Metabolic Footprint of Treponema phagedenis and Treponema pedis Reveals Potential Interaction Towards Community Succession and Pathogenesis in Bovine Digital Dermatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolates and Culture Medium
2.2. Metabolomic Profiling
2.3. Data Analysis
3. Results
3.1. Organic Acids (OAs)
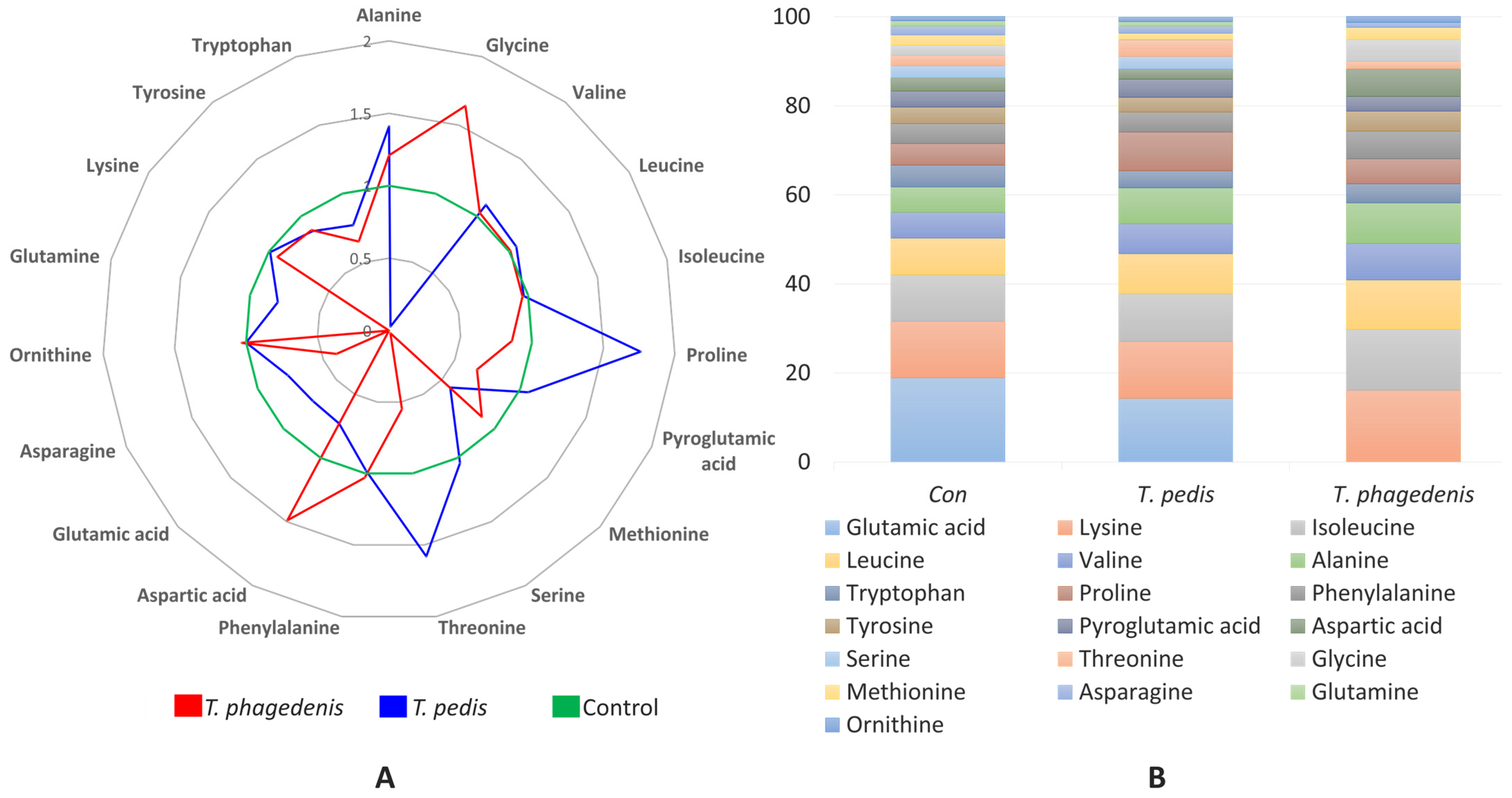
3.2. Amino Acids (AAs)
3.3. Fatty Acids (FAs/SCFAs)
4. Discussion
4.1. Organic Acids
4.2. Amino Acids
4.3. Fatty Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lubbe, A.; Bowen, B.P.; Northen, T. Exometabolomic Analysis of Cross-Feeding Metabolites. Metabolites 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Zelezniak, A.; Andrejev, S.; Ponomarova, O.; Mende, D.R.; Bork, P.; Patil, K.R. Metabolic Dependencies Drive Species Co-Occurrence in Diverse Microbial Communities. Proc. Natl. Acad. Sci. USA 2015, 112, 6449–6454. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.A.; Vilchez, S. Cooperation and Bacterial Pathogenicity: An Approach to Social Evolution. Rev. Chil. Hist. Nat. 2014, 87, 14. [Google Scholar] [CrossRef][Green Version]
- Gabrilska, R.A.; Rumbaugh, K.P. Biofilm Models of Polymicrobial Infection. Future Microbiol. 2015, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Krull, A.C.; Shearer, J.K.; Gorden, P.J.; Cooper, V.L.; Phillips, G.J.; Plummera, P.J. Deep Sequencing Analysis Reveals Temporal Microbiota Changes Associated with Development of Bovine Digital Dermatitis. Infect. Immun. 2014, 82, 3359–3373. [Google Scholar] [CrossRef]
- Espiritu, H.M.; Mamuad, L.L.; Kim, S.; Jin, S.; Lee, S.; Kwon, S.; Cho, Y. Microbiome Shift, Diversity, and Overabundance of Opportunistic Pathogens in Bovine Digital Dermatitis Revealed by 16S RRNA Amplicon Sequencing. Animals 2020, 10, 1798. [Google Scholar] [CrossRef]
- Moreira, T.F.; Facury Filho, E.J.; Carvalho, A.U.; Strube, M.L.; Nielsen, M.W.; Klitgaard, K.; Jensen, T.K. Pathology and Bacteria Related to Digital Dermatitis in Dairy Cattle in All Year-Round Grazing System in Brazil. PLoS ONE 2018, 13, e0193870. [Google Scholar] [CrossRef]
- Bay, V.; Griffiths, B.; Carter, S.; Evans, N.J.; Lenzi, L.; Bicalho, R.C.; Oikonomou, G. 16S RRNA Amplicon Sequencing Reveals a Polymicrobial Nature of Complicated Claw Horn Disruption Lesions and Interdigital Phlegmon in Dairy Cattle. Sci. Rep. 2018, 8, 15529. [Google Scholar] [CrossRef]
- Zinicola, M.; Lima, F.; Lima, S.; Machado, V.; Gomez, M. Altered Microbiomes in Bovine Digital Dermatitis Lesions, and the Gut as a Pathogen Reservoir. PLoS ONE 2015, 10, e0120504. [Google Scholar] [CrossRef]
- Schrank, K.; Choi, B.K.; Grund, S.; Moter, A.; Heuner, K.; Nattermann, H.; Göbel, U.B. Treponema brennaborense sp. nov., a Novel Spirochaete Isolated from a Dairy Cow Suffering from Digital Dermatitis. Int. J. Syst. Bacteriol. 1999, 49, 43–50. [Google Scholar] [CrossRef]
- Nally, J.E.; Hornsby, R.L.; Alt, D.P.; Whitelegge, J.P. Phenotypic and Proteomic Characterization of Treponemes Associated with Bovine Digital Dermatitis. Vet. Microbiol. 2019, 235, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Demirkan, I.; Murray, R.D.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Treponema pedis sp. nov., a Spirochaete Isolated from Bovine Digital Dermatitis Lesions. Int. J. Syst. Evol. Microbiol. 2009, 59, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, P.; Brodard, I.; Alsaaod, M.; Steiner, A.; Stoffel, M.H.; Jores, J. Treponema phagedenis (Ex Noguchi 1912) Brumpt 1922 sp. nov., nom. rev., Isolated from Bovine Digital Dermatitis. Int. J. Syst. Evol. Microbiol. 2020, 70, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Staton, G.J.; Clegg, S.R.; Ainsworth, S.; Armstrong, S.; Carter, S.D.; Radford, A.D.; Darby, A.; Wastling, J.; Hall, N.; Evans, N.J. Dissecting the Molecular Diversity and Commonality of Bovine and Human Treponemes Identifies Key Survival and Adhesion Mechanisms. PLoS Pathog. 2021, 17, e1009464. [Google Scholar] [CrossRef]
- Nielsen, M.W.; Strube, M.L.; Isbrand, A.; Al-Medrasi, W.D.H.M.; Boye, M.; Jensen, T.K.; Klitgaard, K. Potential Bacterial Core Species Associated with Digital Dermatitis in Cattle Herds Identified by Molecular Profiling of Interdigital Skin Samples. Vet. Microbiol. 2016, 186, 139–149. [Google Scholar] [CrossRef]
- Demirkan, I.; Evans, N.J.; Singh, P.; Brown, J.M.; Getty, B.; Carter, S.D.; Timofte, D.; Hart, C.A.; Vink, W.D.; Birtles, R.J.; et al. Association of Unique, Isolated Treponemes with Bovine Digital Dermatitis Lesions. J. Clin. Microbiol. 2009, 47, 689–696. [Google Scholar] [CrossRef]
- Espiritu, H.M.; Mamuad, L.L.; Jin, S.; Kim, S.; Kwon, S.; Lee, S.; Lee, S.; Cho, Y. Genotypic and Phenotypic Characterization of Treponema phagedenis from Bovine Digital Dermatitis. Microorganisms 2020, 8, 1520. [Google Scholar] [CrossRef]
- Espiritu, H.; Mamuad, L.; Valete, E.J.; Jung, M.; Lee, S.S.; Cho, Y.L. Complete Genome Sequence of Treponema pedis GNW45 Isolated from Dairy Cattle with Active Bovine Digital Dermatitis in Korea. J. Anim. Sci. Technol. 2023. pISSN: 2055-0391, eISSN: 2672-0191. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.H.; Oh, S.J.; Lee, H.S.; Ji, M.; Choi, S.; Lee, S.S.; Paik, M.J. Metabolomic Analysis of Organic Acids, Amino Acids, and Fatty Acids in Plasma of Hanwoo Beef on a High-Protein Diet. Metabolomics 2020, 16, 114. [Google Scholar] [CrossRef]
- Silva, L.P.; Northen, T.R. Exometabolomics and MSI: Deconstructing How Cells Interact to Transform Their Small Molecule Environment. Curr. Opin. Biotechnol. 2015, 34, 209–216. [Google Scholar] [CrossRef]
- Freilich, S.; Zarecki, R.; Eilam, O.; Segal, E.S.; Henry, C.S.; Kupiec, M.; Gophna, U.; Sharan, R.; Ruppin, E. Competitive and Cooperative Metabolic Interactions in Bacterial Communities. Nat. Commun. 2011, 2, 589. [Google Scholar] [CrossRef]
- Doelle, H.W. Carbohydrate Metabolism. In Bacterial Metabolism; Elsevier: Amsterdam, The Netherlands, 1975; pp. 208–311. [Google Scholar]
- Kreth, J.; Lengeler, J.W.; Jahreis, K. Characterization of Pyruvate Uptake in Escherichia Coli K-12. PLoS ONE 2013, 8, e67125. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.C.; Baseman, J.B. Carbon Sources Utilized by Virulent Treponema pallidum. Infect. Immun. 1975, 12, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Dong, Z.; Xu, X.; Cochran, D.L.; Ebersole, J.L. Role of Glutathione Metabolism of Treponema denticola in Bacterial Growth and Virulence Expression. Infect. Immun. 2002, 70, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Blötz, C.; Stülke, J. Glycerol Metabolism and Its Implication in Virulence in Mycoplasma. FEMS Microbiol. Rev. 2017, 41, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.J.; Wu, L.; Huang, W.; Chen, C.; Chen, Y.; Lu, X.L.; Zhang, X.L.; Yang, B.F.; Dong, D.L. Glycolic Acid Modulates the Mechanical Property and Degradation of Poly(Glycerol, Sebacate, Glycolic Acid). J. Biomed. Mater. Res. A 2010, 92, 332–339. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, F.; Thomas, S.C.; Zhang, Y.; Paul, B.; Sakilam, S.; Chae, S.; Li, P.; Almeter, C.; Kamer, A.R.; et al. Targeting the Succinate Receptor Effectively Inhibits Periodontitis. Cell Rep. 2022, 40, 111389. [Google Scholar] [CrossRef]
- Rotstein, O.D.; Pruett, T.L.; Fiegel, V.D.; Nelson, R.D.; Simmons, R.L. Succinic Acid, a Metabolic by-Product of Bacteroides Species, Inhibits Polymorphonuclear Leukocyte Function. Infect. Immun. 1985, 48, 402. [Google Scholar] [CrossRef]
- Sharma, A. Virulence Mechanisms of Tannerella forsythia. Periodontol. 2000 2010, 54, 106. [Google Scholar] [CrossRef]
- Konze, S.A.; Abraham, W.R.; Goethe, E.; Surges, E.; Kuypers, M.M.M.; Hoeltig, D.; Meens, J.; Vogel, C.; Stiesch, M.; Valentin-Weigand, P.; et al. Link between Heterotrophic Carbon Fixation and Virulence in the Porcine Lung Pathogen Actinobacillus pleuropneumoniae. Infect. Immun. 2019, 87, 10–1128. [Google Scholar] [CrossRef]
- Pierzynowski, S.; Pierzynowska, K. Alpha-Ketoglutarate, a Key Molecule Involved in Nitrogen Circulation in Both Animals and Plants, in the Context of Human Gut Microbiota and Protein Metabolism. Adv. Med. Sci. 2022, 67, 142–147. [Google Scholar] [CrossRef]
- Sidiq, K.R.; Chow, M.W.; Zhao, Z.; Daniel, R.A. Alanine Metabolism in Bacillus Subtilis. Mol. Microbiol. 2020, 115, 739–757. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J.; et al. Amino Acids as Mediators of Metabolic Cross Talk between Host and Pathogen. Front. Immunol. 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Citterio, F.; Romano, F.; Meoni, G.; Iaderosa, G.; Grossi, S.; Sobrero, A.; Dego, F.; Corana, M.; Berta, G.N.; Tenori, L.; et al. Changes in the Salivary Metabolic Profile of Generalized Periodontitis Patients after Non-Surgical Periodontal Therapy: A Metabolomic Analysis Using Nuclear Magnetic Resonance Spectroscopy. J. Clin. Med. 2020, 9, 3977. [Google Scholar] [CrossRef] [PubMed]
- Szafrański, S.P.; Deng, Z.L.; Tomasch, J.; Jarek, M.; Bhuju, S.; Meisinger, C.; Kühnisch, J.; Sztajer, H.; Wagner-Döbler, I. Functional Biomarkers for Chronic Periodontitis and Insights into the Roles of Prevotella nigrescens and Fusobacterium nucleatum; a Metatranscriptome Analysis. npj Biofilms Microbiomes 2015, 1, 15017. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.L.; Johnson, R.C.; Peterson, D. Metabolism of Common Substrates by the Reiter Strain of Treponema pallidum. Infect. Immun. 1971, 3, 727–734. [Google Scholar] [CrossRef]
- Miao, R.; Fieldsteel, A.H. Genetics of Treponema: Relationship between Treponema pallidum and Five Cultivable Treponemes. J. Bacteriol. 1978, 133, 101–107. [Google Scholar] [CrossRef]
- Silva Junior, A.R.D.; Semenoff Segundo, A.; Semenoff, T.A.D.V.; Silva, N.F.D.; CoporossiI, C. Effect of Glutamine Ingestion on the Progression of Induced Periodontitis: Experimental Study in Rats. Rev. Odontol. UNESP 2018, 47, 119–123. [Google Scholar] [CrossRef]
- Téllez, N.; Aguilera, N.; Quiñónez, B.; Silva, E.; González, L.E.; Hernández, L. Arginine and Glutamate Levels in the Gingival Crevicular Fluid from Patients with Chronic Periodontitis. Braz. Dent. J. 2008, 19, 318–322. [Google Scholar] [CrossRef][Green Version]
- Kang, D.; Shi, B.; Erfe, M.C.; Craft, N.; Li, H. Vitamin B12 Modulates the Transcriptome of the Skin Microbiota in Acne Pathogenesis. Sci. Transl. Med. 2015, 7, 293ra103. [Google Scholar] [CrossRef]
- Tsuchida, S.; Nakayama, T. Metabolomics Research in Periodontal Disease by Mass Spectrometry. Molecules 2022, 27, 2864. [Google Scholar] [CrossRef]
- Tan, K.H.; Seers, C.A.; Dashper, S.G.; Mitchell, H.L.; Pyke, J.S.; Meuric, V.; Slakeski, N.; Cleal, S.M.; Chambers, J.L.; McConville, M.J.; et al. Porphyromonas gingivalis and Treponema denticola Exhibit Metabolic Symbioses. PLoS Pathog. 2014, 10, e1003955. [Google Scholar] [CrossRef] [PubMed]
- Dashper, S.G.; Brownfield, L.; Slakeski, N.; Zilm, P.S.; Rogers, A.H.; Reynolds, E.C. Sodium Ion-Driven Serine/Threonine Transport in Porphyromonas gingivalis. J. Bacteriol. 2001, 183, 4142–4148. [Google Scholar] [CrossRef]
- Jelsbak, L.; Hartman, H.; Schroll, C.; Rosenkrantz, J.T.; Lemire, S.; Wallrodt, I.; Thomsen, L.E.; Poolman, M.; Kilstrup, M.; Jensen, P.R.; et al. Identification of Metabolic Pathways Essential for Fitness of Salmonella Typhimurium in Vivo. PLoS ONE 2014, 9, e101869. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, L.M.; Moazzez, R.V.; Carpenter, G.H. Evidence for Proline Utilization by Oral Bacterial Biofilms Grown in Saliva. Front. Microbiol. 2021, 11, 619968. [Google Scholar] [CrossRef]
- Fukamachi, H.; Nakano, Y.; Okano, S.; Shibata, Y.; Abiko, Y.; Yamashita, Y. High Production of Methyl Mercaptan by L-Methionine-α-Deamino-γ-Mercaptomethane Lyase from Treponema denticola. Biochem. Biophys. Res. Commun. 2005, 331, 127–131. [Google Scholar] [CrossRef]
- Stephen, A.S.; Millhouse, E.; Sherry, L.; Aduse-Opoku, J.; Culshaw, S.; Ramage, G.; Bradshaw, D.J.; Burnett, G.R.; Allaker, R.P. In Vitro Effect of Porphyromonas gingivalis Methionine Gamma Lyase on Biofilm Composition and Oral Inflammatory Response. PLoS ONE 2016, 11, e0169157. [Google Scholar] [CrossRef]
- De Ciccio, A.; McLaughlin, R.; Chan, E.C.S. Factors Affecting the Formation of Spherical Bodies in the Spirochete Treponema denticola. Oral Microbiol. Immunol. 1999, 14, 384–386. [Google Scholar] [CrossRef]
- Van Horn, K.G.; Smibert, R.M. Fatty Acid Requirement of Treponema denticola and Treponema vincentii. Can. J. Microbiol. 1982, 28, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.K.; Ellermann, M. Long Chain Fatty Acids and Virulence Repression in Intestinal Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2022, 12, 801. [Google Scholar] [CrossRef]
- Wyss, C. Fatty Acids Synthesized by Oral Treponemes in Chemically Defined Media. FEMS Microbiol. Lett. 2007, 269, 70–76. [Google Scholar] [CrossRef][Green Version]
- Teoh, W.P.; Chen, X.; Laczkovich, I.; Alonzo, F. Staphylococcus aureus Adapts to the Host Nutritional Landscape to Overcome Tissue-Specific Branched-Chain Fatty Acid Requirement. Proc. Natl. Acad. Sci. USA 2021, 118, e2022720118. [Google Scholar] [CrossRef] [PubMed]
- Shikama, Y.; Kudo, Y.; Ishimaru, N.; Funaki, M. Possible Involvement of Palmitate in Pathogenesis of Periodontitis. J. Cell. Physiol. 2015, 230, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, F.; Guo, X.; Liu, F.; Liu, Z.; Wu, X.; Zhao, M.; Ma, M.; Liu, H.; Qin, L.; et al. Interception of Host Fatty Acid Metabolism by Mycobacteria under Hypoxia to Suppress Anti-TB Immunity. Cell Discov. 2021, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Krieg, N.R.; Staley, J.T.; Brown, D.R.; Hedlund, B.P.; Paster, B.J.; Ward, N.L.; Ludwig, W.; Whitman, W.B.; Parte, A.C. Bergey’s Manual of Systematic Bacteriology Volume 4: The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes; Springer: New York, NY, USA, 2011; ISBN 9780387950426. [Google Scholar]
- O’Leary, W. Practical Handbook of Microbiology; CRC Press: New York, NY, USA; Washington, DC, USA, 1989; ISBN 9781466587403. [Google Scholar]
- Tax, G.; Urbán, E.; Palotás, Z.; Puskás, R.; Kónya, Z.; Bíró, T.; Kemény, L.; Szabó, K. Propionic Acid Produced by Propionibacterium acnes Strains Contributes to Their Pathogenicity. Acta Derm. Venereol. 2016, 96, 43–49. [Google Scholar] [CrossRef]
- Wilson-Welder, J.H.; Elliott, M.K.; Zuerner, R.L.; Bayles, D.O.; Alt, D.P.; Stanton, T.B. Biochemical and Molecular Characterization of Treponema phagedenis-like Spirochetes Isolated from a Bovine Digital Dermatitis Lesion. BMC Microbiol. 2013, 13, 280. [Google Scholar] [CrossRef]
- Shirasugi, M.; Nakagawa, M.; Nishioka, K.; Yamamoto, T.; Nakaya, T.; Kanamura, N. Relationship between Periodontal Disease and Butyric Acid Produced by Periodontopathic Bacteria. Inflamm. Regen. 2018, 38, 23. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Guo, W.; Li, H.; Lei, L. Periodontitis-Level Butyrate-Induced Ferroptosis in Periodontal Ligament Fibroblasts by Activation of Ferritinophagy. Cell Death Discov. 2020, 6, 119. [Google Scholar] [CrossRef]
- Hou, J.; Xu, J.; Liu, Y.; Zhang, H.; Wang, S.; Jiao, Y.; Guo, L.; Li, S. Sodium Butyrate Inhibits Osteogenesis in Human Periodontal Ligament Stem Cells by Suppressing Smad1 Expression. BMC Oral Health 2022, 22, 301. [Google Scholar] [CrossRef]
- Guan, X.; Li, W.; Meng, H. A Double-Edged Sword: Role of Butyrate in the Oral Cavity and the Gut. Mol. Oral Microbiol. 2021, 36, 121–131. [Google Scholar] [CrossRef]
- VanHook, A.M. Formate for Tumor Progression. Sci. Signal. 2022, 15, eadd1844. [Google Scholar] [CrossRef]
- Hatanaka, K.; Shirahase, Y.; Yoshida, T.; Kono, M.; Toya, N.; Sakasegawa, S.I.; Konishi, K.; Yamamoto, T.; Ochiai, K.; Takashiba, S. Enzymatic Measurement of Short-Chain Fatty Acids and Application in Periodontal Disease Diagnosis. PLoS ONE 2022, 17, e0268671. [Google Scholar] [CrossRef] [PubMed]


| OA (ng/5 µL) | Control | T. pedis | T. phagedenis | SEM | p-Value |
|---|---|---|---|---|---|
| Pyruvic acid | 1258.4 a | 484.2 b | 239.6 b | 64.76 | <0.001 |
| Glycolic acid | 1666.9 a | 1680.9 a | 1302.5 b | 48.0 | <0.001 |
| 2-Hydroxybutyric acid | 8.0 b | 47.5 a | 8.5 b | 0.69 | <0.001 |
| 3-Hydroxypropionic acid | 365.8 a | 322.3 a | 198.5 b | 28.69 | 0.019 |
| Succinic acid | 217 b | 284.9 a | 213.1 b | 8.74 | <0.001 |
| Fumaric acid | 12 b | 16.4 a | 6.2 c | 0.77 | <0.001 |
| Oxaloacetic acid | 17.1 a | 19.8 a | 12.1 b | 0.85 | <0.001 |
| α-Ketoglutaric acid | 34.1 a | 32.8 a | 13.4 b | 1.56 | <0.001 |
| Malic acid | 95.6 | 114.5 | 132.7 | 10.89 | 0.205 |
| 2-Hydroxyglutaric acid | 82.2 | 121.6 | 76.3 | 15.45 | 0.231 |
| OA (ng/2 µL) | Control | T. pedis | T. phagedenis | SEM | p-Value |
|---|---|---|---|---|---|
| Alanine | 59.1 c | 83.3 a | 71.4 b | 52.09 | <0.001 |
| Glycine | 23.4 b | 0.6 c | 38.4 a | 13.79 | <0.001 |
| Valine | 62.3 | 68.7 | 64.4 | 46.39 | 0.556 |
| Leucine | 86.9 | 92.4 | 87.3 | 60.69 | 0.188 |
| Isoleucine | 112.5 | 109.4 | 107.8 | 77.06 | 0.933 |
| Proline | 50.8 b | 89.7 a | 43.9 b | 46.78 | <0.001 |
| Pyroglutamic acid | 39.2 | 41.7 | 26.3 | 24.73 | 0.060 |
| Methionine | 24.4 a | 14.2 b | 21.5 ab | 12.5 | 0.047 |
| Serine | 28.0 a | 29.1 a | 0.6 b | 12.17 | 0.004 |
| Threonine | 24.0 b | 38.1 a | 13.2 b | 18.93 | 0.008 |
| Phenylalanine | 47.0 | 46.1 | 48.6 | 0.75 | 0.165 |
| Aspartic acid | 32.4 ab | 23.7 b | 48.4 a | 3.96 | 0.030 |
| Glutamic acid | 205.8 a | 147.8 a | 0 b | 17.46 | 0.003 |
| Asparagine | 21.4 | 16.4 | 8.6 | 2.29 | 0.078 |
| Ornithine | 10.7 | 10.7 | 11.0 | 0.62 | 0.931 |
| Glutamine | 12.5 a | 10.1 a | 0 b | 0.87 | 0.001 |
| Lysine | 135.4 | 133.4 | 126.6 | 15.01 | 0.925 |
| Tyrosine | 39.8 | 34.5 | 35.0 | 3.66 | 0.633 |
| Tryptophan | 52.0 | 39.9 | 33.9 | 7.35 | 0.383 |
| FA (ng/2 µL) | Control | T. pedis | T. phagedenis | SEM | p-Value |
|---|---|---|---|---|---|
| cis-9-Hexadecenoic acid (C16:1) | 146.9 a | 28.8 b | 18.6 c | 2.12 | <0.001 |
| Palmitic acid (C16:0) | 1115.3 a | 191.4 b | 142.2 b | 79.98 | <0.001 |
| Linoleic acid (C18:2) | 135.1 a | 18.5 c | 70.5 b | 2.29 | <0.001 |
| Oleic acid (C18:1) | 745.1 a | 431.4 b | 386.2 b | 14.24 | <0.001 |
| Octadecanoic acid (C18:0) | 730.9 | 405.7 | 228.4 | 159.48 | 0.203 |
| Octacosanoic acid (C28:0) | 257.0 | 93.4 | 310.7 | 100.56 | 0.523 |
| SCFA (mM) | |||||
| Lactic acid | 26.1 a | 15.28 b | 12.99 b | 2.15 | 0.002 |
| Formic acid | 0 b | 0 b | 5.1 a | 0.85 | <0.001 |
| Acetic acid | 22.08 b | 50.96 a | 26.59 b | 4.72 | <0.001 |
| Propionic acid | 5.73 c | 59.18 a | 28.41 b | 7.79 | <0.001 |
| Butyric acid | 16.19 c | 67.55 b | 116.58 a | 14.50 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espiritu, H.M.; Valete, E.J.P.; Mamuad, L.L.; Jung, M.; Paik, M.-J.; Lee, S.-S.; Cho, Y.-I. Metabolic Footprint of Treponema phagedenis and Treponema pedis Reveals Potential Interaction Towards Community Succession and Pathogenesis in Bovine Digital Dermatitis. Pathogens 2024, 13, 796. https://doi.org/10.3390/pathogens13090796
Espiritu HM, Valete EJP, Mamuad LL, Jung M, Paik M-J, Lee S-S, Cho Y-I. Metabolic Footprint of Treponema phagedenis and Treponema pedis Reveals Potential Interaction Towards Community Succession and Pathogenesis in Bovine Digital Dermatitis. Pathogens. 2024; 13(9):796. https://doi.org/10.3390/pathogens13090796
Chicago/Turabian StyleEspiritu, Hector M., Edeneil Jerome P. Valete, Lovelia L. Mamuad, Myunghwan Jung, Man-Jeong Paik, Sang-Suk Lee, and Yong-Il Cho. 2024. "Metabolic Footprint of Treponema phagedenis and Treponema pedis Reveals Potential Interaction Towards Community Succession and Pathogenesis in Bovine Digital Dermatitis" Pathogens 13, no. 9: 796. https://doi.org/10.3390/pathogens13090796
APA StyleEspiritu, H. M., Valete, E. J. P., Mamuad, L. L., Jung, M., Paik, M.-J., Lee, S.-S., & Cho, Y.-I. (2024). Metabolic Footprint of Treponema phagedenis and Treponema pedis Reveals Potential Interaction Towards Community Succession and Pathogenesis in Bovine Digital Dermatitis. Pathogens, 13(9), 796. https://doi.org/10.3390/pathogens13090796





