Role of Poly(A)-Binding Protein Cytoplasmic 1, a tRNA-Derived RNA Fragment-Bound Protein, in Respiratory Syncytial Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Virus Preparation
2.2. Preparation of Cytosolic Fraction
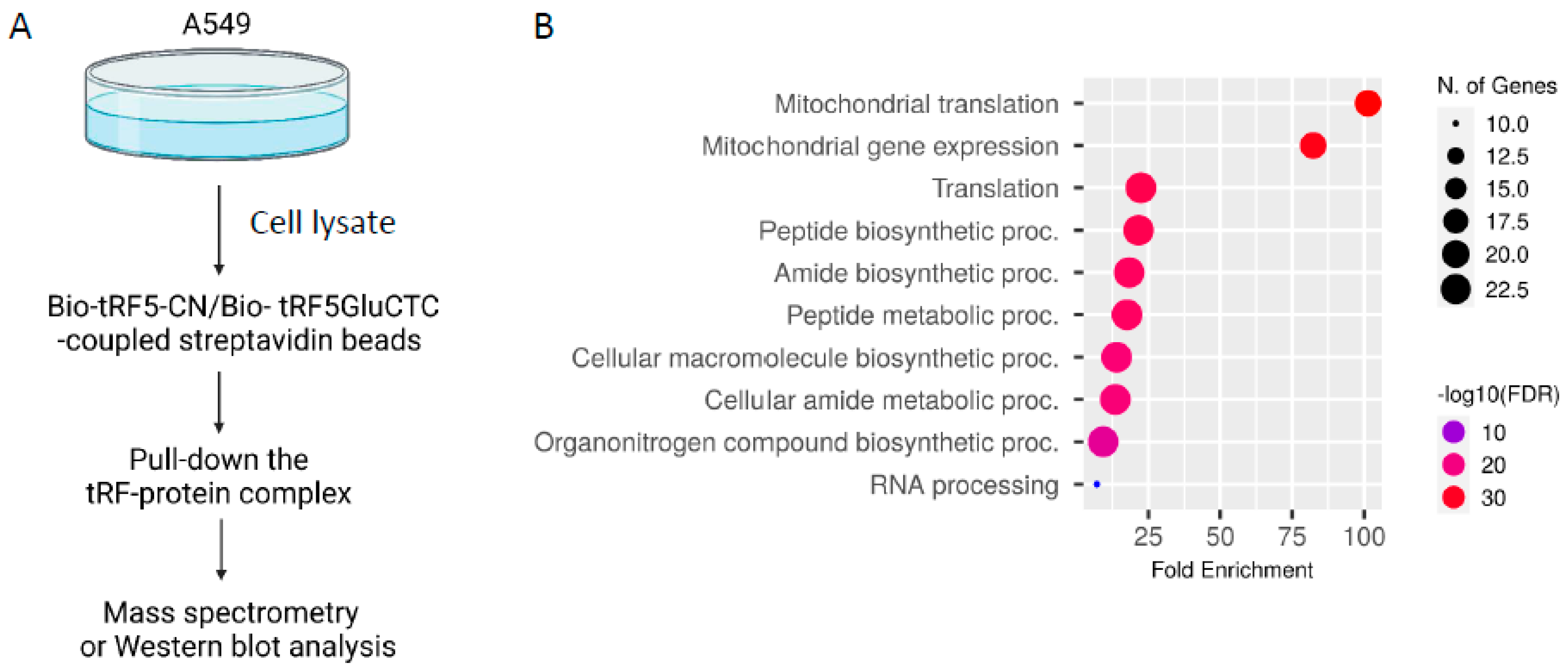
2.3. tRF-GluCTC-Bound Proteins Identification by Proteomics
2.4. Validation of PABPC1 in tRF5-GluCTC Complex
2.5. Immunoprecipitation
2.6. siRNA Transfection and Viral Infection
2.7. RNA Extraction and qRT-PCR
2.8. Virus Titration Assay
2.9. Statistical Analysis
3. Results
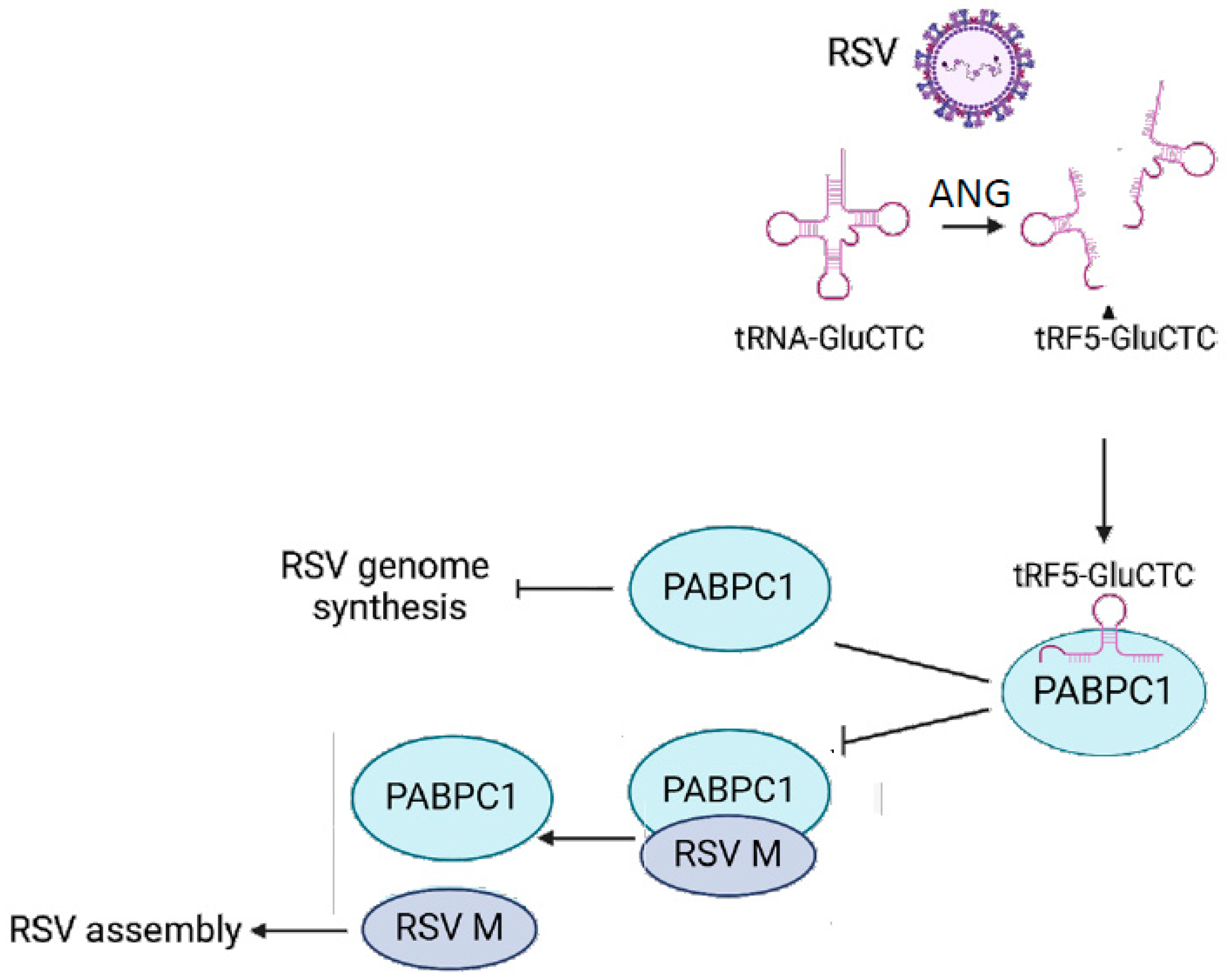
3.1. PABPC1 Interacts with tRF5-GluCTC
3.2. The Impact of PABPC1 on RSV Infection
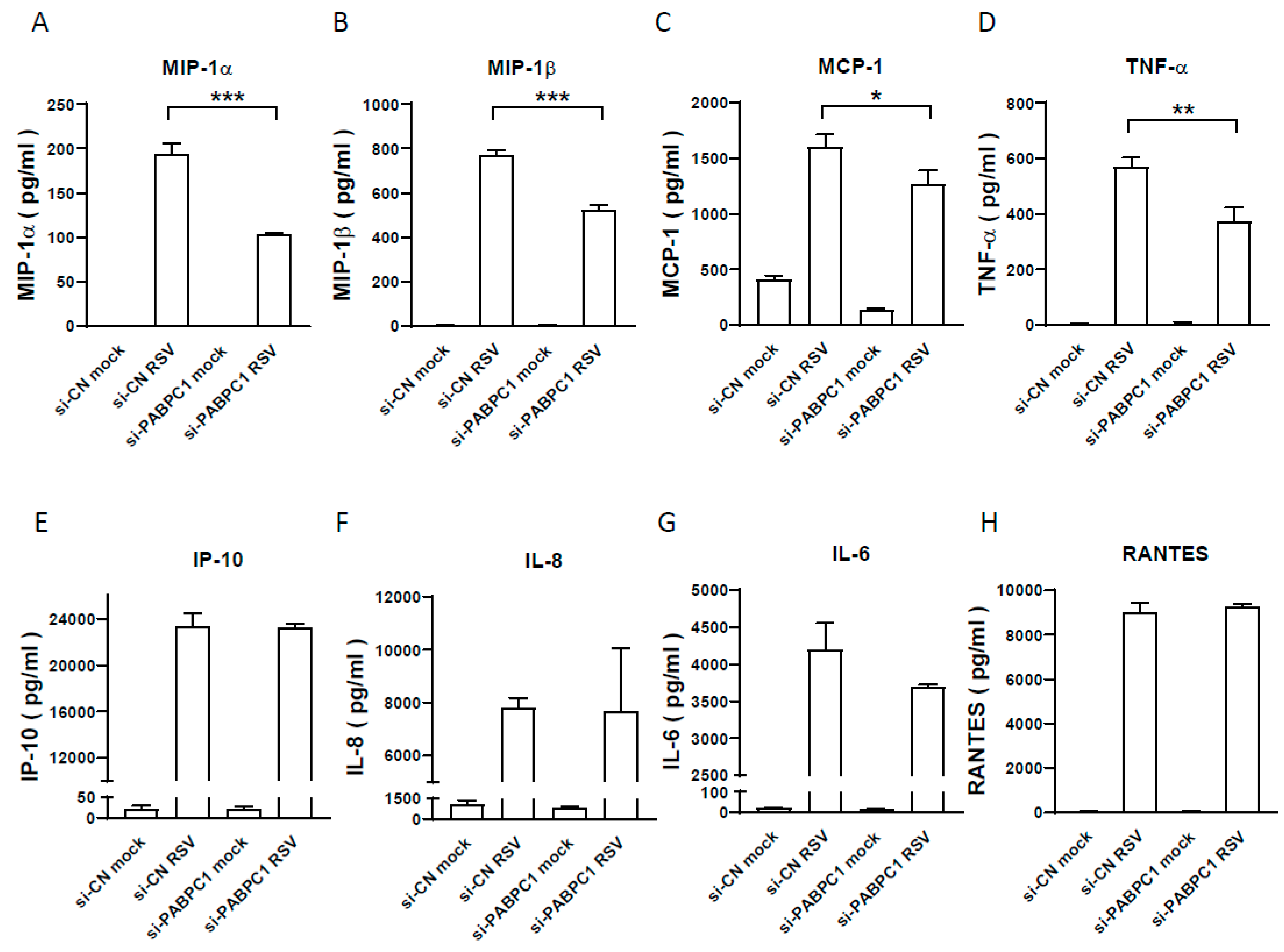
3.3. The Effect of PABPC1 on RSV-Induced Cytokine/Chemokine Induction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suh, M.; Movva, N.; Jiang, X.; Reichert, H.; Bylsma, L.C.; Fryzek, J.P.; Nelson, C.B. Respiratory Syncytial Virus Burden and Healthcare Utilization in United States Infants <1 Year of Age: Study of Nationally Representative Databases, 2011–2019. J. Infect. Dis. 2022, 226 (Suppl. S2), S184–S194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suh, M.; Movva, N.; Jiang, X.; Bylsma, L.C.; Reichert, H.; Fryzek, J.P.; Nelson, C.B. Respiratory Syncytial Virus Is the Leading Cause of United States Infant Hospitalizations, 2009–2019: A Study of the National (Nationwide) Inpatient Sample. J. Infect. Dis. 2022, 226 (Suppl. S2), S154–S163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suh, M.; Movva, N.; Bylsma, L.C.; Fryzek, J.P.; Nelson, C.B. A Systematic Literature Review of the Burden of Respiratory Syncytial Virus and Health Care Utilization Among United States Infants Younger Than 1 Year. J. Infect. Dis. 2022, 226 (Suppl. S2), S195–S212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osei-Yeboah, R.; Spreeuwenberg, P.; Del Riccio, M.; Fischer, T.K.; Egeskov-Cavling, A.M.; Boas, H.; van Boven, M.; Wang, X.; Lehtonen, T.; Bangert, M.; et al. Estimation of the Number of Respiratory Syncytial Virus-Associated Hospitalizations in Adults in the European Union. J. Infect. Dis. 2023, 228, 1539–1548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falsey, A.R.; Walsh, E.E. Respiratory syncytial virus infection in elderly adults. Drugs Aging 2005, 22, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Chatzis, O.; Darbre, S.; Pasquier, J.; Meylan, P.; Manuel, O.; Aubert, J.D.; Beck-Popovic, M.; Masouridi-Levrat, S.; Ansari, M.; Kaiser, L.; et al. Burden of severe RSV disease among immunocompromised children and adults: A 10 year retrospective study. BMC Infect. Dis. 2018, 18, 111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, J.M.; Fleming-Dutra, K.E.; Prill, M.M.; Roper, L.E.; Brooks, O.; Sanchez, P.J.; Kotton, C.N.; Mahon, B.E.; Meyer, S.; Long, S.S.; et al. Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices-United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 920–925. [Google Scholar] [CrossRef] [PubMed Central]
- Walsh, E.E.; Perez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef]
- Melgar, M.; Britton, A.; Roper, L.E.; Talbot, H.K.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices-United States, 2023. Am. J. Transpl. 2023, 23, 1631–1640. [Google Scholar] [CrossRef]
- Kampmann, B.; Radley, D.; Munjal, I. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. Reply. N. Engl. J. Med. 2023, 389, 1053–1055. [Google Scholar] [CrossRef]
- Harris, E. FDA Approves RSV Monoclonal Antibody for Infants and Young Children. JAMA 2023, 330, 586. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Morichika, K.; Kirino, Y. Selective amplification and sequencing of cyclic phosphate-containing RNAs by the cP-RNA-seq method. Nat. Protoc. 2016, 11, 476–489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pekarsky, Y.; Balatti, V.; Croce, C.M. tRNA-derived fragments (tRFs) in cancer. J. Cell Commun. Signal 2023, 17, 47–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karaiskos, S.; Grigoriev, A. Dynamics of tRNA fragments and their targets in aging mammalian brain. F1000Research 2016, 5, ISCB Comm J-2758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prehn, J.H.M.; Jirstrom, E. Angiogenin and tRNA fragments in Parkinson’s disease and neurodegeneration. Acta. Pharmacol. Sin. 2020, 41, 442–446. [Google Scholar] [CrossRef] [PubMed Central]
- Wu, W.; Shen, A.; Lee, I.; Miranda, E.G.; Spratt, H.; Pappolla, M.; Fang, X.; Bao, X. Changes of tRNA-derived Fragments by Alzheimer’s Disease in Cerebrospinal Fluid and Blood Serum. J. Alzheimer’s Dis. 2023, 93, 1285–1304. [Google Scholar] [CrossRef]
- Wang, Q.; Lee, I.; Ren, J.; Ajay, S.S.; Lee, Y.S.; Bao, X. Identification and functional characterization of tRNA-derived RNA fragments (tRFs) in respiratory syncytial virus infection. Mol. Ther. 2013, 21, 368–379. [Google Scholar] [CrossRef] [PubMed Central]
- Lee, Y.S.; Shibata, Y.; Malhotra, A.; Dutta, A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes. Dev. 2009, 23, 2639–2649. [Google Scholar] [CrossRef]
- Martens-Uzunova, E.S.; Jalava, S.E.; Dits, N.F.; van Leenders, G.J.; Moller, S.; Trapman, J.; Bangma, C.H.; Litman, T.; Visakorpi, T.; Jenster, G. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene 2012, 31, 978–991. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. GtRNAdb: A database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 2009, 37, D93–D97. [Google Scholar] [CrossRef]
- Deng, J.; Ptashkin, R.N.; Chen, Y.; Cheng, Z.; Liu, G.; Phan, T.; Deng, X.; Zhou, J.; Lee, I.; Lee, Y.S.; et al. Respiratory Syncytial Virus Utilizes a tRNA Fragment to Suppress Antiviral Responses Through a Novel Targeting Mechanism. Mol. Ther. 2015, 23, 1622–1629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeon, S.H.; Lee, K.; Lee, K.S.; Kunkeaw, N.; Johnson, B.H.; Holthauzen, L.M.; Gong, B.; Leelayuwat, C.; Lee, Y.S. Characterization of the direct physical interaction of nc886, a cellular non-coding RNA, and PKR. FEBS Lett. 2012, 586, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Qin, W.; Dong, S.; Yang, X.; Zhai, X.; Tong, W.; Liu, C.; Zheng, H.; Yu, H.; Kong, N.; et al. PEDV N protein capture protein translation element PABPC1 and eIF4F to promote viral replication. Vet. Microbiol. 2023, 284, 109844. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wei, X.; Zheng, S.; She, G.; Han, Z.; Xu, Z.; Cao, Y.; Xue, C. Poly(A)-Binding Protein Cytoplasmic 1 Inhibits Porcine Epidemic Diarrhea Virus Replication by Interacting with Nucleocapsid Protein. Viruses 2022, 14, 1196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, Q.; Liu, H.; Zhu, Z.; Xue, Z.; Liu, X.; Zheng, H. Seneca Valley Virus 3C(pro) Cleaves PABPC1 to Promote Viral Replication. Pathogens 2020, 9, 443. [Google Scholar] [CrossRef] [PubMed Central]
- Borah, S.; Darricarrere, N.; Darnell, A.; Myoung, J.; Steitz, J.A. A viral nuclear noncoding RNA binds re-localized poly(A) binding protein and is required for late KSHV gene expression. PLoS Pathog. 2011, 7, e1002300. [Google Scholar] [CrossRef] [PubMed Central]
- Choi, E.J.; Ren, Y.; Chen, Y.; Liu, S.; Wu, W.; Ren, J.; Wang, P.; Garofalo, R.P.; Zhou, J.; Bao, X. Exchange Proteins Directly Activated by cAMP and Their Roles in Respiratory Syncytial Virus Infection. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef] [PubMed Central]
- Choi, E.J.; Ren, J.; Zhang, K.; Wu, W.; Lee, Y.S.; Lee, I.; Bao, X. The Importance of AGO 1 and 4 in Post-Transcriptional Gene Regulatory Function of tRF5-GluCTC, an Respiratory Syncytial Virus-Induced tRNA-Derived RNA Fragment. Int. J. Mol. Sci. 2020, 21, 8766. [Google Scholar] [CrossRef] [PubMed Central]
- Ren, J.; Liu, T.; Pang, L.; Li, K.; Garofalo, R.P.; Casola, A.; Bao, X. A novel mechanism for the inhibition of interferon regulatory factor-3-dependent gene expression by human respiratory syncytial virus NS1 protein. J. Gen. Virol. 2011, 92 Pt 9, 2153–2159. [Google Scholar] [CrossRef]
- Lee, K.; Kunkeaw, N.; Jeon, S.H.; Lee, I.; Johnson, B.H.; Kang, G.Y.; Bang, J.Y.; Park, H.S.; Leelayuwat, C.; Lee, Y.S. Precursor miR-886, a novel noncoding RNA repressed in cancer, associates with PKR and modulates its activity. RNA 2011, 17, 1076–1089. [Google Scholar] [CrossRef] [PubMed Central]
- Choi, E.J.; Wu, W.; Cong, X.; Zhang, K.; Luo, J.; Ye, S.; Wang, P.; Suresh, A.; Ullah, U.M.; Zhou, J.; et al. Broad Impact of Exchange Protein Directly Activated by cAMP 2 (EPAC2) on Respiratory Viral Infections. Viruses 2021, 13, 1179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, J.; Wang, Q.; Kolli, D.; Prusak, D.J.; Tseng, C.T.; Chen, Z.J.; Li, K.; Wood, T.G.; Bao, X. Human metapneumovirus M2-2 protein inhibits innate cellular signaling by targeting MAVS. J. Virol. 2012, 86, 13049–13061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, J.; Tang, Y.D.; Hu, W.; Zheng, C. When Poly(A) Binding Proteins Meet Viral Infections, Including SARS-CoV-2. J. Virol. 2022, 96, e0013622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Behm-Ansmant, I.; Gatfield, D.; Rehwinkel, J.; Hilgers, V.; Izaurralde, E. A conserved role for cytoplasmic poly(A)-binding protein 1 (PABPC1) in nonsense-mediated mRNA decay. EMBO J. 2007, 26, 1591–1601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Funakoshi, Y.; Doi, Y.; Hosoda, N.; Uchida, N.; Osawa, M.; Shimada, I.; Tsujimoto, M.; Suzuki, T.; Katada, T.; Hoshino, S. Mechanism of mRNA deadenylation: Evidence for a molecular interplay between translation termination factor eRF3 and mRNA deadenylases. Genes. Dev. 2007, 21, 3135–3148. [Google Scholar] [CrossRef] [PubMed Central]
- Massimelli, M.J.; Kang, J.G.; Majerciak, V.; Le, S.Y.; Liewehr, D.J.; Steinberg, S.M.; Zheng, Z.M. Stability of a long noncoding viral RNA depends on a 9-nt core element at the RNA 5′ end to interact with viral ORF57 and cellular PABPC1. Int. J. Biol. Sci. 2011, 7, 1145–1160. [Google Scholar] [CrossRef] [PubMed Central]
- Farouk, I.A.; Batra, J.; Choo, W.S.; Lal, S. Influenza A virus nucleoprotein requires the human polyadenylate binding protein (PABPC1) for successful virus replication. Int. J. Infect. Dis. 2023, 130, S101. [Google Scholar] [CrossRef]
- Bouillier, C.; Cosentino, G.; Leger, T.; Rincheval, V.; Richard, C.A.; Desquesnes, A.; Sitterlin, D.; Blouquit-Laye, S.; Eleouet, J.F.; Gault, E.; et al. The Interactome analysis of the Respiratory Syncytial Virus protein M2-1 suggests a new role in viral mRNA metabolism post-transcription. Sci. Rep. 2019, 9, 15258. [Google Scholar] [CrossRef] [PubMed Central]
- Sobala, A.; Hutvagner, G. Transfer RNA-derived fragments: Origins, processing, and functions. Wiley Interdiscip. Rev. RNA 2011, 2, 853–862. [Google Scholar] [CrossRef]
- Fu, Y.; Lee, I.; Lee, Y.S.; Bao, X. Small Non-coding Transfer RNA-Derived RNA Fragments (tRFs): Their Biogenesis, Function and Implication in Human Diseases. Genom. Inform. 2015, 13, 94–101. [Google Scholar] [CrossRef] [PubMed Central]
- Wu, W.; Choi, E.J.; Wang, B.; Zhang, K.; Adam, A.; Huang, G.; Tunkle, L.; Huang, P.; Goru, R.; Imirowicz, I.; et al. Changes of Small Non-coding RNAs by Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Front. Mol. Biosci. 2022, 9, 821137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, E.J.; Wu, W.; Zhang, K.; Yuan, X.; Deng, J.; Ismail, D.; Buck, D.L.; Thomason, K.S.; Garofalo, R.P.; Zhang, S.; et al. Parent tRNA Modification Status Determines the Induction of Functional tRNA-Derived RNA by Respiratory Syncytial Virus Infection. Viruses 2022, 15, 57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selitsky, S.R.; Baran-Gale, J.; Honda, M.; Yamane, D.; Masaki, T.; Fannin, E.E.; Guerra, B.; Shirasaki, T.; Shimakami, T.; Kaneko, S.; et al. Small tRNA-derived RNAs are increased and more abundant than microRNAs in chronic hepatitis B and C. Sci. Rep. 2015, 5, 7675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, H.; Zheng, L.; Li, H.; Feng, C.; Zhang, W. Identification of functional tRNA-derived fragments in senescence-accelerated mouse prone 8 brain. Aging 2019, 11, 10485–10498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, W.; Lee, I.; Spratt, H.; Fang, X.; Bao, X. tRNA-Derived Fragments in Alzheimer’s Disease: Implications for New Disease Biomarkers and Neuropathological Mechanisms. J. Alzheimer’s Dis. 2021, 79, 793–806. [Google Scholar] [CrossRef]
- Su, Z.; Monshaugen, I.; Klungland, A.; Ougland, R.; Dutta, A. Characterization of novel small non-coding RNAs and their modifications in bladder cancer using an updated small RNA-seq workflow. Front. Mol. Biosci. 2022, 9, 887686. [Google Scholar] [CrossRef] [PubMed Central]
- Olvedy, M.; Scaravilli, M.; Hoogstrate, Y.; Visakorpi, T.; Jenster, G.; Martens-Uzunova, E. A comprehensive repertoire of tRNA-derived fragments in prostate cancer. Oncotarget 2016, 7, 24766. [Google Scholar] [CrossRef]
- Zhu, P.; Yu, J.; Zhou, P. Role of tRNA-derived fragments in cancer: Novel diagnostic and therapeutic targets tRFs in cancer. Am. J. Cancer Res. 2020, 10, 393–402. [Google Scholar] [PubMed Central]
- Kuhle, B.; Chen, Q.; Schimmel, P. tRNA renovatio: Rebirth through fragmentation. Mol. Cell 2023, 83, 3953–3971. [Google Scholar] [CrossRef] [PubMed Central]
- Lorenz, C.; Lunse, C.E.; Morl, M. tRNA Modifications: Impact on Structure and Thermal Adaptation. Biomolecules 2017, 7, 35. [Google Scholar] [CrossRef] [PubMed Central]
- Cozen, A.E.; Quartley, E.; Holmes, A.D.; Hrabeta-Robinson, E.; Phizicky, E.M.; Lowe, T.M. ARM-seq: AlkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat. Methods 2015, 12, 879–884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernandez-Alias, X.; Katanski, C.D.; Zhang, W.; Assari, M.; Watkins, C.P.; Schaefer, M.H.; Serrano, L.; Pan, T. Single-read tRNA-seq analysis reveals coordination of tRNA modification and aminoacylation and fragmentation. Nucleic Acids Res. 2023, 51, e17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guzzi, N.; Ciesla, M.; Ngoc, P.C.T.; Lang, S.; Arora, S.; Dimitriou, M.; Pimkova, K.; Sommarin, M.N.E.; Munita, R.; Lubas, M.; et al. Pseudouridylation of tRNA-Derived Fragments Steers Translational Control in Stem Cells. Cell 2018, 173, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Ivanov, P.; Hu, G.F.; Anderson, P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 2009, 185, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.M.; Gudanis, D.; Coyne, S.M.; Gdaniec, Z.; Ivanov, P. Identification of functional tetramolecular RNA G-quadruplexes derived from transfer RNAs. Nat. Commun. 2017, 8, 1127. [Google Scholar] [CrossRef] [PubMed Central]
- Lyons, S.M.; Kharel, P.; Akiyama, Y.; Ojha, S.; Dave, D.; Tsvetkov, V.; Merrick, W.; Ivanov, P.; Anderson, P. eIF4G has intrinsic G-quadruplex binding activity that is required for tiRNA function. Nucleic Acids Res. 2020, 48, 6223–6233. [Google Scholar] [CrossRef] [PubMed Central]
- Suzuki, T.; Yashiro, Y.; Kikuchi, I.; Ishigami, Y.; Saito, H.; Matsuzawa, I.; Okada, S.; Mito, M.; Iwasaki, S.; Ma, D.; et al. Complete chemical structures of human mitochondrial tRNAs. Nat. Commun. 2020, 11, 4269. [Google Scholar] [CrossRef] [PubMed Central]
- Zhou, J.; Liu, S.; Chen, Y.; Fu, Y.; Silver, A.J.; Hill, M.S.; Lee, I.; Lee, Y.S.; Bao, X. Identification of two novel functional tRNA-derived fragments induced in response to respiratory syncytial virus infection. J. Gen. Virol. 2017, 98, 1600–1610. [Google Scholar] [CrossRef] [PubMed Central]
- Kahvejian, A.; Svitkin, Y.V.; Sukarieh, R.; M’Boutchou, M.N.; Sonenberg, N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes. Dev. 2005, 19, 104–113. [Google Scholar] [CrossRef] [PubMed Central]
- Mangus, D.A.; Evans, M.C.; Jacobson, A. Poly(A)-binding proteins: Multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 2003, 4, 223. [Google Scholar] [CrossRef] [PubMed Central]
- Wise, E.L.; Samolej, J.; Elliott, G. Herpes Simplex Virus 1 Expressing GFP-Tagged Virion Host Shutoff (vhs) Protein Uncouples the Activities of RNA Degradation and Differential Nuclear Retention of the Virus Transcriptome. J. Virol. 2022, 96, e0192621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dobrikova, E.; Shveygert, M.; Walters, R.; Gromeier, M. Herpes simplex virus proteins ICP27 and UL47 associate with polyadenylate-binding protein and control its subcellular distribution. J. Virol. 2010, 84, 270–279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burgui, I.; Aragon, T.; Ortin, J.; Nieto, A. PABP1 and eIF4GI associate with influenza virus NS1 protein in viral mRNA translation initiation complexes. J. Gen. Virol. 2003, 84 Pt 12, 3263–3274. [Google Scholar] [CrossRef] [PubMed]
- Ghildyal, R.; Mills, J.; Murray, M.; Vardaxis, N.; Meanger, J. Respiratory syncytial virus matrix protein associates with nucleocapsids in infected cells. J. Gen. Virol. 2002, 83 Pt 4, 753–757. [Google Scholar] [CrossRef]


| Target | Primer | Sequence (5′-3′) |
|---|---|---|
| PABPC1 | Forward primer | GCCAGTACGCATCATGTGGTCTC |
| Reverse primer | CATACAGTGCTTTATTATCAATGG | |
| RSV N | RT primer | CTGCGATGAGTGGCAGGCTTTTTTTTTTTTAACTYAAAGCTC |
| Forward primer | ACTACAGTGTATTAGACTTRACAGCAGAAG | |
| Reverse primer | CTGCGATGAGTGGCAGGC | |
| RSV L | RT primer | CTGCGATGAGTGGCAGGCTTTTTTTTTTTTCATTATTCATTATG |
| Forward primer | CTTACCTAAGTGAATTGTTAAACAGCTTGAC | |
| Reverse primer | CTGCGATGAGTGGCAGGC | |
| RSV (-) | RT primer | CTGCGATGAGTGGCAGGCACTACAGTGTATTAGACTTRACAGCAGAAG |
| Forward primer | GCATCTTCTCCATGRAATTCAGG | |
| Reverse primer | CTGCGATGAGTGGCAGGC | |
| tRF5-GluCTC | RT primer | CGTCGGACTGTAGAACTCTCAAAGC |
| Foward primer | TCCCTGGTGGTCTAGTG | |
| Reverse primer | CGTCGGACTGTAGAACTCTCAAAGC | |
| Bio-tRF5-GluCTC | UCCCUGGUGGUCUAGUGGUUAGGAUUCGG-Biotin | |
| Bio-tRF5-CN | AGGUCCAACUAAAUCACUAAUAAUAAACCGC-Biotin |
| GeneID | GeneSymbol | iBAQ | Fold | |
|---|---|---|---|---|
| Bio-tRF5-CN | Bio-tRF5-GluCTC | Bio-tRF5-GluCTC/Bio-tRF5-CN | ||
| 9406 | ZRANB2 | 0.3415 | ||
| 122704 | MRPL52 | 0.0587 | ||
| 55794 | DDX28 | 0.0399 | ||
| 65003 | MRPL11 | 0.0383 | ||
| 6231 | RPS26 | 0.0011 | 0.0369 | 34.17 |
| 84545 | MRPL43 | 0.0368 | ||
| 84311 | MRPL45 | 0.0350 | ||
| 29088 | MRPL15 | 0.0332 | ||
| 2926 | PAIP1 | 0.0324 | ||
| 9130 | FAM50A | 0.0297 | ||
| 1478 | CSTF2 | 0.0243 | ||
| 64928 | MRPL14 | 0.0227 | ||
| 3185 | HNRNPF | 0.0022 | 0.0220 | 10.00 |
| 51649 | MRPS23 | 0.0210 | ||
| 90480 | GADD45GIP1 | 0.0198 | ||
| 23438 | HARS2 | 0.0193 | ||
| 26986 | PABPC1 | 0.0012 | 0.0183 | 15.69 |
| 28957 | MRPS28 | 0.0017 | 0.0182 | 10.48 |
| 51335 | NGRN | 0.0178 | ||
| 28998 | MRPL13 | 0.0172 | ||
| 55178 | MRM3 | 0.0011 | 0.0169 | 15.54 |
| 29093 | MRPL22 | 0.0164 | ||
| 51263 | MRPL30 | 0.0156 | ||
| 28977 | MRPL42 | 0.0106 | ||
| 26995 | TRUB2 | 0.0084 | ||
| 27349 | MCAT | 0.0078 | ||
| 51258 | MRPL51 | 0.0070 | ||
| 64969 | MRPS5 | 0.0069 | ||
| 51073 | MRPL4 | 0.0069 | ||
| 84881 | RPUSD4 | 0.0057 | ||
| 26024 | PTCD1 | 0.0056 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, D.V.; Choi, E.-J.; Ismail, D.; Hernandez, M.L.; Choi, J.M.; Zhang, K.; Khatkar, K.; Jung, S.Y.; Wu, W.; Bao, X. Role of Poly(A)-Binding Protein Cytoplasmic 1, a tRNA-Derived RNA Fragment-Bound Protein, in Respiratory Syncytial Virus Infection. Pathogens 2024, 13, 791. https://doi.org/10.3390/pathogens13090791
Davis DV, Choi E-J, Ismail D, Hernandez ML, Choi JM, Zhang K, Khatkar K, Jung SY, Wu W, Bao X. Role of Poly(A)-Binding Protein Cytoplasmic 1, a tRNA-Derived RNA Fragment-Bound Protein, in Respiratory Syncytial Virus Infection. Pathogens. 2024; 13(9):791. https://doi.org/10.3390/pathogens13090791
Chicago/Turabian StyleDavis, Devin V., Eun-Jin Choi, Deena Ismail, Miranda L. Hernandez, Jong Min Choi, Ke Zhang, Kashish Khatkar, Sung Yun Jung, Wenzhe Wu, and Xiaoyong Bao. 2024. "Role of Poly(A)-Binding Protein Cytoplasmic 1, a tRNA-Derived RNA Fragment-Bound Protein, in Respiratory Syncytial Virus Infection" Pathogens 13, no. 9: 791. https://doi.org/10.3390/pathogens13090791







