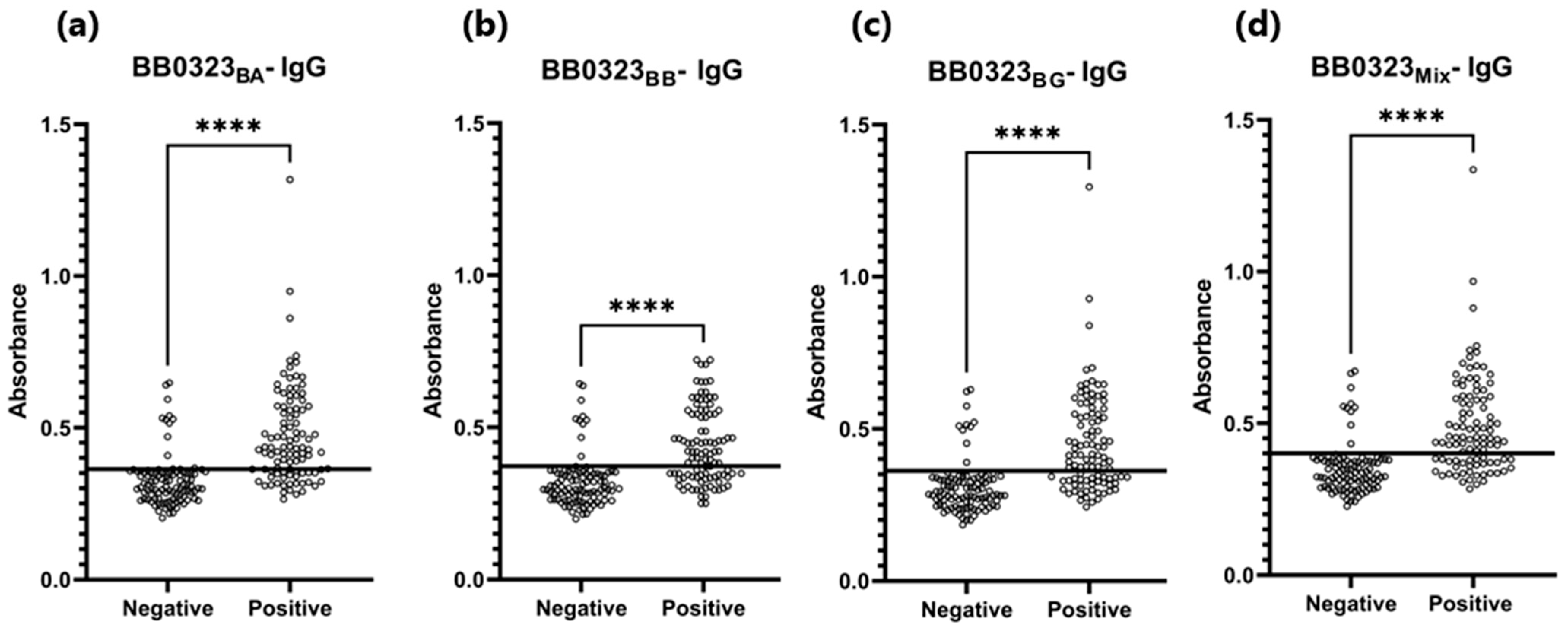
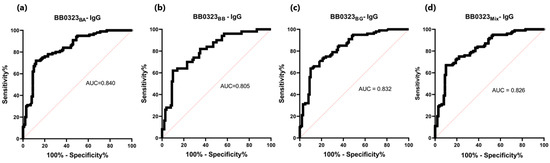
Figure 7.
ROC analysis and AUC: (a) IgG-ELISA-BB0323BA; (b) IgG-ELISA-BB0323BB; (c) IgG- ELISA- BB0323BG; (d) IgG-ELISA-BB0323Mix.
Figure 7.
ROC analysis and AUC: (a) IgG-ELISA-BB0323BA; (b) IgG-ELISA-BB0323BB; (c) IgG- ELISA- BB0323BG; (d) IgG-ELISA-BB0323Mix.
The sensitivity of IgM detection was lower. It ranged for BB0108 variants from 52% to 63% (Table 5). However, unlike the IgG-ELISA here, BB0108BB provided the highest sensitivity. AUC also decreased to 0.724–0.736 (Table 6, Figure 8 and Figure 9). However, a slightly higher specificity of these tests was observed, reaching 91% for BB0108BG. In the IgM-ELISA, using a mix of three protein variants did not significantly affect the sensitivity and specificity; instead, it led to an increase in AUC (Figure 9).

Table 6.
IgM-ELISA results.
Table 6.
IgM-ELISA results.
| Recombinant Protein | Optimal Cut-Off | Sensitivity [%] | Specificity [%] | AUC | Mean Absorbance | Median Absorbance |
|---|---|---|---|---|---|---|
| BB0108BA | 0.219 | 56% (27/48) * | 86% (9/65) * | 0.724 | P 1: 0.277 N 2: 0.171 | P 1: 0.259 N 2: 0.145 |
| BB0108BB | 0.234 | 63% (30/48) * | 83% (12/65) * | 0.736 | P 1: 0.259 N 2: 0.183 | P 1: 0.263 N 2: 0.159 |
| BB0108BG | 0.234 | 52% (25/48) * | 91% (6/65) * | 0.729 | P 1: 0.264 N 2: 0.157 | P 1: 0.269 N 2: 0.144 |
| BB0108Mix | 0.228 | 56% (27/48) * | 86% (9/65) * | 0.765 | P 1: 0.287 N 2: 0.168 | P 1: 0.246 N 2: 0.133 |
| BB0323BA | No statistical difference in absorbance for positive and negative sera (p = 0.575) | 0.533 | P 1: 0.262 N 2: 0.250 | P 1: 0.252 N 2: 0.232 | ||
| BB0323BB | No statistical difference in absorbance for positive and negative sera (p = 0.052) | 0.572 | P 1: 0.294 N 2: 0.246 | P 1: 0.273 N 2: 0.233 | ||
| BB0323BG | No statistical difference in absorbance for positive and negative sera (p = 0.319) | 0.526 | P 1: 0.325 N 2: 0.301 | P 1: 0.304 N 2: 0.284 | ||
| BB0323Mix | 0.248 | 60% (29/48) * | 71% (29/65) * | 0.686 | P 1: 0.333 N 2: 0.261 | P 1: 0.323 N 2: 0.243 |
*—number of sera reactive with antigen/number of tested sera; AUC—area under the curve; 1—positive sera; 2—negative sera; Mix—a mixture of three variants of recombinant protein.
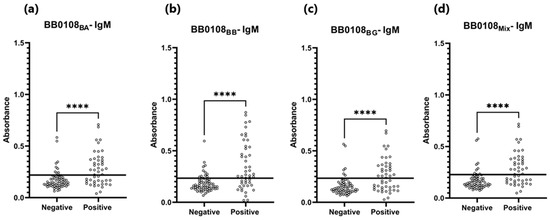
Figure 8.
Absorbance for positive and negative sera: (a) IgM-ELISA-BB0108BA; (b) IgM-ELISA-BB0108BB; (c) IgM-ELISA-BB0108BG; (d) IgM-ELISA-BB0108Mix. The horizontal line marks the cut-off. ****—p ≤ 0.0001.
Figure 8.
Absorbance for positive and negative sera: (a) IgM-ELISA-BB0108BA; (b) IgM-ELISA-BB0108BB; (c) IgM-ELISA-BB0108BG; (d) IgM-ELISA-BB0108Mix. The horizontal line marks the cut-off. ****—p ≤ 0.0001.
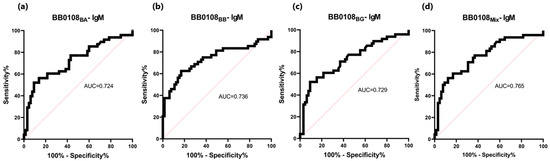
Figure 9.
ROC analysis and AUC: (a) IgM-ELISA-BB0108BA; (b) IgM-ELISA-BB0108BB; (c) IgM-ELISA-BB0108BG; (d) IgM-ELISA-BB0108Mix.
Figure 9.
ROC analysis and AUC: (a) IgM-ELISA-BB0108BA; (b) IgM-ELISA-BB0108BB; (c) IgM-ELISA-BB0108BG; (d) IgM-ELISA-BB0108Mix.
Student’s t-test revealed non-statistically significant differences in absorbance between positive and negative sera in the IgM-ELISA based on single BB0323 variants (p > 0.05) (Table 5, Figure 10), with the acquired AUC values falling within the range of 0.526- 572 (Figure 11). When employing a protein mix in the IgM-ELISA, a statistically significant difference was observed in the absorbance levels between the two groups of sera.
Figure 10.
Absorbance for positive and negative sera: (a) IgM-ELISA-BB0323BA; (b) IgM-ELISA-BB0323BB; (c) IgM-ELISA-BB0323BG; (d) IgM-ELISA-BB0323Mix. The horizontal line marks the cut-off. ***—p ≤ 0.001. ns—p > 0.05.
Figure 10.
Absorbance for positive and negative sera: (a) IgM-ELISA-BB0323BA; (b) IgM-ELISA-BB0323BB; (c) IgM-ELISA-BB0323BG; (d) IgM-ELISA-BB0323Mix. The horizontal line marks the cut-off. ***—p ≤ 0.001. ns—p > 0.05.
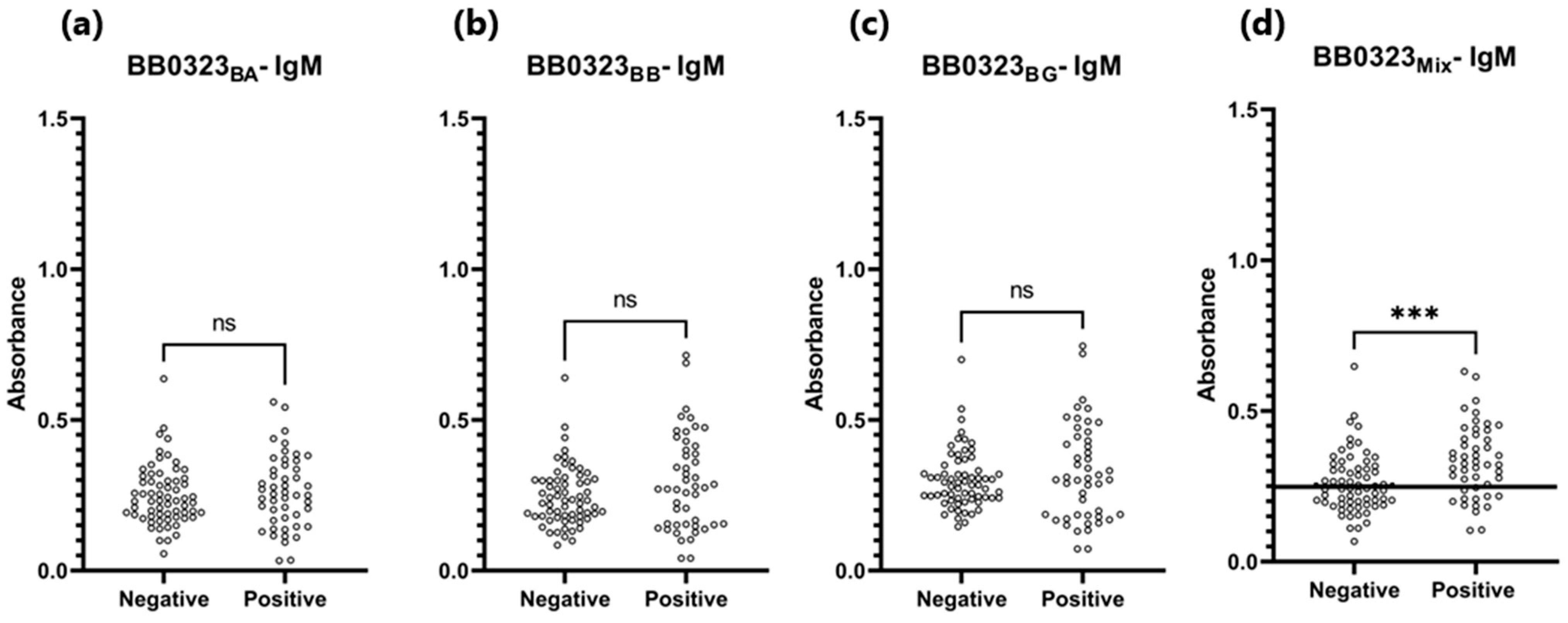
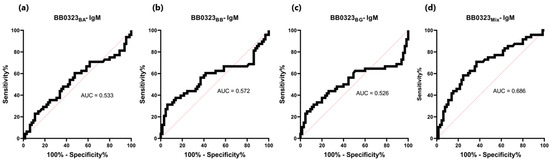
Figure 11.
ROC analysis and AUC: (a) IgM-ELISA-BB0323BA; (b) IgM-ELISA-BB0323BB; (c) IgM-ELISA-BB0323BG; (d) IgM-ELISA-BB0323Mix.
Figure 11.
ROC analysis and AUC: (a) IgM-ELISA-BB0323BA; (b) IgM-ELISA-BB0323BB; (c) IgM-ELISA-BB0323BG; (d) IgM-ELISA-BB0323Mix.
4. Discussion
Despite its widespread use, serodiagnosis faces many challenges. The primary challenges stem from the complex antigenic structure of spirochetes. The high diversity of genospecies within B. burgdorferi s.l. and the low conservation level of amino acid sequences in their proteins mean that utilizing cell lysates from one genospecies in diagnostic tests may not be sufficient for accurate Lyme disease diagnosis [6,18,25]. Moreover, B. burgdorferi s.l. contains many proteins that are homologous to antigens present in other pathogens, which can lead to cross-reactivity. It is precisely these frequent nonspecific interactions that led to the introduction of a two-step serodiagnostic test [26,27,28].
A potential solution to these challenges could involve employing carefully selected recombinant proteins as antigens in serodiagnostic tests. Currently, Western blot tests frequently utilize biotechnologically produced antigens, facilitating improved standardization of antigen preparations and cost reduction. In Europe, to ensure better sensitivity of the ELISA, highly immunogenic B. burgdorferi s.l. recombinant antigens obtained from several genospecies are added to the whole cell lysate [25,29]. Because of its complex structure, the proteome of B. burgdorferi s.l. remains poorly understood. In fact, only a few of its antigens have been tested for diagnostic utility, with the majority being lowly conserved plasmid-encoded antigens, including DbpA, VlsE, BBK32, FlaB, OspA, OspC, OspB, OspF, and OspE. [30,31,32,33,34,35]. Studying the proteome of B. burgdorferi s.l. more closely may lead to the identification of highly conserved chromosome-encoded antigens with diagnostic utility, potentially eliminating the dependency of assay sensitivity on the infecting genospecies. The search for specific proteins can be carried out in different tissues or liquids (for example, in urine) with a high-definition tandem mass spectrometer [36].
For these reasons, in this study, it was decided to evaluate the diagnostic potential of five previously untested B. burgdorferi s.l. antigens whose genes are located on chromosomes and characterized by high-sequence conservation [22]. They were selected based on the literature data indicating their diagnostic potential. BB0323 is an antigen necessary for the transmission of spirochetes throughout the enzootic infection cycle. Additionally, BB0323 has been shown to trigger an immune response in both humans and mice [37].
BB0108 is a membrane lipoprotein, and two independent studies have confirmed the presence of specific antibodies in the sera of individuals with Lyme disease [21,38]. BB0689, localized on surface membrane, has been confirmed that it is an immunogen, as baboons infected with B. burgdorferi s.s. produced BB0689-specific bactericidal antibodies [39,40]. BB0126 and BB0298 are surface proteins, so theoretically they should be well-exposed to the host immune system [40]. Moreover, their up-regulation during tick blood feeding implies their potential involvement in the virulence and pathogenesis of B. burgdorferi s.l. [14]. It was decided to evaluate the reactivity of three variants of each of the antigens derived from B. afzelii, B. burgdorferi s.s., and B. garinii as these genospecies are the most common in Europe [41,42,43].
In this study, we utilized the efficient E. coli expression system developed in our laboratory, along with a simple purification method previously described [44,45], to obtain new recombinant proteins (BB0108, BB0126, BB0298, BB0323, BB0689) from three genospecies of B. burgdorferi s.l. These proteins were then employed in the IgG/IgM Western blot and IgG/IgM ELISA to assess their utility for a serological diagnosis of Borrelia infection in human sera.
The initial assessment of reactivity for the new antigens, conducted using the WB test, revealed that the newly obtained proteins moderately react with antibodies present in human sera. The WB sensitivity reached its peak values for IgG-WB-BB0689; however, this antigen was also frequently recognized by antibodies in the negative sera (specificity range 72–76%). Therefore, it is likely that the relatively high sensitivity of the WB tests is primarily caused by cross-reactivity rather than specific interactions with anti-B. burgdorferi s.l. antibodies.
The most promising results at this stage of research were obtained for BB0108 and BB0323. Comparing these results to those obtained for recombinant proteins commonly used in commercial WB, it turned out that the reactivity of BB0108 and BB0323 was at a similar level as that obtained for IgG-DbpABA (45%; 9/20) in the study by Heikkilä et al. (2002) and exceeded that of the least reactive variant of this protein, B. burgdorferi s.s., which was 20% (4/20). However, when the results for the three DbpA variants in the study were added together, the overall sensitivity for detecting late-stage Lyme disease was 80% (16/20), significantly exceeding the value achieved in the cases of BB0108 and BB0323. In addition, WB based on DbpA was characterized by 100% specificity (0/20), while for BB0108 and BB0323, it was a maximum of 96% (1/25) and 92% (2/25), respectively [17]. WB based on the most reactive FlaA variant derived from B. garinii achieved a sensitivity of 75% (21/28) and a specificity of over 95% (1/23). When the reactivity of the three FlaA variants was added together, the sensitivity increased to 79% (22/28), and specificity decreased to 87% (3/23). FlaA from B. burgdorferi s.s. showed the lowest diagnostic utility, and the sensitivity decreased to 39% (11/28) with a specificity of 91% (2/23), which was a result comparable to that achieved for IgG-WB based on BB0108 and BB0323 [46].
As only BB0108 and BB0323 exhibited statistically significant differences in reactivity between negative and positive sera in the initial WB, it was decided to test these proteins in the ELISA assay on a large pool of sera. In the IgG-ELISA, the BB0108s were highly reactive with the antibodies contained in the positive sera. The sensitivity of the IgG-ELISA even reached above 80% for the most reactive variant of the antigen, which is similar to that obtained by DbpA in the late stages of Lyme disease [17,30]. However, it should be noted that the assay’s high sensitivity was not matched by its satisfactory specificity, as it was 82%. The use of the mixture of three variants of BB0108 in the ELISA did not improve the sensitivity of the test. It turned out to even be slightly lower than that obtained for the most reactive variant of the protein from B. afzelii. This can be explained by the lower content of BB0108BA in the wells, as only 0.33 µg of BB0108BA was used to coat the microtiter plates in this case, while it was 1 µg in the single protein assay. However, using the mixture positively affected the specificity and increased the AUC, significantly improving the diagnostic value of such an immunoenzymatic assay. This approach increased the overall difference in absorbance values between the two groups of sera. It was demonstrated that there is a more significant difference in mean absorbance and median between negative and positive sera in the ELISA assays using a mixture of variant proteins compared to those using single antigens. It is possible that by using three antigen variants, more anti-B. burgdorferi s.l. antibodies from positive sera recognized specific epitopes. This allowed the determination of a higher value of the optimal cut-off (0.254 compared to values oscillating around 0.195), leading to an increase in the specificity of the test, while only slightly lowering the sensitivity.
The sensitivity of the IgG-ELISA based on single variants of BB0323 was in the range of 62–72%. Similar to BB0108, the protein BB0323 obtained from B. afzelii was the most reactive, while the IgG-ELISA-BB0323BB was characterized by the lowest sensitivity. Using the BB0323Mix in the ELISA did not affect the diagnostic usefulness of the enzyme immunoassay. Although sensitivity compared to the ELISA based on single antigens obtained from B. burgdorferi s.s. and B. garinii increased, it did not reach as high a value as it did for BB0323BA. It is assumed that, similarly to the case of the BB0108s, it is related to the lower content of the most reactive protein variant (BB0323BA) in the microtiter plate wells. The specificity remained unchanged at 90%.
There was no statistically significant difference in absorbance between the positive and negative serums in the IgM-ELISA tests based on single variants of BB0323, and the obtained AUC values did not exceed 0.6 (0.533, 0.572, 0.526), indicating the lack of reactivity of this protein with this class of antibodies. Thus, tests based on single variants of BB0323 did not allow for distinguishing between IgM-negative and IgM-positive sera. The use of a mixture of three variants of BB0323 had an observable positive effect. The IgM-ELISA-BB0323Mix showed a statistically significant difference in the absorbance of the positive and negative sera and the AUC increased to 0.686. Perhaps by utilizing a mixture of proteins from three genospecies in the ELISA, it was possible to detect infections with various representatives of B. burgdorferi s.l. in a single assay.
For the IgM-ELISA based on three variants of BB0108, statistically significant differences in absorbance were obtained for both sera groups, which suggests that BB0108 is recognized by specific IgM, which was not observed in the Western blot. This implies that conformational epitopes may play a major role in these interactions, as in the IgM-WB-BB0108 there was no statistically significant difference in the reactivity between negative and positive sera.
In both enzyme immunoassay tests (WB, ELISA), the obtained proteins were not recognized by specific IgM. This is likely due to the nature of IgM, as they are the primary antibodies produced during an immune response and typically exist at lower concentrations in the blood compared to IgG at their peak. Additionally, IgM levels in the blood decrease rapidly over time, and their affinity for antigens is generally lower as they are produced during the early stages of the immune response when it is still developing. Consequently, IgM antibodies have a narrower spectrum of antigen recognition, primarily targeting proteins and their fragments exposed during the early stages of infection [47,48,49,50,51].
Similarly, as in the previous studies, the sensitivity of ELISA and WB based on new recombinant B. burgdorferi s.l. antigens differed depending on the antigen variant used. It is important to note that differences in ELISA sensitivity were up to 11% and 10% for BB0108 and BB0323, respectively, while in the case of WB, they were even lower. Thus, they were not as meaningful as those observed in studies conducted for DbpA and OspC, where differences in ELISA sensitivity between variants in a single study were 25% and 50%, respectively [17,52]. Moreover, in studies by Heikkilä et al. (2002) [17] and Schulte-Spechtel et al. (2006) [53], it was observed that most of the sera were reactive with only one variant of the DbpA. In the present study, in most cases antibodies in the positive sera recognized all three antigen variants. The sensitivity of the ELISA and WB, taking into account all individual antigen variants, in most cases did not differ from that obtained for the most reactive form of the protein. Only in the case of IgG-BB0689s, when its three variants were considered, did the total WB sensitivity increase to 60% (15/25). The results suggest that in most cases, when antibodies against one variant of the antigen were present in the serum, they recognized its other forms. The observation that the most diverse reactivity of different protein variants concerns BB0689 is in line with the bioinformatic analysis, which showed that this antigen exhibited the lowest sequence conservation among those examined [22]. This indicates that the appropriate selection of conserved antigens may make their reactivity independent of the genospecies causing Lyme disease.
In most cases, similar to other research carried out on sera collected from different areas of Europe, the highest reactivity in the IgG class was shown by variants derived from B. afzelii or B. garinii [16,17,20,52]. This allows us to assume that infections with these genospecies were dominant among the patients from whom the sera were collected. However, these are only conjectures as no specific information was available. This observation is not entirely consistent with the research on the prevalence of B. burgdorferi s.l. genospecies in Poland, which state that B. burgdorferi s.s. is dominant [54,55]. However, it agrees with the reports that B. afzelii and B. garinii are the dominant genospecies in Europe [41,56]. It is noteworthy that this study, aimed at discerning the disparities in reactivity among distinct antigenic variants, has predominantly concentrated on three genospecies: B. afzelii, B. burgdorferi s.l., and B. garinii. Nevertheless, it is imperative to bear in mind the presence of B. spielmanii and B. bavarsiensis within Europe, potentially contributing to a broader spectrum of genospecies diversity [57].
It seems that the main parameter that reduces the diagnostic usefulness of BB0108 and BB0323 in the ELISA is their relatively low specificity compared to the previously characterized recombinant proteins. According to the literature, the specificity of the ELISA based on DbpA, VlsE, and OspC is very high and in many studies, it reached 100%, even when the immunoassays were performed on sera collected from individuals infected with pathogens that are the source of cross-reactions with B. burgdorferi s.l. [20,30,52,53,58,59,60,61]. This phenomenon may be due to the location of the genes encoding these proteins. DbpA, VlsE, and OspC are encoded by low-conserved plasmids and are mainly responsible for the pathogenesis and virulence of B. burgdorferi s.l. and are not directly necessary for the proper functioning of the bacterial cell. The bb0108 and bb0323 genes are located on the chromosome, where there are mainly genes that control the basic life functions of the cell, so there is a possibility that homologs of these proteins may be found in other organisms [15,19]. This means that the encoding of the protein by the chromosome ensures its high conservation and reactivity with antibodies directed against different genospecies of B. burgdorferi s.l.; however, on the other hand, it may be the cause of the increased cross-reactivity of these proteins [22].
It was noted that negative sera gave a false-positive overlap in this study, both for the antigens tested and antibody isotypes. The specificity of the IgG-ELISA-BB0108 for all antigen variants was 82%, in all cases; the same sera were responsible for the false-positive results. The same applies to the IgG-ELISA-BB0323. The same 10 sera were the source of cross-reactions in all BB0323 variants. In the IgM-ELISA-BB0108, the cross-reacting sera were also noted to have a partial overlap. However, it was not as clear as for the IgG-ELISA. It seems that such a correlation was not accidental. Unfortunately, the sera used in this study were not tested for antibodies against other pathogens. However, it seems worth checking whether these negative sera that gave false-positive results contain antibodies against pathogens that are the confirmed source of cross-reactions with B. burgdorferi s.l., i.e., Treponema pallidum, Relapsing fever Borrelia, Cytomegalo virus, Parvovirus B19, Epstein-Bar virus, Anaplasma phagocytophilum, and Yersinia [20,26,27,52,62,63,64,65,66]. It would be worthwhile to additionally examine sera for the presence of rheumatoid factors, the most common autoantibodies found in humans, as they may also recognize B. burgdorferi s.l. antigens [34].
In this study, the diagnostic utility of five newly chromosomally encoded recombinant proteins (BB0108, BB0126, BB0298, BB0323, BB0689) originating from three genospecies of B. burgdorferi s.l. (B. afzelii, B. burgdorferi s.s., B. garinii) was evaluated for the first time. Results obtained for two of these proteins, BB0108 and BB0323, suggest that conserved chromosomally encoded proteins might offer a promising alternative in the quest for new antigens for use in developing novel diagnostic tests. This is particularly significant in the context of creating immunoenzymatic tests that exhibit consistent utility regardless of the Borrelia burgdorferi genospecies causing the infection. Conducting fundamental research on chromosomally encoded proteins, followed by applied studies, constitutes a substantial contribution to a better understanding of the B. burgdorferi s.l. proteome, raising hopes for the development of new diagnostic tools. One avenue worth exploring is the utilization of selected fragments of these proteins in constructing chimeric proteins with diagnostic capabilities. For this purpose, experimental epitope mapping may hold significant value, enabling a precise understanding of antigen–antibody interactions and selection of the most applicable amino acids fragments [67]. By combining the robust immunogenicity of plasmid-encoded proteins with the conserved sequences of chromosomally encoded proteins, maybe there is potential to create immunoenzymatic tests of superior quality.
5. Conclusions
Highly conserved antigens encoded by the chromosome have been marginalized by scientists during the improvement of serodiagnosis of Lyme disease due to their lower immunogenicity when compared to antigens encoded by genes on plasmids. Despite their heightened immunogenicity, assays based on plasmid-encoded proteins have proven inadequate for diagnosing Lyme disease caused by various genospecies, as evidenced by numerous experiments. This limitation poses a significant challenge in Europe. This study demonstrates that conserved, chromosomally encoded antigens can also be recognized by anti-B. burgdorferi s.l. antibodies. Additionally, they exhibit reduced diversity in reactivity depending on the genospecies. Therefore, it seems justified to explore immunogenic proteins conserved within the B. burgdorferi s.l. group to develop universal tests that are easy and safe to produce and simpler to interpret. However, further effort is needed before ELISA assays, utilizing conserved chromosomally encoded recombinant proteins or chimeric/multivalent proteins as tools for epidemiological studies, become commercially available.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13090767/s1, Table S1. Sequences of the primers used to amplify the B. burgdorferi s.l. genes (sequence complementary to the gene is underlined, bold nucleotides have been inserted to shift the ORF), Figure S1: Scheme of pUET1-BB0108BA construction using the In-Fusion system, Figure S2: Scheme of pUET1-BB0126BA construction using the In-Fusion system, Figure S3: Scheme of pUET1-BB0298BA construction using the In-Fusion system, Figure S4: Scheme of pUET1-BB0323BA construction using the In-Fusion system, Figure S5: Scheme of pUET1-BB0689BA construction using the In-Fusion system, Figure S6: Amino acid sequence of the BB0108BA monovalent recombinant protein, Figure S7: Amino acid sequence of the BB0108BB monovalent recombinant protein, Figure S8: Amino acid sequence of the BB0108BG monovalent recombinant protein, Figure S9: Amino acid sequence of the BB0126BA monovalent recombinant protein, Figure S10: Amino acid sequence of the BB0126BB monovalent recombinant protein, Figure S11: Amino acid sequence of the BB0126BG monovalent recombinant protein, Figure S12: Amino acid sequence of the BB0298BA monovalent recombinant protein, Figure S13: Amino acid sequence of the BB0298BB monovalent recombinant protein, Figure S14: Amino acid sequence of the BB0298BG monovalent recombinant protein, Figure S15: Amino acid sequence of the BB0232BA monovalent recombinant protein, Figure S16: Amino acid sequence of the BB0323BB monovalent recombinant protein, Figure S17: Amino acid sequence of the BB0323BG monovalent recombinant protein, Figure S18: Amino acid sequence of the BB0689BA monovalent recombinant protein, Figure S19: Amino acid sequence of the BB0689BB monovalent recombinant protein, Figure S20: Amino acid sequence of the BB0689BG monovalent recombinant protein, Figure S21: SDS-PAGE of B. burgdorferi s.l. recombinant proteins in polyacrylamide gel prepared with the use of modified combs, Figure S22: Production of recombinant proteins. SDS-PAGE of proteins contained in E. coli BL21(DE3)pLysS whole cell lysates, Figure S23: Results of purification of recombinant proteins.
Author Contributions
Conceptualization, W.G. and L.H.-G.; methodology, W.G. and L.H.-G.; formal analysis, L.H.-G.; investigation, W.G.; resources, L.H.-G., T.C. and B.F.; writing—original draft preparation, W.G.; writing—review and editing, L.H.-G., T.C. and B.F.; supervision, L.H.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Science Centre, Poland (https://www.ncn.gov.pl, accessed on 11 June 2024), under the research project no. 2023/49/B/NZ6/02881 granted to L.H.-G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data that support the findings are included in this published article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Trevisan, G.; Cinco, M.; Trevisini, S.; Di Meo, N.; Chersi, K.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 1: Borrelia Lyme group and echidna-reptile group. Biology 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Cirkovic, V.; Veinovic, G.; Stankovic, D.; Mihaljica, D.; Sukara, R.; Tomanovic, S. Evolutionary dynamics and geographical dispersal of Borrelia lusitaniae. Front. Microbiol. 2024, 15, 1330914. [Google Scholar] [CrossRef] [PubMed]
- Steinbrink, A.; Brugger, K.; Margos, G.; Kraiczy, P.; Klimpel, S. The evolving story of Borrelia burgdorferi sensu lato transmission in Europe. Parasitol. Res. 2022, 121, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.S.; Respicio-Kingry, L.B.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; Bjork, J.; Liu, G.; Kingry, L.C.; Mead, P.S.; Neitzel, D.F.; et al. Borrelia mayonii Sp. Nov., a Member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the Upper Midwestern United States. Int. J. Syst. Evol. Microbiol. 2016, 123, 4757–4763. [Google Scholar] [CrossRef]
- Wang, G.; van Dam, A.P.; Schwartz, I.; Dankert, J. Molecular typing of Borrelia burgdorferi sensu lato: Taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev. 1999, 12, 633–653. [Google Scholar] [CrossRef]
- Hofmann, H.; Fingerle, V.; Hunfeld, K.P.; Huppertz, H.I.; Krause, A.; Rauer, S.; Ruf, B. Cutaneous Lyme borreliosis: Guideline of the german dermatology society. Ger. Med. Sci. 2017, 15, 1–31. [Google Scholar] [CrossRef]
- Ružić-Sabljić, E.; Lotrič-Furlan, S.; Maraspin, V.; Cimperman, J.; Logar, M.; Jurca, T.; Strle, F. Comparison of isolation rate of Borrelia burgdorferi sensu lato in MKP and BSK-II Medium. Int. J. Med Microbiol. 2006, 296, 267–273. [Google Scholar] [CrossRef]
- Eldin, C.; Raffetin, A.; Bouiller, K.; Hansmann, Y.; Roblot, F.; Raoult, D.; Parola, P. Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med. Mal. Infect. 2019, 49, 121–132. [Google Scholar] [CrossRef]
- Unlu, A.M.; Andersen, N.S.; Larsen, S.L.; Skarphedinsson, S.; Chrysidis, S.; Knudtzen, F.C.; Lage-Hansen, P.R. Differentiating Lyme arthritis: A case-based review. Rheumatol. Int. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. JAMA 1995, 274, 937. [Google Scholar] [CrossRef]
- Brisson, D.; Drecktrah, D.; Eggers, C.H.; Samuels, D.S. Genetics of Borrelia burgdorferi. Annu. Rev. Genet. 2012, 46, 515–536. [Google Scholar] [CrossRef]
- Ohnishi, J.; Piesman, J.; de Silva, A.M. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 2001, 98, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.R.; Gilcrease, E.B.; Vujadinovic, M.; Mongodin, E.F.; Luft, B.J.; Schutzer, S.E.; Fraser, C.M.; Qiu, W.G. Plasmid diversity and phylogenetic consistency in the Lyme disease agent Borrelia burgdorferi. BMC Genom. 2017, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Ojaimi, C.; Brooks, C.; Casjens, S.; Rosa, P.; Elias, A.; Barbour, A.; Jasinskas, A.; Benach, J.; Katona, L.; Radolf, J.; et al. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 2003, 71, 1689–1705. [Google Scholar] [CrossRef]
- Schwartz, I.; Margos, G.; Casjens, S.R.; Qiu, W.G.; Eggers, C.H. Multipartite genome of Lyme disease Borrelia: Structure, variation and prophages. Curr. Issues Mol. Biol. 2021, 42, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Roessler, D.; Hauser, U.; Wilske, B. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J. Clin. Microbiol. 1997, 35, 2752–2758. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, T.; Seppälä, I.; Saxen, H.; Panelius, J.; Yrjänäinen, H. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J. Clin. Microbiol. 2002, 40, 453–460. [Google Scholar] [CrossRef]
- Goettner, G.; Schulte-Spechtel, U.; Hillermann, R.; Liegl, G.; Wilske, B.; Fingerle, V. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J. Clin. Microbiol. 2005, 43, 3602–3609. [Google Scholar] [CrossRef]
- Fraser, C.M.; Casjens, S.; Huang, W.M.; Sutton, G.G.; Clayton, R.; Lathigra, R.; White, O.; Ketchum, K.A.; Dodson, R.; Hickey, E.K.; et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 1997, 390, 580–586. [Google Scholar] [CrossRef]
- Magnarelli, L.A.; Lawrenz, M.; Fikrig, E.; Norris, S.J. Comparative reactivity of human sera to recombinant VlsE and other Borrelia burgdorferi antigens in class-specific enzyme-linked immunosorbent assays for Lyme borreliosis. J. Med. Microbiol. 2002, 51, 649–655. [Google Scholar] [CrossRef]
- Barbour, A.G.; Jasinskas, A.; Kayala, M.A.; Davies, D.H.; Steere, A.C.; Baldi, P.; Felgner, P.L. A Genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect. Immun. 2008, 76, 3374–3389. [Google Scholar] [CrossRef] [PubMed]
- Grąźlewska, W.; Sołowińska, K.; Holec-Gąsior, L. In silico epitope prediction of Borrelia burgdorferi sensu lato antigens for the detection of specific antibodies. J. Immunol. Methods 2024, 524, 113596. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, S.; Kur, J. Cloning, overexpression, and purification of the recombinant His-Tagged SSB protein of Escherichia coli and use in polymerase chain reaction amplification. Protein Expr. Purif. 1999, 16, 96–102. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef]
- Lohr, B.; Fingerle, V.; Norris, D.E.; Hunfeld, K.-P. Laboratory diagnosis of Lyme borreliosis: Current state of the art and future perspectives. Crit. Rev. Clin. Lab. Sci. 2018, 55, 219–245. [Google Scholar] [CrossRef] [PubMed]
- Golkocheva-Markova, E.; Nenova, R.; Stoilov, R.; Christova, I.; Najdenski, H. Cross-reactivity between Yersinia outer membrane proteins and anti-Francisella and anti-Borrelia antibodies in serodiagnosis of Yersinia-triggered reactive arthritis. Comptes Rendus L’Acad. Bulg. Sci. 2011, 64, 61–66. [Google Scholar]
- Wojciechowska-Koszko, I.; Kwiatkowski, P.; Sienkiewicz, M.; Kowalczyk, M.; Kowalczyk, E.; Dołęgowska, B. Cross-reactive results in serological tests for borreliosis in patients with active viral infections. Pathogens 2022, 11, 203. [Google Scholar] [CrossRef]
- Bruckbauer, H.R.; Preac-Mursic, V.; Fuchs, R.; Wilske, B. Cross-reactive proteins of Borrelia burgdorferi. Eur. J. Clin. Microbiol. 1992, 11, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Smit, P.W.; Kurkela, S.; Kuusi, M.; Vapalahti, O. Evaluation of two commercially available rapid diagnostic tests for Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 34, 109–113. [Google Scholar] [CrossRef]
- Heikkilä, T.; Huppertz, H.I.; Seppälä, I.; Sillanpää, H.; Saxen, H.; Lahdenne, P. Recombinant or peptide antigens in the serology of Lyme arthritis in children. J. Infect. Dis. 2003, 187, 1888–1894. [Google Scholar] [CrossRef]
- Panelius, J.; Lahdenne, P.; Saxén, H.; Carlsson, S.A.; Heikkilä, T.; Peltomaa, M.; Lauhio, A.; Seppälä, I. Diagnosis of Lyme neuroborreliosis with antibodies to recombinant proteins DbpA, BBK32, and OspC, and VIsE IR6peptide. J. Neurol. 2003, 250, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, T.; Seppälä, I.; Saxén, H.; Panelius, J.; Peltomaa, M.; Julin, T.; Carlsson, S.A.; Lahdenne, P. Recombinant BBK32 protein in serodiagnosis of early and late Lyme borreliosis. J. Clin. Microbiol. 2002, 40, 1174–1180. [Google Scholar] [CrossRef]
- Glatz, M.; Fingerle, V.; Wilske, B.; Ambros-Rudolph, C.; Kerl, H.; Müllegger, R.R. Immunoblot analysis of the seroreactivity to recombinant Borrelia burgdorferi sensu lato antigens, including VlsE, in the long-term course of treated patients with erythema migrans. Dermatology 2008, 216, 93–103. [Google Scholar] [CrossRef]
- Hsieh, Y.F.; Liu, H.W.; Hsu, T.C.; Wei, J.C.C.; Shih, C.M.; Krause, P.J.; Tsay, G.J. Serum reactivity against Borrelia burgdorferi OspA in patients with rheumatoid arthritis. Clin. Vaccine Immunol. 2007, 14, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Magnarelli, L.; Ijdo, J.W.; Padula, S.J.; Flavell, R.; Fikrig, E. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 2000, 38, 1735–1739. [Google Scholar] [CrossRef]
- Cornero, R.; Irfan, S.S.; Cachaco, S.; Zhou, W.; Byne, A.; Howard, M.; McIntyre, H.; Birkaya, B.; Liotta, L.; Luchini, A. Identification of unambiguous Borrelia peptides in human urine using affinity capture and mass spectrometry. Methods Mol. Biol. 2024, 2742, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, X.; Kumar, M.; Pal, U. BB0323 function is essential for Borrelia burgdorferi virulence and persistence through tick-rodent transmission cycle. J. Infect. Dis. 2009, 200, 1318–1330. [Google Scholar] [CrossRef]
- Xu, Y.; Bruno, J.F.; Luft, B.J. Profiling the humoral immune response to Borrelia burgdorferi infection with protein microarrays. Microb. Pathog. 2008, 45, 403–407. [Google Scholar] [CrossRef]
- Brangulis, K.; Jaudzems, K.; Petrovskis, I.; Akopjana, I.; Kazaks, A.; Tars, K. Structural and functional analysis of BB0689 from Borrelia burgdorferi, a member of the bacterial CAP superfamily. J. Struct. Biol. 2015, 192, 320–330. [Google Scholar] [CrossRef]
- Brooks, C.S.; Vuppala, S.R.; Jett, A.M.; Akins, D.R. Identification of Borrelia burgdorferi outer surface proteins. Infect. Immun. 2006, 74, 296–304. [Google Scholar] [CrossRef]
- Strand, M.; Hönig, V.; Ružek, D.; Grubhoffer, L.; Regoa, R.O.M. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, K.; Dziekońska-Rynko, J.; Szymańska, H.; Kubiak, D.; Dmitryjuk, M.; Dzika, E. Questing Ixodes ricinus ticks (acari, ixodidae) as a vector of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in an urban area of North-Eastern Poland. Exp. Appl. Acarol. 2019, 78, 113–126. [Google Scholar] [CrossRef]
- Gałęziowska, E.; Rzymowska, J.; Najda, N.; Kołodziej, P.; Domżał-Drzewicka, R.; Rząca, M.; Muraczyńska, B.; Charzyńska-Gula, M.; Szadowska-Szlachetka, Z.; Ślusarska, B.; et al. Prevalence of Borrelia burgdorferi in ticks removed from skin of people and circumstances of being bitten—Research from the area of Poland, 2012–2014. Ann. Agric. Environ. Med. 2018, 25, 31–35. [Google Scholar] [CrossRef]
- Holec-Gąsior, L.; Ferra, B.; Drapała, D. MIC1-MAG1-SAG1 Chimeric protein, a most effective antigen for detection of human toxoplasmosis. J. Clin. Immunol. 2012, 19, 1977–1979. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drapała, D.; Holec-Gasior, L.; Kur, J. New Recombinant chimeric antigens, P35-MAG1, MIC1-ROP1, and MAG1-ROP1, for the serodiagnosis of human toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2015, 82, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Panelius, J.; Lahdenne, P.; Saxen, H.; Heikkila, T.; Seppala, I. Recombinant flagellin A proteins from Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 2001, 39, 4013–4019. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Xiong, E.; Hong, R.; Lu, Q.; Ohno, H.; Wang, J.Y. Role of the IgM Fc receptor in immunity and tolerance. Front. Immunol. 2019, 10, 529. [Google Scholar] [CrossRef]
- Hillerdal, H.; Henningsson, A.J. Serodiagnosis of Lyme Borreliosis—Is IgM in serum more harmful than helpful? Eur. J. Clin. Microbiol. 2021, 40, 1161–1168. [Google Scholar] [CrossRef]
- Mäkelä, O.; Rouslahti, E.; Seppälä, I.J.T. Affinity of IgM and IgG antibodies. Immunochemistry 1970, 7, 917–932. [Google Scholar] [CrossRef]
- Keyt, B.A.; Baliga, R.; Sinclair, A.M.; Carroll, S.F.; Peterson, M.S. Structure, function, and therapeutic use of IgM antibodies. Antibodies 2020, 2020, 53. [Google Scholar] [CrossRef]
- Johnson, B.J.B.; Robbins, K.E.; Bailey, R.E.; Cao, B.L.; Sviat, S.L.; Craven, R.B.; Mayer, L.W.; Dennis, D.T. Serodiagnosis of Lyme disease: Accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J. Infect. Dis. 1996, 174, 346–353. [Google Scholar] [CrossRef]
- Panelius, J.; Lahdenne, P.; Heikkila, T.; Peltomaa, M.; Oksi, J.; Seppala, I. Recombinant OspC from Borrelia burgdorferi sensu stricto B. afzelii and B. garinii in the serodiagnosis of Lyme borreliosis. J. Med. Microbiol. 2002, 51, 731–739. [Google Scholar] [CrossRef]
- Schulte-Spechtel, U.; Fingerle, V.; Goettner, G.; Rogge, S.; Wilske, B. Molecular analysis of decorin-binding protein A (DbpA) reveals five major groups among European Borrelia burgdorferi sensu lato strains with impact for the development of serological assays and indicates lateral gene transfer of the DbpA gene. Int. J. Med. Microbiol. 2006, 296, 250–266. [Google Scholar] [CrossRef]
- Cisak, E.; Wójcik-Fatla, A.; Stojek, N.M.; Chmielewska-Badora, J.; Zwoliński, J.; Buczek, A.; Dutkiewicz, J. Prevalence of Borrelia burgdorferi genospecies in Ixodes ricinus ticks from Lublin Region (Eastern Poland). Ann. Agric. Environ. Med. 2006, 13, 301–306. [Google Scholar]
- Strzelczyk, J.K.; Gaździcka, J.; Cuber, P.; Asman, M.; Trapp, G.; Gołąbek, K.; Zalewska-Ziob, M.; Nowak-Chmura, M.; Siuda, K.; Wiczkowski, A.; et al. Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from Southern Poland. Acta Parasitol. 2015, 60, 666–674. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ortega, C.; Sánchez, N.; DeSimone, L.; Sudre, B.; Suk, J.E.; Semenza, J.C. Correlation of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks with specific abiotic traits in the Western Palearctic. Appl. Environ. Microbiol. 2011, 77, 3838–3845. [Google Scholar] [CrossRef]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex-clinical significance of genomic species? Clin. Infect. Dis. 2011, 17, 487–493. [Google Scholar] [CrossRef]
- Gerber, M.A.; Shapiro, E.D.; Bell, G.L.; Sampieri, A.; Padula, S.J. Recombinant outer surface protein-C ELISA for the diagnosis of early Lyme-disease. J. Infect. Dis. 1995, 171, 724–727. [Google Scholar] [CrossRef]
- Fung, B.P.; McHugh, G.L.; Leong, J.M.; Steere, A.C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: Role of the immunoglobulin M response in the serodiagnosis of early infection. Infect. Immun. 1994, 62, 3213–3221. [Google Scholar] [CrossRef]
- Padula, S.J.; Dias, F.; Sampieri, A.; Craven, R.B.; Ryan, R.W. Use of Recombinant Ospc from Borrelia-burgdorferi for serodiagnosis of early Lyme-disease. J. Clin. Microbiol. 1994, 32, 1733–1738. [Google Scholar] [CrossRef]
- Liang, F.T.; Steere, A.C.; Marques, A.R.; Johnson, B.J.; Miller, J.N.; Philipp, M.T. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 1999, 37, 3990–3996. [Google Scholar] [CrossRef] [PubMed]
- Talagrand-Reboul, E.; Raffetin, A.; Zachary, P.; Jaulhac, B.; Eldin, C. Immunoserological diagnosis of human borreliosis: Current knowledge and perspectives. Front. Cell. Infect. Microbiol. 2020, 10, 241. [Google Scholar] [CrossRef]
- Rawlins, M.L.; Gerstner, C.; Hill, H.R.; Litwin, C.M. Evaluation of a Western blot method for the detection of Yersinia antibodies: Evidence of serological cross-reactivity between Yersinia outer membrane proteins and Borrelia burgdorferi. Clin. Diagn. Lab. Immunol. 2005, 12, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Nohlmans, M.K.; van den Bogaard, E. Epstein-Barr Virus and Cytomegalovirus Infections Cause False-Positive Results in IgM Two-Test Protocol for Early Lyme Borreliosis. Infection 1999, 27, 231. [Google Scholar] [CrossRef]
- Smismans, A.; Goossens, V.J.; Nulens, E.; Bruggeman, C.A. Comparison of five different immunoassays for the detection of Borrelia burgdorferi IgM and IgG antibodies. Clin. Infect. Dis. 2006, 12, 648–655. [Google Scholar] [CrossRef]
- Tuuminen, T.; Hedman, K.; Söderlund-Venermo, M.; Seppälä, I. Acute parvovirus B19 infection causes nonspecificity frequently in Borrelia and less often in Salmonella and Campylobacter serology, posing a problem in diagnosis of infectious arthropathy. Clin. Vaccine Immunol. 2011, 18, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Grąźlewska, W.; Holec-Gąsior, L.; Sołowińska, K.; Chmielewski, T.; Fiecek, B.; Contreras, M. Epitope Mapping of BmpA and BBK32 Borrelia burgdorferi sensu stricto antigens for the design of chimeric proteins with potential diagnostic value. ACS Infect. Dis. 2023, 9, 2160–2172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).