Global Research Trends in the Links between Periodontal Disease and Cancer: A Bibliometric Analysis
Abstract
1. Introduction
2. Materials and Methods
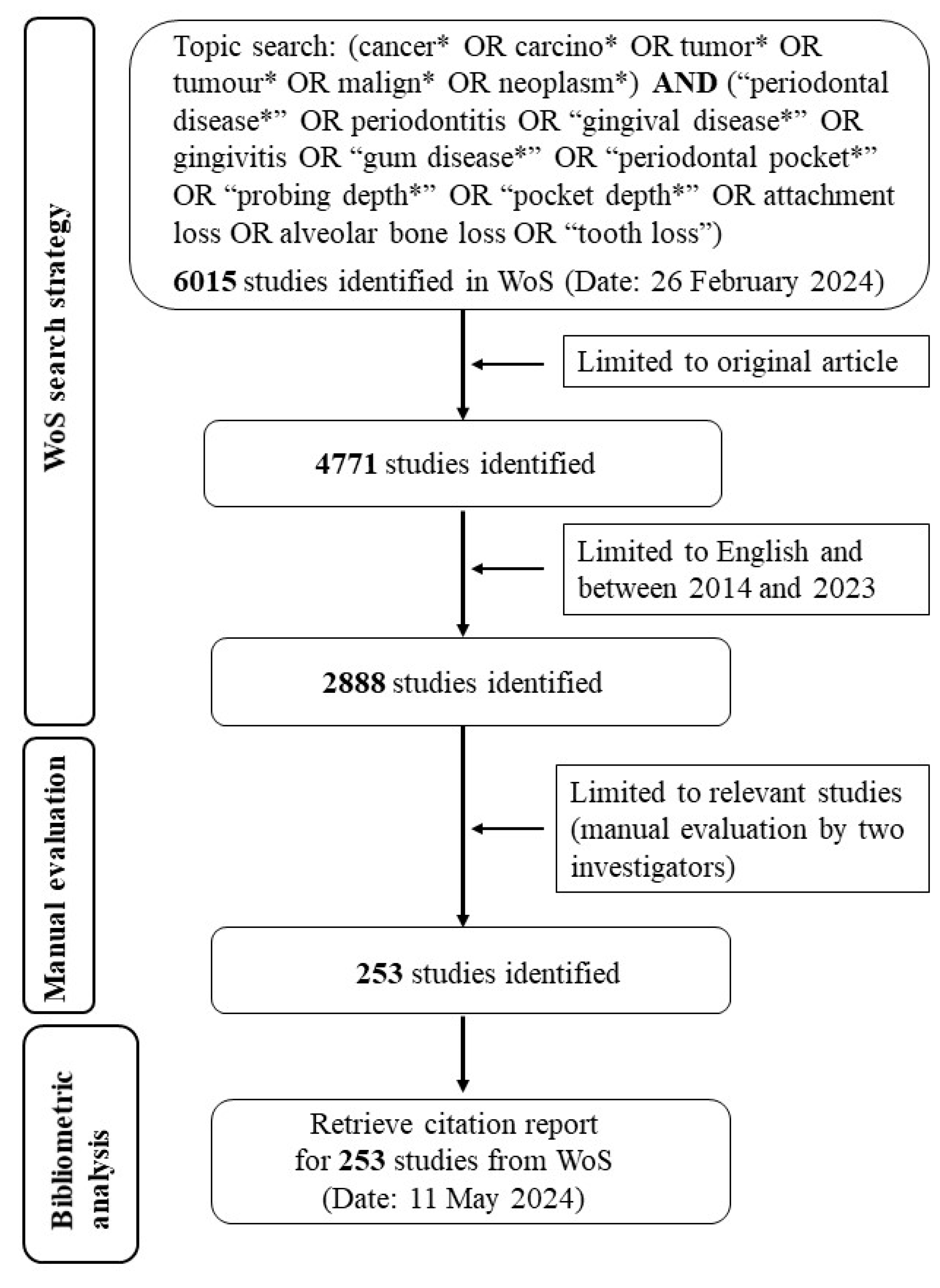
2.1. Data Sources and Search Strategy
2.2. Data Collection and Processing
2.3. Bibliometric Analysis and Visualization
3. Results
3.1. Publications and Their Categories and Study Design
3.2. Publications and Their Citations
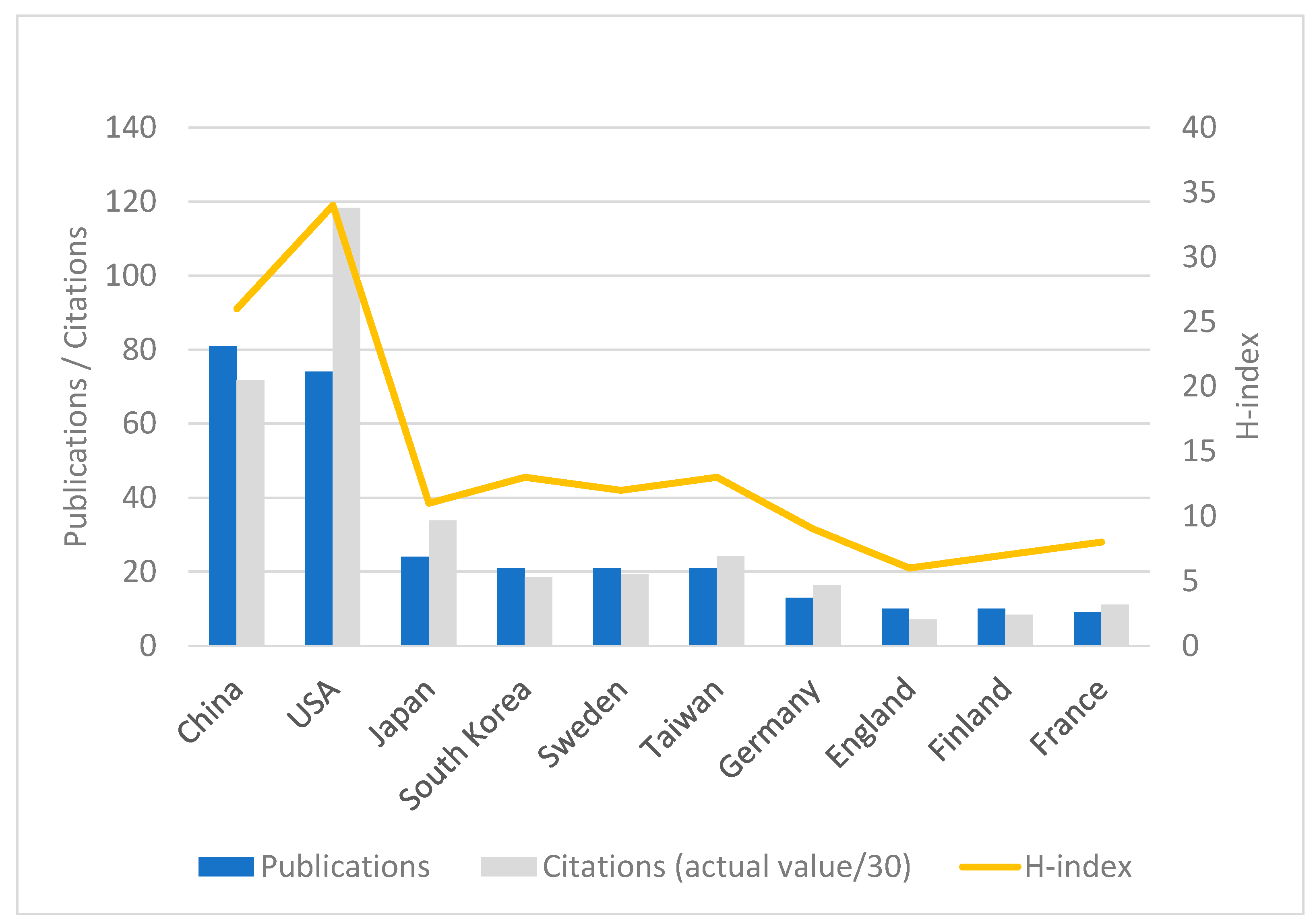
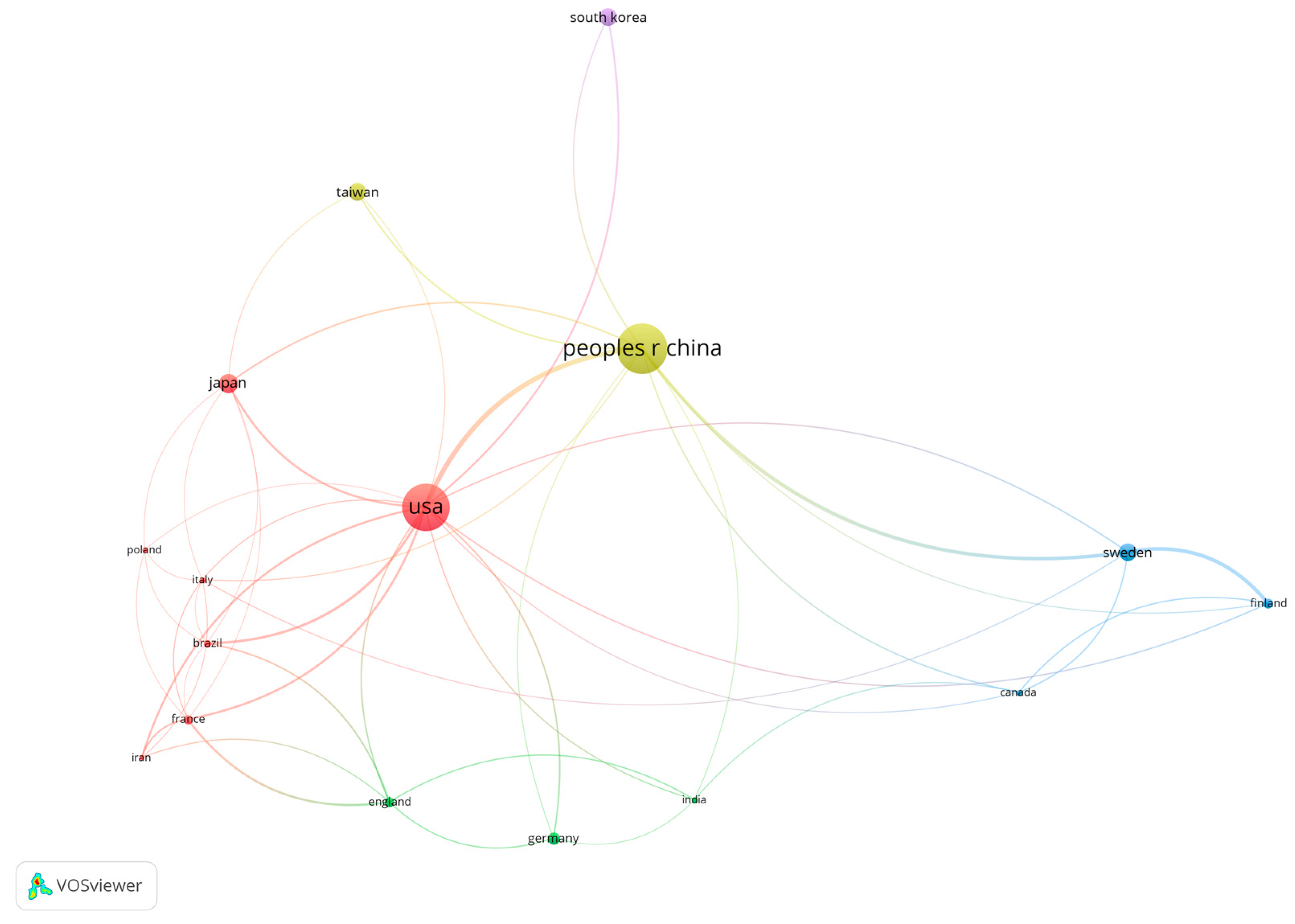
3.3. Publications and Citations by Authors, Affiliations, and Countries/Regions
3.4. Publications and Citations by Journals
3.5. Top Ten Most Cited Articles
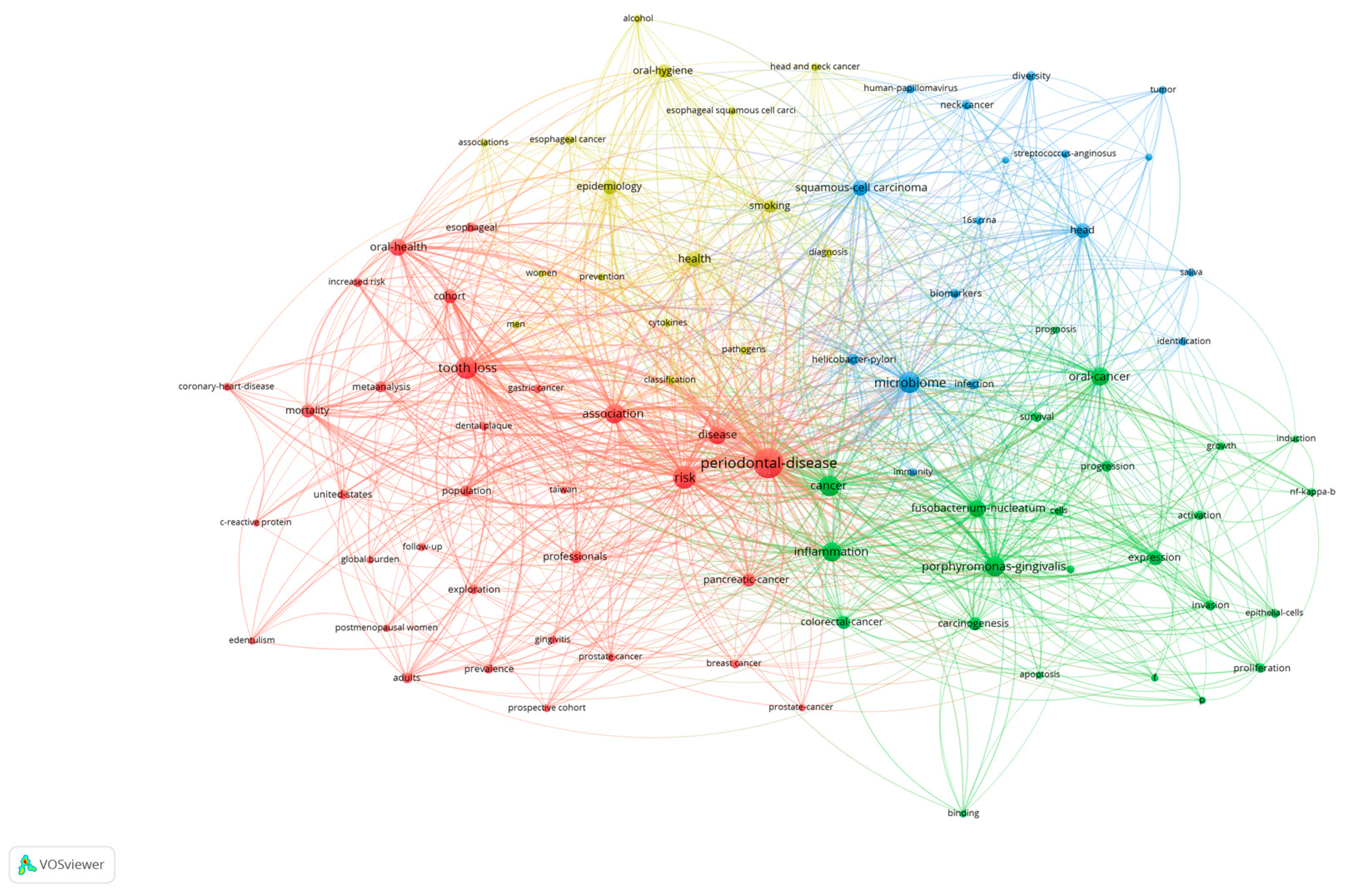
3.6. Keyword Analysis of Research in Periodontal Disease and Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Ibraheem, W.I.; Al-Ak’hali, M.S.; Shamala, A.; Halboub, E.; Alhajj, M.N. Association of periodontitis and tooth loss with liver cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2021, 159, 103221. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Ribaldone, D.G.; Romano, F.; Aimetti, M.; Romandini, M. The gum-gut axis: Periodontitis and the risk of gastrointestinal cancers. Cancers 2023, 15, 4594. [Google Scholar] [CrossRef]
- Gasparoni, L.M.; Alves, F.A.; Holzhausen, M.; Pannuti, C.M.; Serpa, M.S. Periodontitis as a risk factor for head and neck cancer. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e430–e436. [Google Scholar] [CrossRef]
- Gopinath, D.; Kunnath Menon, R.; K Veettil, S.; George Botelho, M.; Johnson, N.W. Periodontal diseases as putative risk factors for head and neck cancer: Systematic review and meta-analysis. Cancers 2020, 12, 1893. [Google Scholar] [CrossRef]
- Guo, Z.; Gu, C.; Li, S.; Gan, S.; Li, Y.; Xiang, S.; Gong, L.; Wang, S. Periodontal disease and the risk of prostate cancer: A meta-analysis of cohort studies. Int. Braz. J. Urol. 2021, 47, 1120–1130. [Google Scholar] [CrossRef]
- Heikkilä, P.; But, A.; Sorsa, T.; Haukka, J. Periodontitis and cancer mortality: Register-based cohort study of 68,273 adults in 10-year follow-up. Int. J. Cancer 2018, 142, 2244–2253. [Google Scholar] [CrossRef]
- Kesharani, P.; Kansara, P.; Kansara, T.; Kini, A.; Bhat, R.; Shetty, P.; Penugonda, B. Is periodontitis a risk factor for lung cancer? A meta-analysis and detailed review of mechanisms of association. Contemp. Clin. Dent. 2022, 13, 297–306. [Google Scholar] [CrossRef]
- Li, T.-J.; Hao, Y.-H.; Tang, Y.-L.; Liang, X.-H. Periodontal pathogens: A crucial link between periodontal diseases and oral cancer. Front. Microbiol. 2022, 13, 919633. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Lu, J.Y.; Peacock-Villada, A.Y.; Barber, J.R.; Joshu, C.E.; Prizment, A.E.; Beck, J.D.; Offenbacher, S.; Platz, E.A. Periodontal disease assessed using clinical dental measurements and cancer risk in the ARIC study. J. Natl. Cancer Inst. 2018, 110, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Nwizu, N.N.; Marshall, J.R.; Moysich, K.; Genco, R.J.; Hovey, K.M.; Mai, X.D.; LaMonte, M.J.; Freudenheim, J.L.; Wactawski-Wende, J. Periodontal disease and incident cancer risk among postmenopausal women: Results from the Women’s Health Initiative Observational Cohort. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontology 2000 2020, 83, 213–233. [Google Scholar] [CrossRef]
- Shin, Y.J.; Choung, H.W.; Lee, J.H.; Rhyu, I.C.; Kim, H.D. Association of periodontitis with oral cancer: A case-control study. J. Dent. Res. 2019, 98, 526–533. [Google Scholar] [CrossRef]
- Verma, U.P.; Singh, P.; Verma, A.K. Correlation between chronic periodontitis and lung cancer: A systematic review with meta-analysis. Cureus 2023, 15, e36476. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, X.; Hu, T.; Cheng, R.; Bhowmick, N.A. The relationship between periodontal disease and breast cancer: From basic mechanism to clinical management and prevention. Oral Health Prev. Dent. 2023, 21, 49–60. [Google Scholar] [CrossRef]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease-Is there cause for consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef]
- Elebyary, O.; Barbour, A.; Fine, N.; Tenenbaum, H.C.; Glogauer, M. The crossroads of periodontitis and oral squamous cell carcinoma: Immune implications and tumor promoting capacities. Front. Oral Health 2020, 1, 584705. [Google Scholar] [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal disease: The good, the bad, and the unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Sobocki, B.K.; Basset, C.A.; Bruhn-Olszewska, B.; Olszewski, P.; Szot, O.; Kazmierczak-Siedlecka, K.; Guziak, M.; Nibali, L.; Leone, A. Molecular mechanisms leading from periodontal disease to cancer. Int. J. Mol. Sci. 2022, 23, 970. [Google Scholar] [CrossRef] [PubMed]
- Surlin, P.; Nicolae, F.M.; Surlin, V.M.; Patrascu, S.; Ungureanu, B.S.; Didilescu, A.C.; Gheonea, D.I. Could periodontal disease through periopathogen Fusobacterium nucleatum be an aggravating factor for gastric cancer? J. Clin. Med. 2020, 9, 885. [Google Scholar] [CrossRef] [PubMed]
- Manoj Kumar, L.; George, R.J.; Anisha, P.S. Bibliometric analysis for medical research. Indian J. Psychol. Med. 2023, 45, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Chadegani, A.A.; Hadi, S.; Melor Md, Y.; Hadi, F.; Masood, F.; Maryam, F.; Nader, A. A comparison between two main academic literature collections: Web of Science and Scopus databases. Asian Soc. Sci. 2013, 9, 18–26. [Google Scholar] [CrossRef]
- Hirsch, J.E. An index to quantify an individual’s scientific research output. Proc. Natl. Acad. Sci. USA 2005, 102, 16569–16572. [Google Scholar] [CrossRef]
- Fan, X.Z.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, Human Papilloma Virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef]
- Yang, C.Y.; Yeh, Y.M.; Yu, H.Y.; Chin, C.Y.; Hsu, C.W.; Liu, H.; Huang, P.J.; Hu, S.N.; Liao, C.T.; Chang, K.P.; et al. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M.; Queiroz, E.L.S.; Nightingale, K.; Kerr, A.R.; DeLacure, M.D.; Veeramachaneni, R.; et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS ONE 2014, 9, e98741. [Google Scholar] [CrossRef]
- Zhao, H.S.; Chu, M.; Huang, Z.W.; Yang, X.; Ran, S.J.; Hu, B.; Zhang, C.P.; Liang, J.P. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017, 7, 11773. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The oral microbiota may have influence on oral cancer. Front. Cell. Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef]
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.; et al. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin. Cancer Res. 2016, 22, 5574–5581. [Google Scholar] [CrossRef] [PubMed]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015, 6, 7209–7220. [Google Scholar] [CrossRef] [PubMed]
- Gallimidi, A.B.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, K.A.; Shingala, J.; Evens, A.; Birmann, B.M.; Giovannucci, E.; Michaud, D.S. Periodontal disease and risk of non-Hodgkin lymphoma in the Health Professionals Follow-up Study. Int. J. Cancer 2017, 140, 1020–1026. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Babic, A.; Tworoger, S.S.; Zhang, L.B.; Wu, K.N.; Smith-Warner, S.A.; Ogino, S.; Chan, A.T.; Meyerhardt, J.; Giovannucci, E.; et al. Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. Int. J. Cancer 2017, 140, 646–652. [Google Scholar] [CrossRef]
- Gerlovin, H.; Michaud, D.S.; Cozier, Y.C.; Palmer, J.R. Oral health in relation to pancreatic cancer risk in African American women. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 675–679. [Google Scholar] [CrossRef]
- Michaud, D.S.; Kelsey, K.T.; Papathanasiou, E.; Genco, C.A.; Giovannucci, E. Periodontal disease and risk of all cancers among male never smokers: An updated analysis of the Health Professionals Follow-up Study. Ann. Oncol. 2016, 27, 941–947. [Google Scholar] [CrossRef]
- Petrick, J.L.; Wilkinson, J.E.; Michaud, D.S.; Cai, Q.Y.; Gerlovin, H.; Signorello, L.B.; Wolpin, B.M.; Ruiz-Narváez, E.A.; Long, J.R.; Yang, Y.H.; et al. The oral microbiome in relation to pancreatic cancer risk in African Americans. Br. J. Cancer 2022, 126, 287–296. [Google Scholar] [CrossRef]
- Zhou, B.J.; Lu, J.Y.; Beck, J.D.; Moss, K.L.; Prizment, A.E.; Demmer, R.T.; Rodriguez, K.A.P.; Joshu, C.E.; Michaud, D.S.; Platz, E.A. Periodontal and other oral bacteria and risk of lung cancer in the atherosclerosis risk in communities (ARIC) study. Cancer Epidemiol. Biomarkers Prev. 2023, 32, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Debertin, J.; Teles, F.; Martin, L.M.; Lu, J.Y.; Koestler, D.C.; Kelsey, K.T.; Beck, J.D.; Platz, E.A.; Michaud, D.S. Antibodies to oral pathobionts and colon cancer risk in the CLUE I cohort study. Int. J. Cancer 2023, 153, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Petrick, J.L.; Abnet, C.C.; Graubard, B.I.; Murphy, G.; Weinstein, S.J.; Männistö, S.; Albanes, D.; McGlynn, K.A. Tooth loss and liver cancer incidence in a Finnish cohort. Cancer Causes Control 2017, 28, 899–904. [Google Scholar] [CrossRef]
- Thistle, J.E.; Yang, B.Y.; Petrick, J.L.; Fan, J.H.; Qiao, Y.L.; Abnet, C.C.; Taylor, P.R.; McGlynn, K.A. Association of tooth loss with liver cancer incidence and chronic liver disease mortality in a rural Chinese population. PLoS ONE 2018, 13, e0203926. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.K.; Wang, J.B.; Zhao, F.H.; Fan, J.H.; Qiao, Y.L.; Taylor, P.R.; Abnet, C.C. Combined risk factors and risk of upper gastrointestinal cancer mortality in the Linxian general population. Int. J. Cancer 2022, 151, 1462–1473. [Google Scholar] [CrossRef]
- Sheikh, M.; Poustchi, H.; Pourshams, A.; Etemadi, A.; Islami, F.; Khoshnia, M.; Gharavi, A.; Hashemian, M.; Roshandel, G.; Khademi, H.; et al. Individual and combined effects of environmental risk factors for esophageal cancer based on results from the Golestan Cohort Study. Gastroenterology 2019, 156, 1416–1427. [Google Scholar] [CrossRef]
- Yano, Y.; Abnet, C.C.; Poustchi, H.; Roshandel, G.; Pourshams, A.; Islami, F.; Khoshnia, M.; Amiriani, T.; Norouzi, A.; Kamangar, F.; et al. Oral health and risk of upper gastrointestinal cancers in a large prospective study from a high-risk region: Golestan cohort study. Cancer Prev. Res. 2021, 14, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Vogtmann, E.; Etemadi, A.; Kamangar, F.; Islami, F.; Roshandel, G.; Poustchi, H.; Pourshams, A.; Khoshnia, M.; Gharravi, A.; Brennan, P.J.; et al. Oral health and mortality in the Golestan Cohort Study. Int. J. Epidemiol. 2017, 46, 2028–2035. [Google Scholar] [CrossRef]
- Vogtmann, E.; Han, Y.L.; Caporaso, J.G.; Bokulich, N.; Mohamadkhani, A.; Moayyedkazemi, A.; Hua, X.; Kamangar, F.; Wan, Y.H.; Suman, S.; et al. Oral microbial community composition is associated with pancreatic cancer: A case-control study in Iran. Cancer Med. 2020, 9, 797–806. [Google Scholar] [CrossRef]
- Shao, D.T.; Vogtmann, E.; Liu, A.Q.; Qin, J.J.; Chen, W.; Abnet, C.C.; Wei, W.Q. Microbial characterization of esophageal squamous cell carcinoma and gastric cardia adenocarcinoma from a high-risk region of China. Cancer 2019, 125, 3993–4002. [Google Scholar] [CrossRef]
- Pignatelli, P.; Iezzi, L.; Pennese, M.; Raimondi, P.; Cichella, A.; Bondi, D.; Grande, R.; Cotellese, R.; Di Bartolomeo, N.; Innocenti, P.; et al. The Potential of Colonic Tumor Tissue Fusobacterium nucleatum to Predict Staging and Its Interplay with Oral Abundance in Colon Cancer Patients. Cancers 2021, 13, 1032. [Google Scholar] [CrossRef] [PubMed]

| Authors | Publications | Citations | Average Cites | H-Index | Affiliation |
|---|---|---|---|---|---|
| Michaud DS | 12 | 348 | 29.0 | 8 | Tufts Univ, USA |
| Abnet CC | 10 | 749 | 74.9 | 8 | National Cancer Institute, NIH, USA |
| Ye WM | 9 | 316 | 35.1 | 6 | Karolinska Institutet, Sweden |
| Park HR | 8 | 298 | 37.3 | 8 | Pusan National Univ, South Korea |
| Vogtmann E | 8 | 167 | 20.9 | 7 | National Cancer Institute, NIH, USA |
| Affiliations | Publications | Citations | Average Cites | H-Index |
|---|---|---|---|---|
| Karolinska Institutet, Sweden | 19 | 536 | 28.2 | 11 |
| National Institutes of Health, USA | 19 | 901 | 47.4 | 10 |
| Harvard University, USA | 16 | 890 | 55.6 | 10 |
| Tufts University, USA | 13 | 361 | 27.8 | 8 |
| University of Michigan, USA | 13 | 398 | 30.6 | 9 |
| Journal | Publications | Citations | Average Cites | IF | Publisher | Category |
|---|---|---|---|---|---|---|
| International Journal of Cancer | 12 | 414 | 34.5 | 5.7 | Wiley | Oncology |
| Frontiers in Cellular and Infection Microbiology | 11 | 526 | 47.8 | 4.6 | Frontiers Media SA | Immunology; Microbiology |
| Scientific Reports | 11 | 530 | 48.2 | 3.8 | Nature Portfolio | Multidisciplinary Sciences |
| BMC Oral Health | 9 | 81 | 9.0 | 2.6 | BMC | Dentistry, Oral Surgery & Medicine |
| Cancer Causes Control | 9 | 192 | 21.3 | 2.2 | Springer | Oncology; Public, Environmental & Occupational Health |
| Cancers | 9 | 158 | 17.6 | 4.5 | MDPI | Oncology |
| International Journal of Molecular Sciences | 9 | 71 | 7.9 | 4.9 | MDPI | Biochemistry & Molecular Biology; Chemistry, Multidisciplinary |
| PLoS ONE | 8 | 427 | 53.4 | 2.9 | Public Library Science | Multidisciplinary Sciences |
| Cancer Epidemiology Biomarkers Prevention | 7 | 194 | 27.7 | 3.7 | American Association for Cancer Research | Oncology; Public, Environmental & Occupational Health |
| Journal of Dental Research | 6 | 174 | 29.0 | 5.7 | Sage Publications Inc. | Dentistry, Oral Surgery & Medicine |
| Rank | Article Title | Citations | Corresponding Authors | Year of Publication | Country/ Region | Journal (IF) | Cancer Type | Bacteria | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study | 461 | Ahn J | 2018 | USA | Gut (IF = 23) | Pancreatic cancer | Oral microbiome (oral wash) | [27] |
| 2 | Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis | 271 | Baba H | 2016 | Japan | Clinical Cancer Research (IF = 10) | Esophageal cancer | F. nucleatum (cancer tissue) | [33] |
| 3 | Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression | 260 | Bachrach G | 2020 | Israel | Nature Communications (IF = 14.7) | Breast cancer | F. nucleatum (cancer tissue, animal study) | [34] |
| 4a | Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model | 246 | Elkin M | 2015 | Israel | Oncotarget (IF = 5.168) | Oral cancer | P.gingivalis, F. nucleatum (animal study) | [36] |
| 4b | Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis | 246 | Nosho K | 2015 | Japan | Oncotarget (IF = 5.168) | Pancreatic cancer | Fusobacterium (cancer tissue) | [35] |
| 6 | Changes in abundance of oral microbiota associated with oral cancer | 240 | Albertson DG | 2014 | USA | PLoS ONE (IF = 2.9) | Oral cancer | Oral microbiome (swabs) | [30] |
| 7 | Variations in oral microbiota associated with oral cancer | 211 | Hu B, Liang JP, Zhang CP | 2017 | China | Scientific Reports (IF = 3.8) | Oral cancer | Oral microbiome (swabs) | [31] |
| 8 | 16s rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papillomavirus infection and surgical treatment | 202 | Guerrero-Preston R, Sidransky D | 2016 | USA | Oncotarget (IF = 5.168) | Oral cancer | Oral microbiome (saliva) | [28] |
| 9 | Oral microbiota community dynamics associated with oral squamous cell carcinoma staging | 190 | Chang KP, Chang YL | 2018 | Taiwan | Frontiers in Microbiology (IF = 4) | Oral cancer | Oral microbiome (oral rinse) | [29] |
| 10 | The oral microbiota may have influence on oral cancer | 185 | Zhang CP, Zheng HJ | 2020 | China | Frontiers in Cellular and Infection Microbiology (IF = 4.6) | Oral cancer | Oral microbiome (swabs) | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.-W.; Yang, J.-J.; Lin, Y.-Y. Global Research Trends in the Links between Periodontal Disease and Cancer: A Bibliometric Analysis. Pathogens 2024, 13, 789. https://doi.org/10.3390/pathogens13090789
Hu S-W, Yang J-J, Lin Y-Y. Global Research Trends in the Links between Periodontal Disease and Cancer: A Bibliometric Analysis. Pathogens. 2024; 13(9):789. https://doi.org/10.3390/pathogens13090789
Chicago/Turabian StyleHu, Suh-Woan, Jaw-Ji Yang, and Yuh-Yih Lin. 2024. "Global Research Trends in the Links between Periodontal Disease and Cancer: A Bibliometric Analysis" Pathogens 13, no. 9: 789. https://doi.org/10.3390/pathogens13090789
APA StyleHu, S.-W., Yang, J.-J., & Lin, Y.-Y. (2024). Global Research Trends in the Links between Periodontal Disease and Cancer: A Bibliometric Analysis. Pathogens, 13(9), 789. https://doi.org/10.3390/pathogens13090789








