Comprehensive Genomic Analysis of Uropathogenic E. coli: Virulence Factors, Antimicrobial Resistance, and Mobile Genetic Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Uropathogenic E. coli
2.2. Determination of Antibiotic Susceptibility
2.3. Effect of Phenylalanine-Arginine β-Naphthylamide (PaβN) on Ciprofloxacin Minimum Inhibitory Concentration (MIC) Using the Broth Microdilution Method
2.4. Quantitation of the Efflux Pump and Outer Membrane Gene Expression via Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
2.5. Whole-Genome Sequencing
2.6. Identification of Multilocus Sequence Types (MLST), Core Genome MLST (cgMLST), Serotype, FimH and FumC Type, Antibiotic Resistance, Mobile Genetic Elements (MGEs), Phages, CRISPR-Cas Systems, and Virulence
3. Results
3.1. Phylogenetic, MLST, cgMLST, Serotype, and CH (fimH/fumC) Type Analysis
3.2. Phenotypic Antimicrobial Susceptibility
3.3. Analysis of Quantification of RNA Expression by qRT-PCR
3.4. Genotypic Antimicrobial Analysis of UPEC WGS
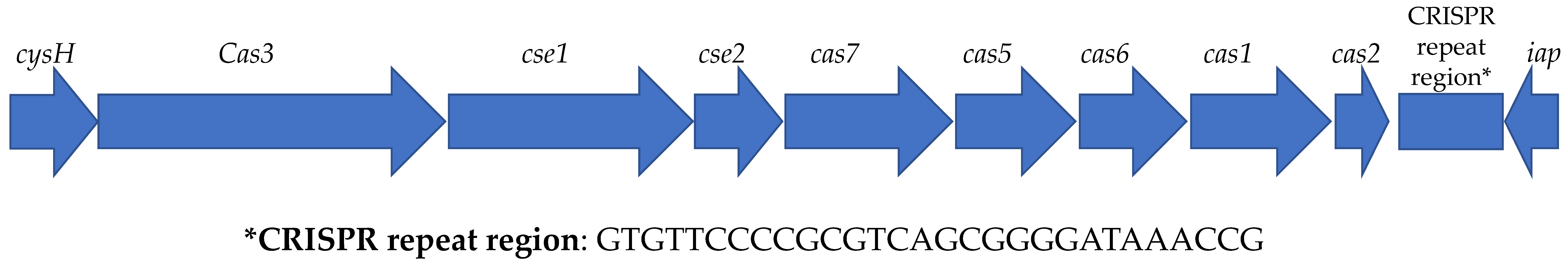
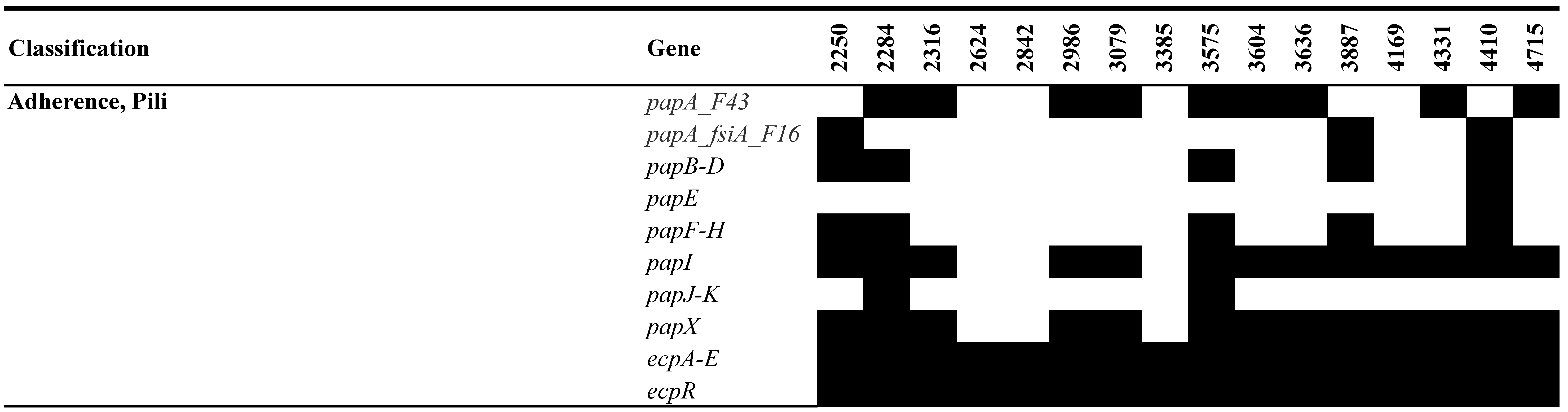
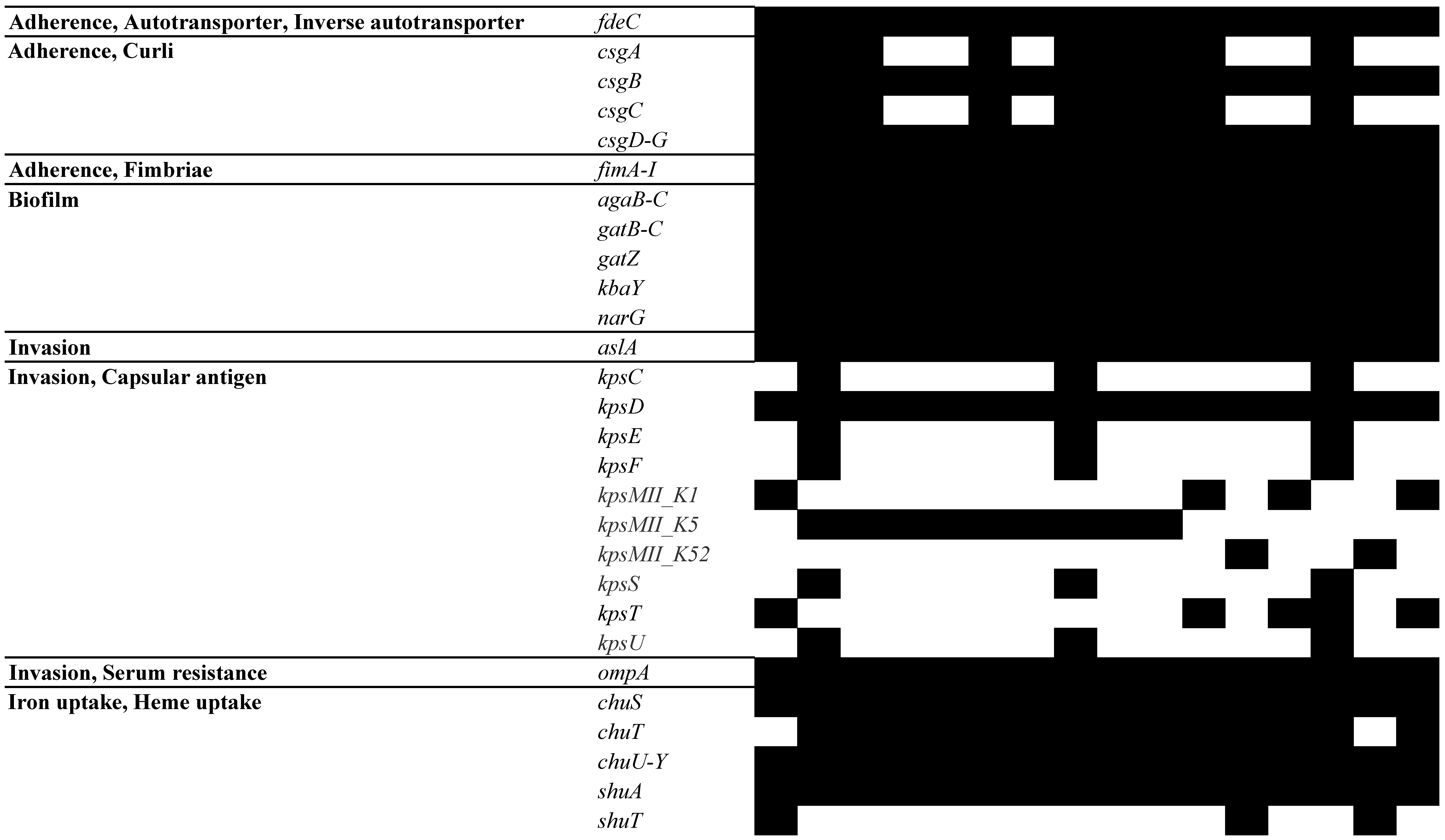
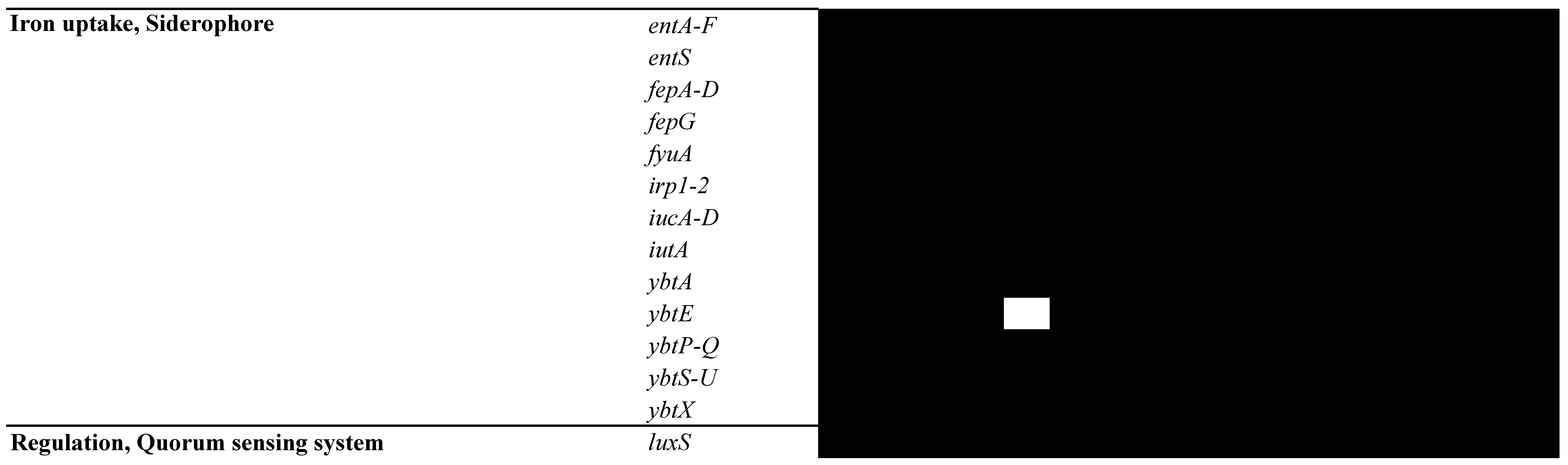
3.5. Analysis of MGEs, Insertion Elements (ISs), Phages, and CRISPR-Cas Systems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schappert, S.M.; Rechtsteiner, E.A. Ambulatory medical care utilization estimates for 2007. Vital Health Stat. 13 2011, 169, 1–38. [Google Scholar]
- Kahlmeter, G. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: The ECO.SENS Project. J. Antimicrob. Chemother. 2003, 51, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Brown, P. Epidemiology of urinary tract infections: Transmission and risk factors, incidence, and costs. Infect. Dis. Clin. N. Am. 2003, 17, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef]
- Prevention, C.F.D.C.A. Multidrug-resistant E. coli. Available online: https://arpsp.cdc.gov/profile/antibiotic-resistance/mdr-ecoli (accessed on 15 April 2024).
- Asadi Karam, M.R.; Habibi, M.; Bouzari, S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol. Immunol. 2019, 108, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Mazzariol, A.; Bazaj, A.; Cornaglia, G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: A review. J. Chemother. 2017, 29, 2–9. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Oelschlaeger, T.A.; Dobrindt, U.; Hacker, J. Virulence factors of uropathogens. Curr. Opin. Urol. 2002, 12, 33–38. [Google Scholar] [CrossRef]
- Bhandari, M.; Poelstra, J.W.; Kauffman, M.; Varghese, B.; Helmy, Y.A.; Scaria, J.; Rajashekara, G. Genomic Diversity, Antimicrobial Resistance, Plasmidome, and Virulence Profiles of Salmonella Isolated from Small Specialty Crop Farms Revealed by Whole-Genome Sequencing. Antibiotics 2023, 12, 1637. [Google Scholar] [CrossRef]
- Rizk, A.M.; Elsayed, M.M.; Abd El Tawab, A.A.; Elhofy, F.I.; Soliman, E.A.; Kozytska, T.; Brangsch, H.; Sprague, L.D.; Neubauer, H.; Wareth, G. Phenotypic and genotypic characterization of resistance and virulence in Pseudomonas aeruginosa isolated from poultry farms in Egypt using whole genome sequencing. Vet. Microbiol. 2024, 292, 110063. [Google Scholar] [CrossRef]
- Zouharova, M.; Matiasovic, J.; Gebauer, J.; Matiaskova, K.; Nedbalcova, K. Survey of Genotype Diversity, Virulence, and Antimicrobial Resistance Genes in Mastitis-Causing Streptococcus uberis in Dairy Herds Using Whole-Genome Sequencing. Pathogens 2023, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019.
- Alotaibi, K.; Khan, A.A.; Marasa, B.; Khan, S.A.; Johnson, J.; Johnston, B.; Sung, K.; Nawaz, M. Draft Genome Sequences of Sixteen Fluoroquinolone-Resistant Extraintestinal Escherichia coli Isolates from Human Patients. Microbiol. Resour. Announc. 2022, 11, e0000322. [Google Scholar] [CrossRef]
- Center for Genomic Epidemiology. Available online: https://www.genomicepidemiology.org/services/ (accessed on 20 February 2024).
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Olson, R.D.; Assaf, R.; Brettin, T.; Conrad, N.; Cucinell, C.; Davis, J.J.; Dempsey, D.M.; Dickerman, A.; Dietrich, E.M.; Kenyon, R.W.; et al. Introducing the Bacterial and Viral Bioinformatics Resource Center (BV-BRC): A resource combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2023, 51, D678–D689. [Google Scholar] [CrossRef]
- Antonopoulos, D.A.; Assaf, R.; Aziz, R.K.; Brettin, T.; Bun, C.; Conrad, N.; Davis, J.J.; Dietrich, E.M.; Disz, T.; Gerdes, S.; et al. PATRIC as a unique resource for studying antimicrobial resistance. Brief. Bioinform. 2019, 20, 1094–1102. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- McArthur, A.G.; Waglechner, N.; Nizam, F.; Yan, A.; Azad, M.A.; Baylay, A.J.; Bhullar, K.; Canova, M.J.; De Pascale, G.; Ejim, L.; et al. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 2013, 57, 3348–3357. [Google Scholar] [CrossRef]
- Bharat, A.; Petkau, A.; Avery, B.P.; Chen, J.C.; Folster, J.P.; Carson, C.A.; Kearney, A.; Nadon, C.; Mabon, P.; Thiessen, J.; et al. Correlation between Phenotypic and In Silico Detection of Antimicrobial Resistance in Salmonella enterica in Canada Using Staramr. Microorganisms 2022, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Goh, Y.X.; Tai, C.; Wang, H.; Deng, Z.; Ou, H.Y. VRprofile2: Detection of antibiotic resistance-associated mobilome in bacterial pathogens. Nucleic Acids Res. 2022, 50, W768–W773. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.H.K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J. Antimicrob. Chemother. 2021, 76, 101–109. [Google Scholar] [CrossRef]
- Brown, C.L.; Mullet, J.; Hindi, F.; Stoll, J.E.; Gupta, S.; Choi, M.; Keenum, I.; Vikesland, P.; Pruden, A.; Zhang, L. mobileOG-db: A Manually Curated Database of Protein Families Mediating the Life Cycle of Bacterial Mobile Genetic Elements. Appl. Environ. Microbiol. 2022, 88, e0099122. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [PubMed]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Neron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef]
- Wattam, A.R.; Davis, J.J.; Assaf, R.; Boisvert, S.; Brettin, T.; Bun, C.; Conrad, N.; Dietrich, E.M.; Disz, T.; Gabbard, J.L.; et al. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res. 2017, 45, D535–D542. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef] [PubMed]
- Sayers, S.; Li, L.; Ong, E.; Deng, S.; Fu, G.; Lin, Y.; Yang, B.; Zhang, S.; Fa, Z.; Zhao, B.; et al. Victors: A web-based knowledge base of virulence factors in human and animal pathogens. Nucleic Acids Res. 2019, 47, D693–D700. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.A.; Sharp, P.M. Enterobacterial repetitive intergenic consensus (ERIC) sequences in Escherichia coli: Evolution and implications for ERIC-PCR. Mol. Biol. Evol. 2006, 23, 1156–1168. [Google Scholar] [CrossRef]
- Manges, A.R.; Geum, H.M.; Guo, A.; Edens, T.J.; Fibke, C.D.; Pitout, J.D.D. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin. Microbiol. Rev. 2019, 32, e00135-18. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Peirano, G.; Chen, L.; DeVinney, R.; Matsumura, Y. Escherichia coli ST1193: Following in the Footsteps of E. coli ST131. Antimicrob. Agents Chemother. 2022, 66, e0051122. [Google Scholar] [CrossRef]
- Nicolas-Chanoine, M.H.; Blanco, J.; Leflon-Guibout, V.; Demarty, R.; Alonso, M.P.; Canica, M.M.; Park, Y.J.; Lavigne, J.P.; Pitout, J.; Johnson, J.R. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 2008, 61, 273–281. [Google Scholar] [CrossRef]
- Kondratyeva, K.; Salmon-Divon, M.; Navon-Venezia, S. Meta-analysis of Pandemic Escherichia coli ST131 Plasmidome Proves Restricted Plasmid-clade Associations. Sci. Rep. 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Ruppe, E.; Hem, S.; Lath, S.; Gautier, V.; Ariey, F.; Sarthou, J.L.; Monchy, D.; Arlet, G. CTX-M beta-lactamases in Escherichia coli from community-acquired urinary tract infections, Cambodia. Emerg. Infect. Dis. 2009, 15, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hossain, M.M.K.; Rubaya, R.; Halder, J.; Karim, M.E.; Bhuiya, A.A.; Khatun, A.; Alam, J. Association of Antibiotic Resistance Traits in Uropathogenic Escherichia coli (UPEC) Isolates. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 4251486. [Google Scholar] [CrossRef]
- Somorin, Y.M.; Weir, N.M.; Pattison, S.H.; Crockard, M.A.; Hughes, C.M.; Tunney, M.M.; Gilpin, D.F. Antimicrobial resistance in urinary pathogens and culture-independent detection of trimethoprim resistance in urine from patients with urinary tract infection. BMC Microbiol. 2022, 22, 144. [Google Scholar] [CrossRef]
- Anes, J.; McCusker, M.P.; Fanning, S.; Martins, M. The ins and outs of RND efflux pumps in Escherichia coli. Front. Microbiol. 2015, 6, 587. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Kobylka, J.; Kuth, M.S.; Pos, K.M.; Picard, M.; Blair, J.M.A.; Bavro, V.N. Structure, Assembly, and Function of Tripartite Efflux and Type 1 Secretion Systems in Gram-Negative Bacteria. Chem. Rev. 2021, 121, 5479–5596. [Google Scholar] [CrossRef]
- Lopez, C.A.; Travers, T.; Pos, K.M.; Zgurskaya, H.I.; Gnanakaran, S. Dynamics of Intact MexAB-OprM Efflux Pump: Focusing on the MexA-OprM Interface. Sci. Rep. 2017, 7, 16521. [Google Scholar] [CrossRef]
- Saint, S.; Veenstra, D.L.; Sullivan, S.D.; Chenoweth, C.; Fendrick, A.M. The potential clinical and economic benefits of silver alloy urinary catheters in preventing urinary tract infection. Arch. Intern. Med. 2000, 160, 2670–2675. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids and the spread of resistance. Int. J. Med. Microbiol. 2013, 303, 298–304. [Google Scholar] [CrossRef]
- Yao, M.; Zhu, Q.; Zou, J.; Shenkutie, A.M.; Hu, S.; Qu, J.; He, Z.; Leung, P.H.M. Genomic Characterization of a Uropathogenic Escherichia coli ST405 Isolate Harboring bla (CTX-M-15)-Encoding IncFIA-FIB Plasmid, bla (CTX-M-24)-Encoding IncI1 Plasmid, and Phage-Like Plasmid. Front. Microbiol. 2022, 13, 845045. [Google Scholar] [CrossRef]
- Johnson, T.J.; Nolan, L.K. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol. Mol. Biol. Rev. 2009, 73, 750–774. [Google Scholar] [CrossRef]
- Creuzburg, K.; Recktenwald, J.; Kuhle, V.; Herold, S.; Hensel, M.; Schmidt, H. The Shiga toxin 1-converting bacteriophage BP-4795 encodes an NleA-like type III effector protein. J. Bacteriol. 2005, 187, 8494–8498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, S.; Jensen, M.A.; Bai, J.; Debroy, C.; Barrangou, R.; Dudley, E.G. The evolutionary divergence of Shiga toxin-producing Escherichia coli is reflected in clustered regularly interspaced short palindromic repeat (CRISPR) spacer composition. Appl. Environ. Microbiol. 2013, 79, 5710–5720. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Ooka, T.; Iguchi, A.; Toh, H.; Asadulghani, M.; Oshima, K.; Kodama, T.; Abe, H.; Nakayama, K.; Kurokawa, K.; et al. Comparative genomics reveal the mechanism of the parallel evolution of O157 and non-O157 enterohemorrhagic Escherichia coli. Proc. Natl. Acad. Sci. USA 2009, 106, 17939–17944. [Google Scholar] [CrossRef]
- Lindenstrauss, A.G.; Pavlovic, M.; Bringmann, A.; Behr, J.; Ehrmann, M.A.; Vogel, R.F. Comparison of genotypic and phenotypic cluster analyses of virulence determinants and possible role of CRISPR elements towards their incidence in Enterococcus faecalis and Enterococcus faecium. Syst. Appl. Microbiol. 2011, 34, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.N.; Zhang, L.; Zollner, S.; Srinivasan, U.; Abbas, K.; Marrs, C.F.; Foxman, B. Uropathogenic Escherichia coli are less likely than paired fecal E. coli to have CRISPR loci. Infect. Genet. Evol. 2013, 19, 212–218. [Google Scholar] [CrossRef]
- Schmidt, H.; Hensel, M. Pathogenicity islands in bacterial pathogenesis. Clin. Microbiol. Rev. 2004, 17, 14–56. [Google Scholar] [CrossRef]
- Dobrindt, U.; Hochhut, B.; Hentschel, U.; Hacker, J. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2004, 2, 414–424. [Google Scholar] [CrossRef]
- Luthje, P.; Brauner, A. Virulence factors of uropathogenic E. coli and their interaction with the host. Adv. Microb. Physiol. 2014, 65, 337–372. [Google Scholar] [CrossRef]
- Saldana, Z.; De la Cruz, M.A.; Carrillo-Casas, E.M.; Duran, L.; Zhang, Y.; Hernandez-Castro, R.; Puente, J.L.; Daaka, Y.; Giron, J.A. Production of the Escherichia coli common pilus by uropathogenic E. coli is associated with adherence to HeLa and HTB-4 cells and invasion of mouse bladder urothelium. PLoS ONE 2014, 9, e101200. [Google Scholar] [CrossRef]
- Mirzahosseini, H.K.; Najmeddin, F.; Najafi, A.; Ahmadi, A.; Sharifnia, H.; Khaledi, A.; Mojtahedzadeh, M. Correlation of biofilm formation, virulence factors, and phylogenetic groups among Escherichia coli strains causing urinary tract infection: A global systematic review and meta-analysis. J. Res. Med. Sci. 2023, 28, 66. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Ulett, G.C.; Totsika, M.; Phan, M.D.; Schembri, M.A. Role of capsule and O antigen in the virulence of uropathogenic Escherichia coli. PLoS ONE 2014, 9, e94786. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Ba-Thein, W.; Kumao, T.; Akaza, H.; Hayashi, H. Identification of a type III secretion system in uropathogenic Escherichia coli. FEMS Microbiol. Lett. 2002, 212, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.C.; Brumbaugh, A.R.; Mobley, H.L. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect. Immun. 2011, 79, 1225–1235. [Google Scholar] [CrossRef]
- Lane, M.C.; Lockatell, V.; Monterosso, G.; Lamphier, D.; Weinert, J.; Hebel, J.R.; Johnson, D.E.; Mobley, H.L. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 2005, 73, 7644–7656. [Google Scholar] [CrossRef]
- Sandkvist, M. Type II secretion and pathogenesis. Infect. Immun. 2001, 69, 3523–3535. [Google Scholar] [CrossRef]
- De Souza Santos, M.; Orth, K. The Role of the Type III Secretion System in the Intracellular Lifestyle of Enteric Pathogens. Microbiol. Spectr. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.A.; Silva, R.M.; Ruiz, R.C.; Pimenta, D.C.; Bryant, J.A.; Henderson, I.R.; Barbosa, A.S.; Elias, W.P. Secreted Autotransporter Toxin (Sat) Mediates Innate Immune System Evasion. Front. Immunol. 2022, 13, 844878. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.H.; Lee, Y. Virulence Factors Associated with Escherichia coli Bacteremia and Urinary Tract Infection. Ann. Lab. Med. 2022, 42, 203–212. [Google Scholar] [CrossRef]
- Abe, C.M.; Salvador, F.A.; Falsetti, I.N.; Vieira, M.A.; Blanco, J.; Blanco, J.E.; Blanco, M.; Machado, A.M.; Elias, W.P.; Hernandes, R.T.; et al. Uropathogenic Escherichia coli (UPEC) strains may carry virulence properties of diarrhoeagenic E. coli. FEMS Immunol. Med. Microbiol. 2008, 52, 397–406. [Google Scholar] [CrossRef]
- Yun, K.W.; Kim, H.Y.; Park, H.K.; Kim, W.; Lim, I.S. Virulence factors of uropathogenic Escherichia coli of urinary tract infections and asymptomatic bacteriuria in children. J. Microbiol. Immunol. Infect. 2014, 47, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Blanco, J.E.; Alonso, M.P.; Mora, A.; Balsalobre, C.; Munoa, F.; Juarez, A.; Blanco, J. Detection of pap, sfa and afa adhesin-encoding operons in uropathogenic Escherichia coli strains: Relationship with expression of adhesins and production of toxins. Res. Microbiol. 1997, 148, 745–755. [Google Scholar] [CrossRef] [PubMed]
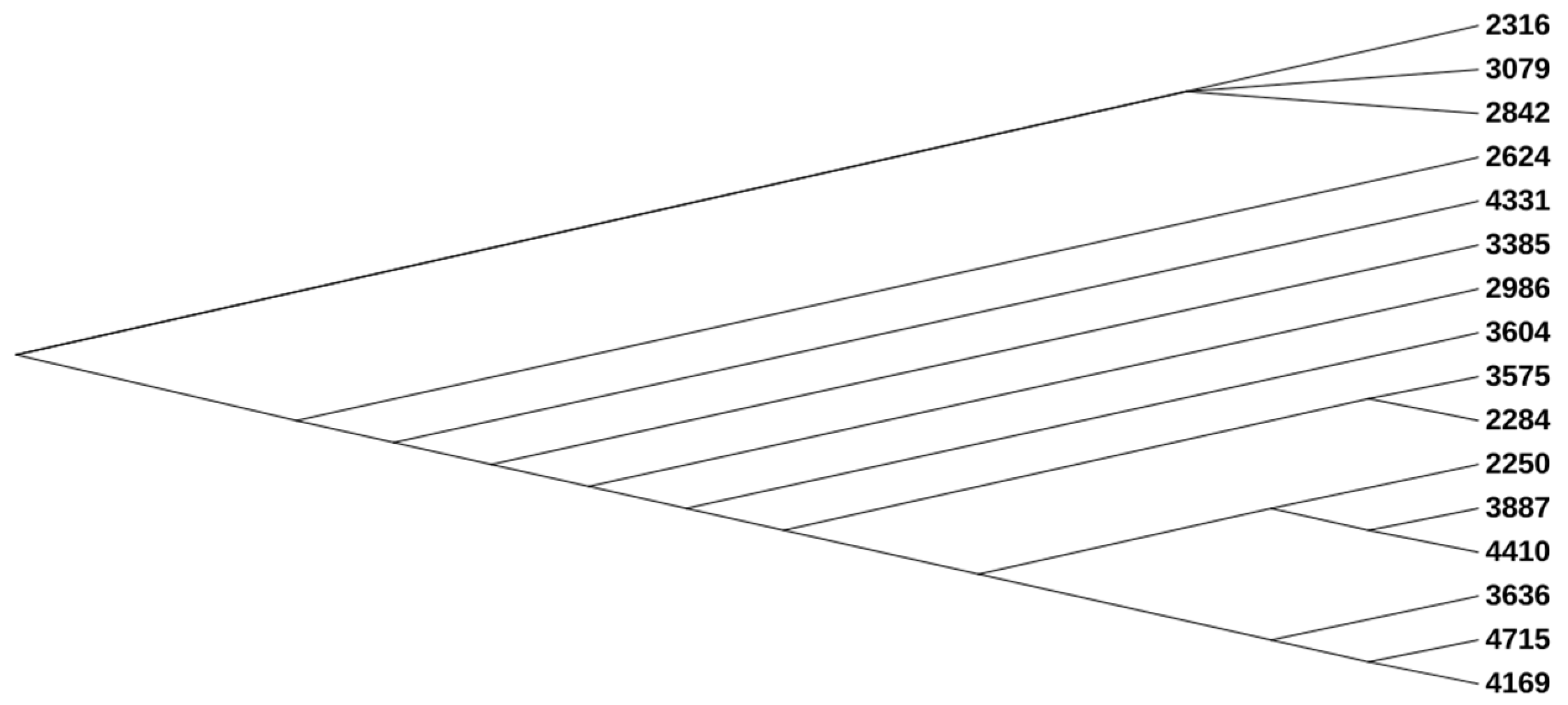
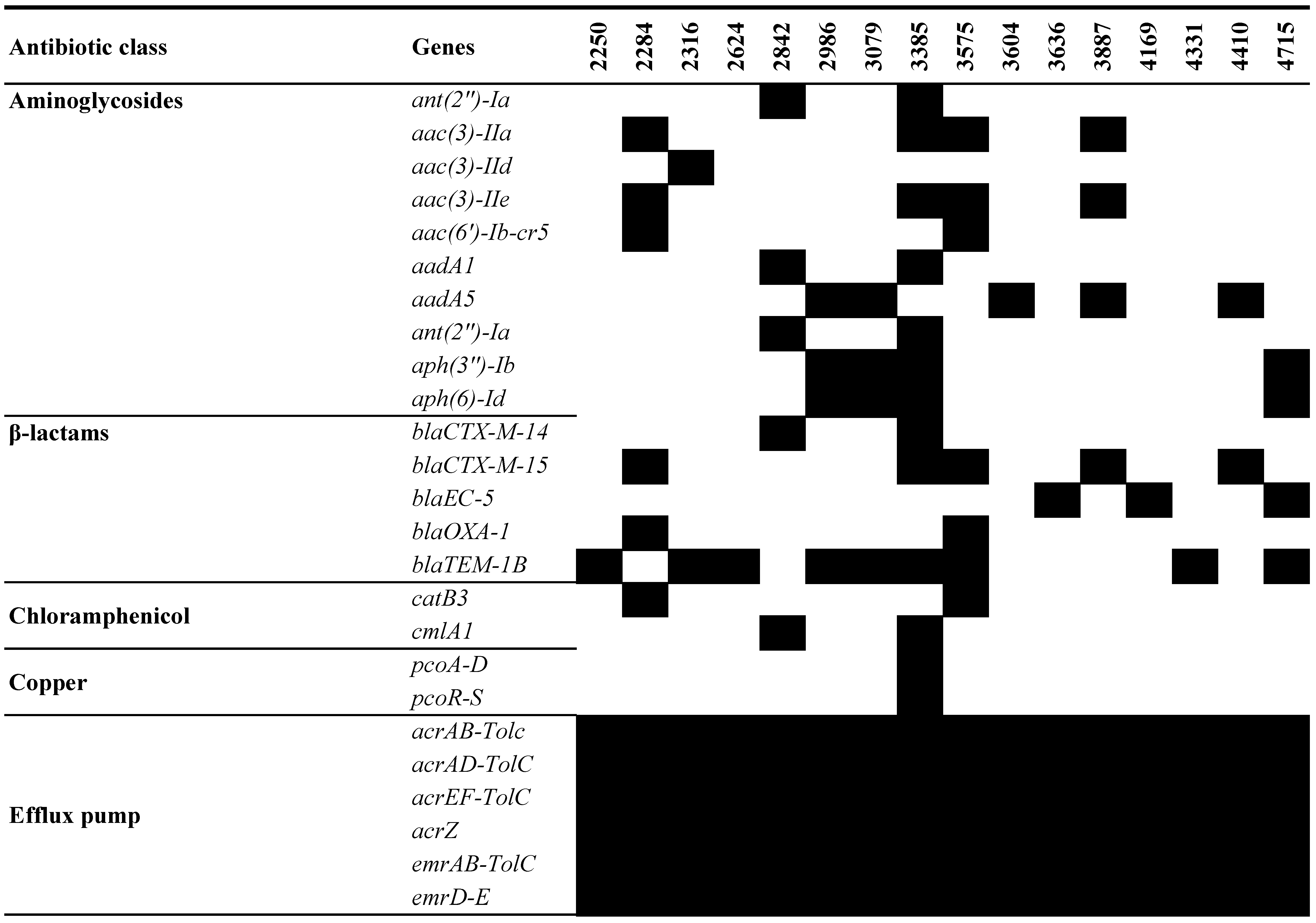

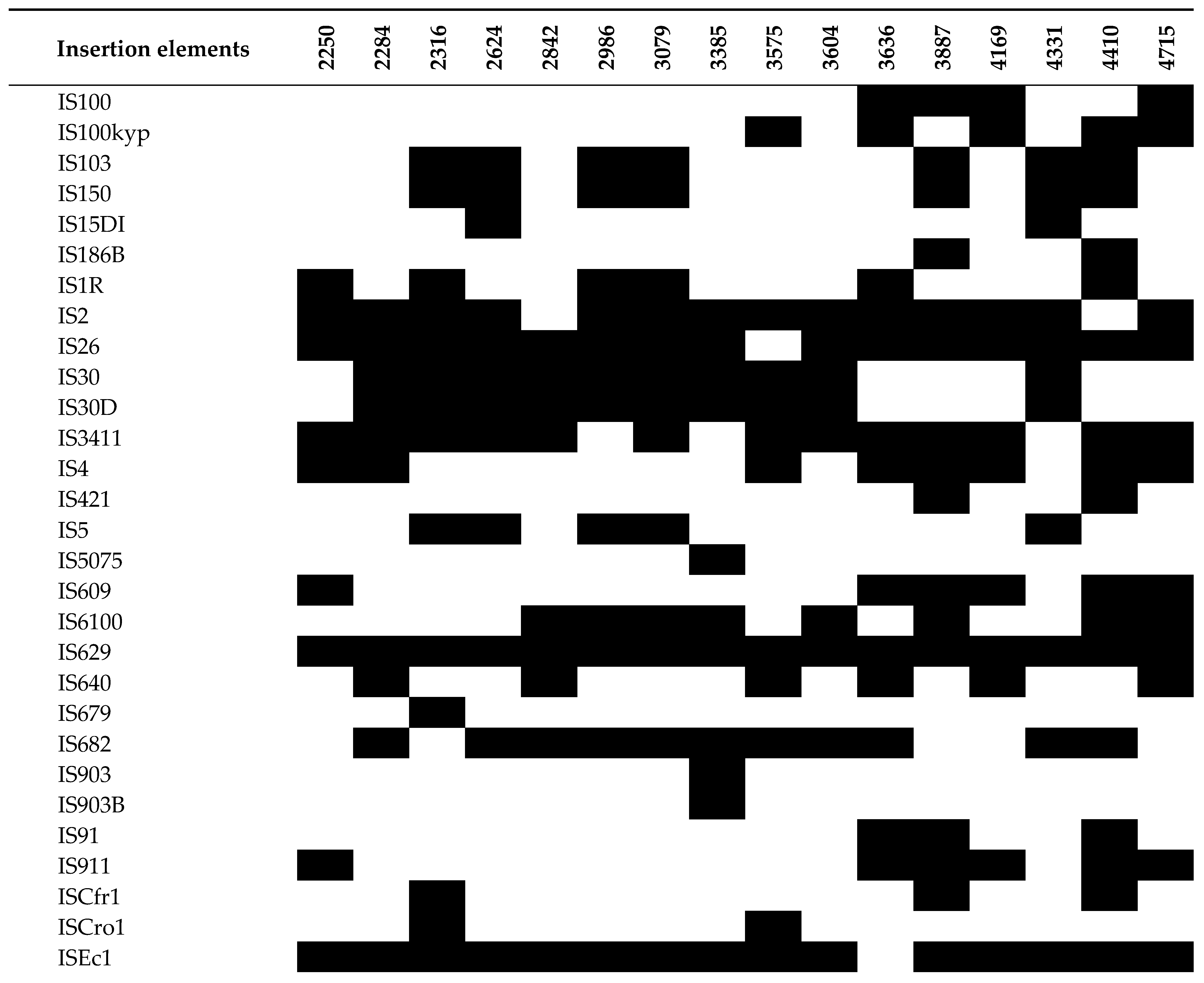
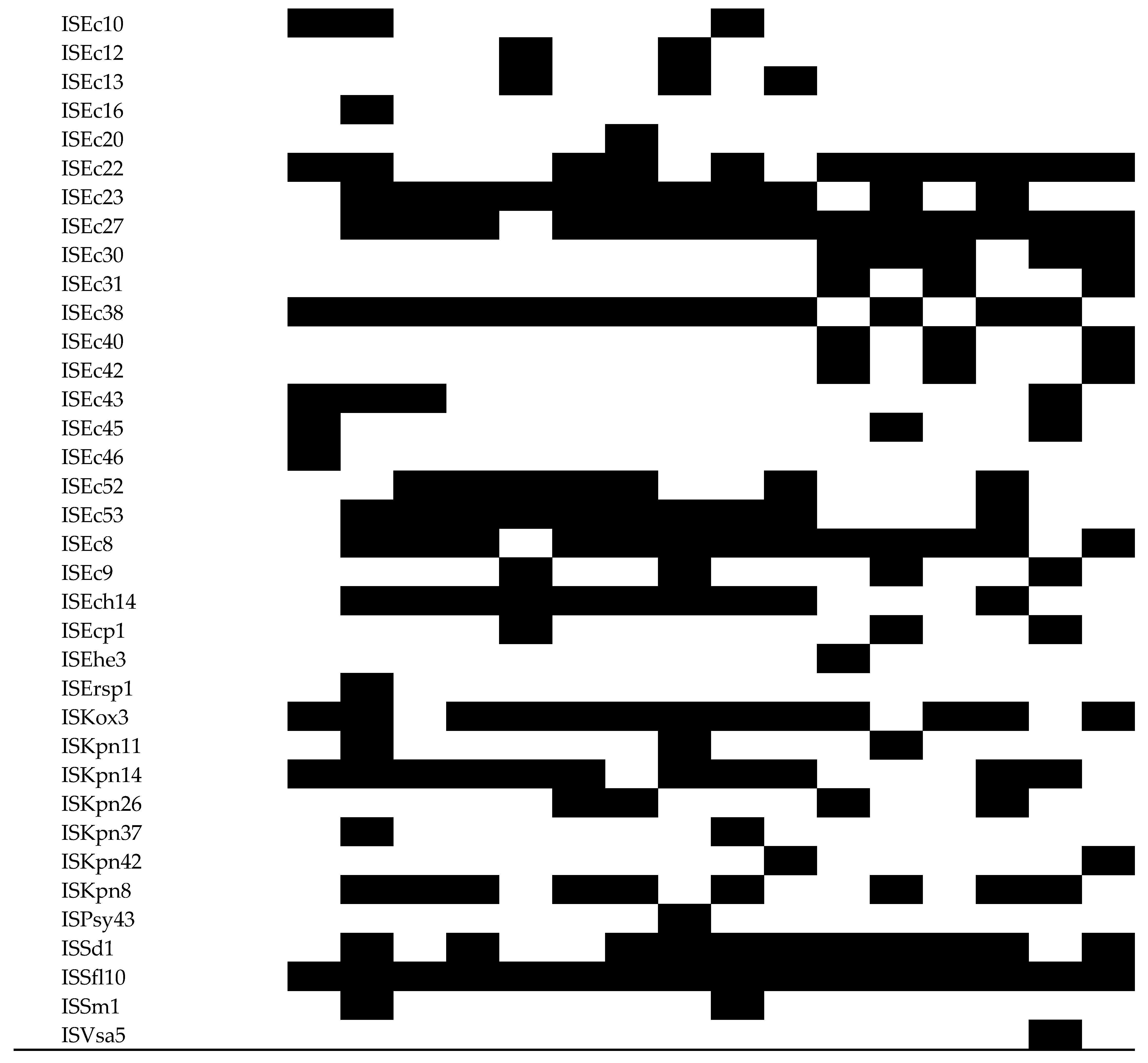
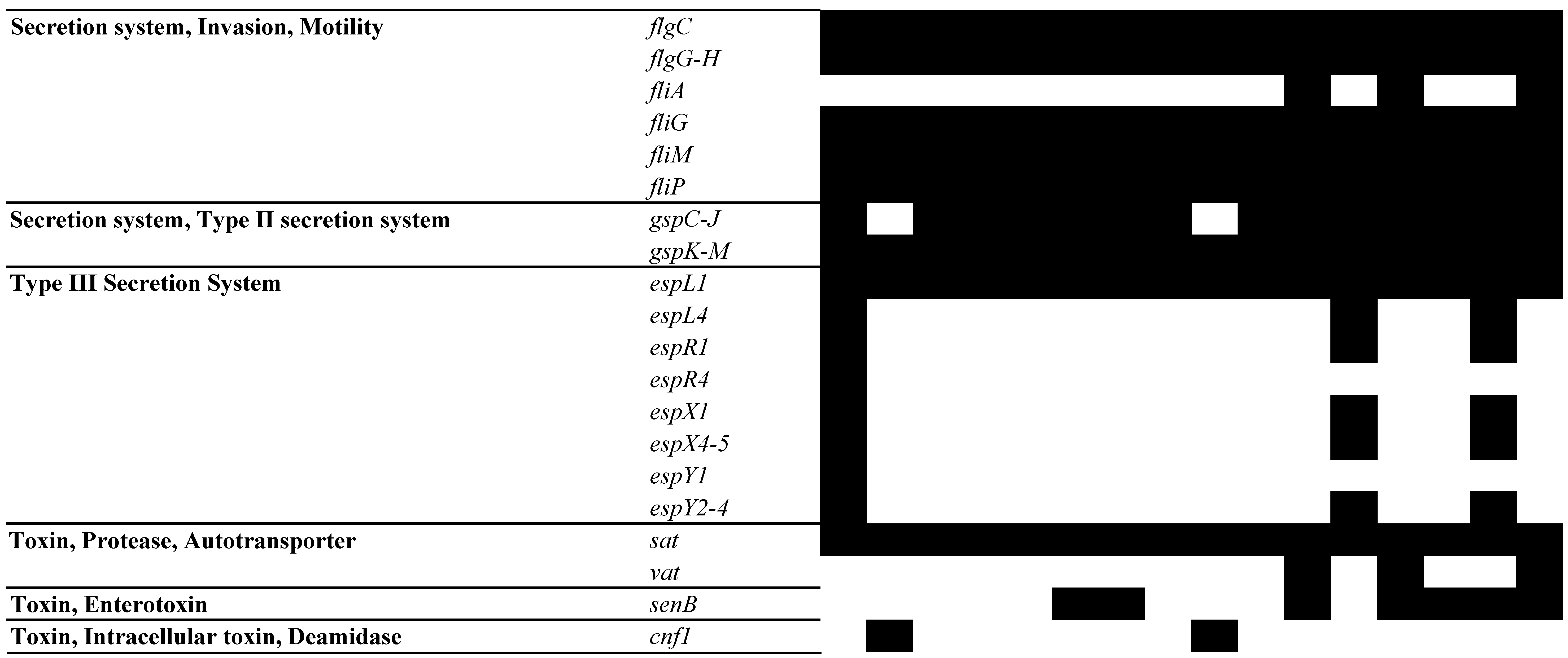
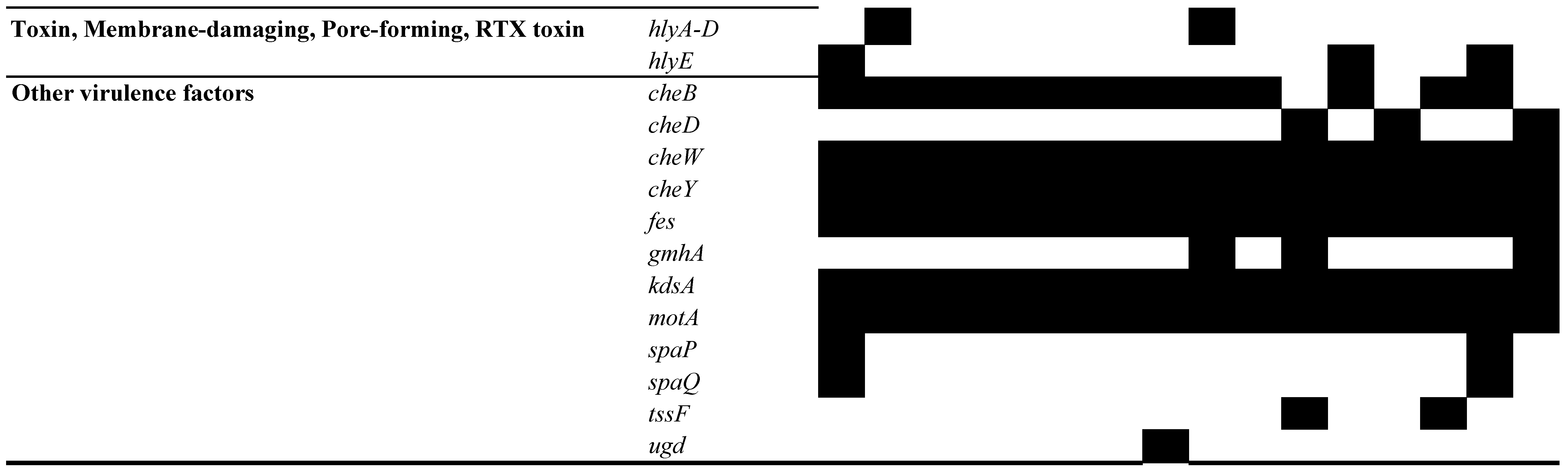
| 2250 | 2284 | 2316 | 2624 | 2842 | 2986 | 3079 | 3385 | 3575 | 3604 | 3636 | 3887 | 4169 | 4331 | 4410 | 4715 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multilocus sequence typing | adk | 18 | 53 | 53 | 53 | 53 | 53 | 53 | 53 | 53 | 53 | 14 | 35 | 14 | 53 | 35 | 14 |
| fumC | 106 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 14 | 37 | 14 | 40 | 37 | 14 | |
| gyrB | 17 | 47 | 47 | 47 | 47 | 47 | 47 | 47 | 47 | 47 | 10 | 29 | 10 | 47 | 29 | 10 | |
| icd | 6 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 200 | 25 | 200 | 13 | 25 | 200 | |
| mdh | 5 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 36 | 17 | 4 | 17 | 36 | 4 | 17 | |
| purA | 5 | 28 | 28 | 28 | 28 | 28 | 28 | 28 | 28 | 28 | 7 | 5 | 7 | 28 | 5 | 7 | |
| recA | 4 | 29 | 29 | 29 | 29 | 29 | 29 | 29 | 29 | 29 | 10 | 73 | 10 | 29 | 73 | 10 | |
| ST | 393 | 131 | 131 | 131 | 131 | 131 | 131 | 131 | 131 | 131 | 1193 | 405 | 1193 | 131 | 405 | 1193 | |
| cgMLST | cgST | 152,922 | 152,915 | 152,921 | 152,912 | 152,913 | 152,924 | 152,918 | 152,917 | 152,920 | 152,911 | 72,154 | 152,916 | 152,923 | 152,914 | 158,995 | 152,919 |
| Sero typing | O type | O25 | O25 | O25 | O25 | O25 | O25 | O25 | O25 | O25 | O25 | O75 | O102 | O75 | O25 | O102 | O75 |
| H type | H1 | H4 | H4 | H4 | H4 | H4 | H4 | H4 | H4 | H4 | H5 | H6 | H5 | H4 | H6 | H5 | |
| CH typing | FumC | 106 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 14 | 37 | 14 | 40 | 37 | 14 |
| FimH | 54 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 64 | 29 | 64 | 30 | 29 | 64 |
| Resistant | Intermediate | Susceptible | |
|---|---|---|---|
| AZR | 7 | 2 | 7 |
| GEN | 15 | 1 | 0 |
| KAN | 10 | 5 | 1 |
| STR | 4 | 0 | 12 |
| AMP | 16 | 0 | 0 |
| CEF | 16 | 0 | 0 |
| CFP | 6 | 4 | 6 |
| IMI | 5 | 4 | 7 |
| CHL | 0 | 0 | 16 |
| NAL | 16 | 0 | 0 |
| TET | 13 | 3 | 1 |
| DOX | 6 | 4 | 6 |
| FOS | 0 | 0 | 16 |
| TMP-SMX | 7 | 1 | 8 |
| POL | 15 | 0 | 1 |
| Overexpression of Efflux Pump Genes | Alterations of Outer Membrane Protein | ||||||
|---|---|---|---|---|---|---|---|
| Strains | Ciprofloxacin | Ciprofloxacin + PAβN (100 ug/mL) | acrA | acrB | mdfA | norE | ompF |
| 2250 | 32 | 32 | 3.18 | 4.97 | 14.61 | 4.59 | 2.24 |
| 2284 | 256 | 128 | 1.90 | 3.20 | 2.91 | 3.09 | −1.43 |
| 2316 | 256 | 256 | 2.93 | 2.85 | 3.28 | 3.77 | −1.29 |
| 2624 | 64 | 64 | 1.62 | 1.34 | −1.65 | 1.76 | −2.75 |
| 2842 | 64 | 64 | 1.50 | 1.94 | 3.42 | 3.12 | −1.95 |
| 2986 | 256 | 128 | 1.71 | 1.89 | −1.35 | 2.12 | −2.61 |
| 3079 | 128 | 64 | 1.35 | 3.01 | 1.56 | 1.98 | −2.17 |
| 3385 | 256 | 128 | 1.70 | 1.43 | −1.67 | 1.58 | −2.77 |
| 3575 | 256 | 128 | −1.16 | −1.17 | −1.48 | 1.53 | −2.58 |
| 3604 | 128 | 128 | 2.28 | 2.69 | 1.86 | 3.43 | −2.73 |
| 3636 | 256 | 128 | 2.83 | 4.29 | 4.33 | 2.43 | −1.07 |
| 3887 | 256 | 64 | 3.54 | 4.92 | 4.85 | 4.02 | −14.61 |
| 4169 | 256 | 256 | 3.09 | 4.18 | 2.88 | 3.03 | −1.40 |
| 4331 | 256 | 256 | 2.12 | 1.76 | 1.74 | 2.57 | −2.09 |
| 4410 | 128 | 32 | 3.91 | 4.06 | 1.18 | 2.08 | −3.64 |
| 4715 | 256 | 64 | 1.18 | 1.32 | −2.45 | −1.57 | −3.18 |
| Strains | Plasmid | Composite Transposons | Transposons | Miniature Inverted Repeats | Antibiotic Resistance Genes |
|---|---|---|---|---|---|
| 2250 | Col(BS512), Col156, IncFIA, IncFIB, IncP1 | cn_5813_IS911 | TnpA_Tn3, TnpR_Tn3 | MITEEc1 | blaTEM-1B |
| 2284 | Col(BS512), Col156, IncFIA, IncFIB, IncFII | cn_4876_IS629, cn_15494_IS629 | Tn5403, TnpA_Tn5403, TnpA_TnAs1, TnpR_Tn5403 | MITEEc1 | aac(3)-Ila, tet(A) |
| 2316 | Col(BS512), Col(MG828), Col156, IncFIA, IncFIB, IncFII | MITEEc1 | |||
| 2624 | Col(BS512), Col(MG828), Col156, IncFIA, IncFII | TnpA_Tn3 | MITEEc1 | ||
| 2842 | Col(BS512), Col156, IncFIA, IncFIB, IncFII | MITEEc1 | ant(2″)-Ia, ant(3″)-Ia, blaCTX-M-14, cmlA1, mph(A), qacE, sul1 | ||
| 2986 | Col(BS512), Col156, IncFIA, IncFIB, IncFII | MITEEc1 | aadA5, dfrA17, mph(A), qacE, sul1 | ||
| 3079 | Col(BS512), Col(MG828), Col156, IncFIA, IncFIB, IncFII | MITEEc1 | aadA5, aph(3″)-Ib, aph(6)-Id, dfrA17, mph(A), qacE, sul2, tet(A) | ||
| 3385 | Col(BS512), Col156, IncFIA, IncFIB(K), IncFII | Tn5403, TnpA_ISPa38, TnpA_Tn3, TnpA_Tn3000, TnpA_Tn5403, TnpR_Tn3, TnpR_Tn5403 | MITEEc1 | aadA1, ant(2″)-Ia, aph(3″)-Ib, aph(6)-Id, blaCTX-M-15, blaTEM-1B, cmlA1, qacE, qnrB1, sul2 | |
| 3575 | Col(BS512), Col(MG828), Col156, IncFIA, IncFIB, IncFII, IncX1 | Tn5403, TnpA_Tn5403, TnpR_Tn5403 | MITEEc1 | ||
| 3604 | Col(BS512), IncFIA, IncFIB, IncFII | Tn5403, TnpA_Tn5403, TnpR_Tn20, TnpR_Tn5403 | MITEEc1 | aadA5, dfrA17, mph(A), qacE, sul1 | |
| 3636 | Col(BS512), Col156, IncFIA, IncFIB, IncI1-I(α) | MITEEc1 | |||
| 3887 | Col(BS512), Col(MG828), Col156, IncFIA, IncFIB, IncFII | cn_3324_ISEc1 | TnpA_Tn3 | MITEEc1 | aac(3)-Ila, aadA5, blaCTX-M-15, dfrA17, mph(A), qacE, sul1 |
| 4169 | Col(BS512), Col156, IncFIA, IncFIB | cn_1487_IS629, cn_1794_IS26, cn_8908_IS911, cn_8908_ISEc31 | MITEEc1 | ||
| 4331 | Col(BS512), Col156, IncFIA, IncFIB, IncFII | MITEEc1 | blaTEM-1B | ||
| 4410 | Col(BS512), Col156, IncFIA, IncFIB | cn_3324_ISEc1, cn_5813_IS911 | TnpA_Tn3, TnpR_Tn3 | MITEEc1 | aadA5, blaCTX-M-15, dfrA17, mph(A), qacE, sul1 |
| 4715 | Col(BS512), Col(MG828), Col156, IncFIA, IncFIB, IncQ1 | MITEEc1 | dfrA14, tet(B) |
| Strains | Type | Cas Genes | CRISPR Consensus Repeat | Numbers of Spacer | Repeat Length | Mean Size of Spacers | CRISPR Length |
|---|---|---|---|---|---|---|---|
| 2250 | I, I-E | cas3, cse1, cse2, cas7, cas5, cas6, cas1, cas2 | CGCGTCTTATCAGGCCTACGAGTTCGGTGCTGTGTAGGTCGGATAAGGCGTTCA | 1 | 54 | 42 | 149 |
| GAGTTCCCCGCGCCAGCGGGGATAAACCG | 3 | 29 | 32 | 211 | |||
| GTGTTCCCCGCGTCAGCGGGGATAAACCG | 11 | 29 | 32 | 699 | |||
| CGACCCCCACCATGTCAAGGTGGTGCTCTAACCAACTGAGCTA | 1 | 43 | 38 | 123 | |||
| GTTCACTGCCGTACAGGCAGCTTAGAAA | 3 | 28 | 32 | 207 | |||
| 2284 | ND | ND | GCCGGATGCGGCGTGAACGCCTTATCCGGCCTACAAAAGAAATGCAG | 1 | 47 | 48 | 141 |
| AGTTCACTGCCGTACAGGCAGCT | 1 | 23 | 37 | 82 | |||
| CGACCCCCACCATGTCAAGGTGGTGCTCTAACCAACTGAGCTA | 1 | 43 | 38 | 123 | |||
| 2316 | I | cas3 | AGTTCACTGCCGTACAGGCAGCT | 1 | 23 | 37 | 82 |
| AATGCCTGATGCGACGCTTGTCGCGTCTTATCATGCCTACAAGT | 1 | 44 | 57 | 144 | |||
| 2624 | I | cas3 | GCCGGATGCGGCGTGAACGCCTTATCCGGCCTACAAAAGAAATGCAG | 1 | 47 | 48 | 141 |
| CCACCTTTTTTACCTGCTTCAGATGC | 1 | 26 | 40 | 91 | |||
| AGTTCACTGCCGTACAGGCAGCT | 1 | 23 | 37 | 82 | |||
| 2842 | I | cas3 | CGACCCCCACCATGTCAAGGTGGTGCTCTAACCAACTGAGCTA | 1 | 43 | 38 | 123 |
| ATCTGCCTGTACGGCAGTGAACT | 1 | 23 | 37 | 82 | |||
| 2986 | ND | ND | CCACCTTTTTTACCTGCTTCAGATGC | 1 | 26 | 40 | 91 |
| ATCTGCCTGTACGGCAGTGAACT | 1 | 23 | 37 | 82 | |||
| 3079 | I | cas3 | CGACCCCCACCATGTCAAGGTGGTGCTCTAACCAACTGAGCTA | 1 | 43 | 38 | 123 |
| AGTTCACTGCCGTACAGGCAGCT | 1 | 23 | 37 | 82 | |||
| GCCGGATGCGGCGTGAACGCCTTATCCGGCCTACAAAAGAAATGCAG | 1 | 47 | 48 | 141 | |||
| 3385 | ND | ND | GCCGGATGCGGCGTGAACGCCTTATCCGGCCTACAAAAGAAATGCAG | 1 | 47 | 48 | 141 |
| AGTTCACTGCCGTACAGGCAGCT | 1 | 23 | 37 | 82 | |||
| CGACCCCCACCATGTCAAGGTGGTGCTCTAACCAACTGAGCTA | 1 | 43 | 38 | 123 | |||
| 3575 | I | cas3 | GCCGGATGCGGCGTGAACGCCTTATCCGGCCTACAAAAGAAATGCAG | 1 | 47 | 48 | 141 |
| AGTTCACTGCCGTACAGGCAGCT | 1 | 23 | 37 | 82 | |||
| CCACCTTTTTTACCTGCTTCAGATGC | 1 | 26 | 40 | 91 | |||
| 3604 | ND | ND | CGACCCCCACCATGTCAAGGTGGTGCTCTAACCAACTGAGCTA | 1 | 43 | 38 | 123 |
| GCCGGATGCGGCGTGAACGCCTTATCCGGCCTACAAAAGAAATGCAG | 1 | 47 | 48 | 141 | |||
| AGTTCACTGCCGTACAGGCAGCT | 1 | 23 | 37 | 82 | |||
| 3636 | I | cas3 | GTTCACTGCCGTACAGGCAGCTTAGAAA | 2 | 28 | 32 | 147 |
| CCGAGCCGTAGGCCGGATAAGGCGTTCACGC | 1 | 31 | 56 | 117 | |||
| CGACCCCCACCATGTCAAGGTGGTGCTCTAACCAACTGAGCTA | 1 | 43 | 38 | 123 | |||
| TGAACGCCTTATCCGACCTACACAGCACTGAACTCGTAGGCCTGATAAGACGCG | 1 | 54 | 42 | 149 | |||
| 3887 | I, I-E | cas3, cse1, cse2, cas7, cas5, cas6, cas1, cas2 | TGAACGCCTTATCCGACCTACACAGCACTGAACTCGTAGGCCTGATAAGACGCG | 1 | 54 | 42 | 149 |
| GTGTTCCCCGCGCCAGCGGGGATAAA | 9 | 26 | 35 | 574 | |||
| CCACCTTTTTTACCTGCTTCAGATGC | 1 | 26 | 40 | 91 | |||
| CAGCGTCGCATCAGGCATTGTGCACGATTGCCGGATGCGGCGTGAACGCCTT | 1 | 52 | 47 | 150 | |||
| 4169 | I | cas3 | TTTCTAAGCTGCCTGTACGGCAGTGAAC | 2 | 28 | 32 | 147 |
| ACGCTGCCGCGTCTTATCGGGCCTACAAAAGTTCTGAACCGT | 1 | 42 | 49 | 132 | |||
| TTGATTGCCGGATGCGGCACGAGTGCCTTATCCGGCCTAC | 2 | 40 | 60.5 | 240 | |||
| CGCGTCTTATCAGGCCTACGAGTTCGGTGCTGTGTAGGTCGGATAAGGCGTTCA | 1 | 54 | 42 | 149 | |||
| CCGAGCCGTAGGCCGGATAAGGCGTTCACGC | 1 | 31 | 56 | 117 | |||
| CGACCCCCACCATGTCAAGGTGGTGCTCTAACCAACTGAGCTA | 1 | 43 | 38 | 123 | |||
| 4331 | ND | ND | CCACCTTTTTTACCTGCTTCAGATGC | 1 | 26 | 40 | 91 |
| ATCTGCCTGTACGGCAGTGAACT | 1 | 23 | 37 | 82 | |||
| 4410 | I, I-E | cas3, cse1, cse2, cas7, cas5, cas6, cas1, cas2 | AAGGCGTTCACGCCGCATCCGGCAATCGTGCATAATGCCTGATGCGACGCTG | 1 | 52 | 47 | 150 |
| CCACCTTTTTTACCTGCTTCAGATGC | 1 | 26 | 40 | 91 | |||
| GTGTTCCCCGCGCCAGCGGGGATAAA | 9 | 26 | 35 | 574 | |||
| TGAACGCCTTATCCGACCTACACAGCACTGAACTCGTAGGCCTGATAAGACGCG | 1 | 54 | 42 | 149 | |||
| CGACCCCCACCATGTCAAGGTGGTGCTCTAACCAACTGAGCTA | 1 | 43 | 38 | 123 | |||
| CCCAATTAAGTGAAGAGCAGGTGACGAAGTTACTGCATCGCAAA | 1 | 44 | 58 | 145 | |||
| GAGTTCCCCGCGCCAGCGGGGATAAACCG | 10 | 29 | 32 | 638 | |||
| 4715 | ND | ND | TTTGTAGGCCTGATAAGACGCGCCAGCGTCGCATCAGGC | 1 | 39 | 49 | 126 |
| GTTCACTGCCGTACAGGCAGCTTAGAAA | 2 | 28 | 32 | 147 | |||
| GTAGGCCGGATAAGGCACTTGTGCCGCATCCGGCA | 1 | 35 | 57 | 126 | |||
| TGAACGCCTTATCCGACCTACACAGCACTGAACTCGTAGGCCTGATAAGACGCG | 1 | 54 | 42 | 149 | |||
| ACGCTGCCGCGTCTTATCGGGCCTACAAAAGTTCTGAACCGT | 1 | 42 | 49 | 132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, K.; Nawaz, M.; Park, M.; Chon, J.; Khan, S.A.; Alotaibi, K.; Khan, A.A. Comprehensive Genomic Analysis of Uropathogenic E. coli: Virulence Factors, Antimicrobial Resistance, and Mobile Genetic Elements. Pathogens 2024, 13, 794. https://doi.org/10.3390/pathogens13090794
Sung K, Nawaz M, Park M, Chon J, Khan SA, Alotaibi K, Khan AA. Comprehensive Genomic Analysis of Uropathogenic E. coli: Virulence Factors, Antimicrobial Resistance, and Mobile Genetic Elements. Pathogens. 2024; 13(9):794. https://doi.org/10.3390/pathogens13090794
Chicago/Turabian StyleSung, Kidon, Mohamed Nawaz, Miseon Park, Jungwhan Chon, Saeed A. Khan, Khulud Alotaibi, and Ashraf A. Khan. 2024. "Comprehensive Genomic Analysis of Uropathogenic E. coli: Virulence Factors, Antimicrobial Resistance, and Mobile Genetic Elements" Pathogens 13, no. 9: 794. https://doi.org/10.3390/pathogens13090794
APA StyleSung, K., Nawaz, M., Park, M., Chon, J., Khan, S. A., Alotaibi, K., & Khan, A. A. (2024). Comprehensive Genomic Analysis of Uropathogenic E. coli: Virulence Factors, Antimicrobial Resistance, and Mobile Genetic Elements. Pathogens, 13(9), 794. https://doi.org/10.3390/pathogens13090794








