Antimicrobial Evaluation of Asphodelus microcephalus Extracts and Fine Powder of Dried Organs Against Fusarium and Oomycetes Responsible for Apple and Peach Decline Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogens Used
2.2. Characteristics of Asphodelus microcephalus
2.3. Preparation of Extracts
2.3.1. Preparation of Aqueous Extracts
2.3.2. Preparation of Methanolic Extracts
2.4. Effect of Aqueous Extracts on Mycelial Growth of Pathogens Associated with Apple and Peach Plants
2.5. Effect of Methanolic Extracts on Mycelial Growth of Pathogens Associated with Apple and Peach Plants
2.6. Effect of Powdered Preparation of Dried A. microcephalus Organs on Disease Severity
2.7. Statistical Analysis
3. Results
3.1. Methanolic Extracts Yield
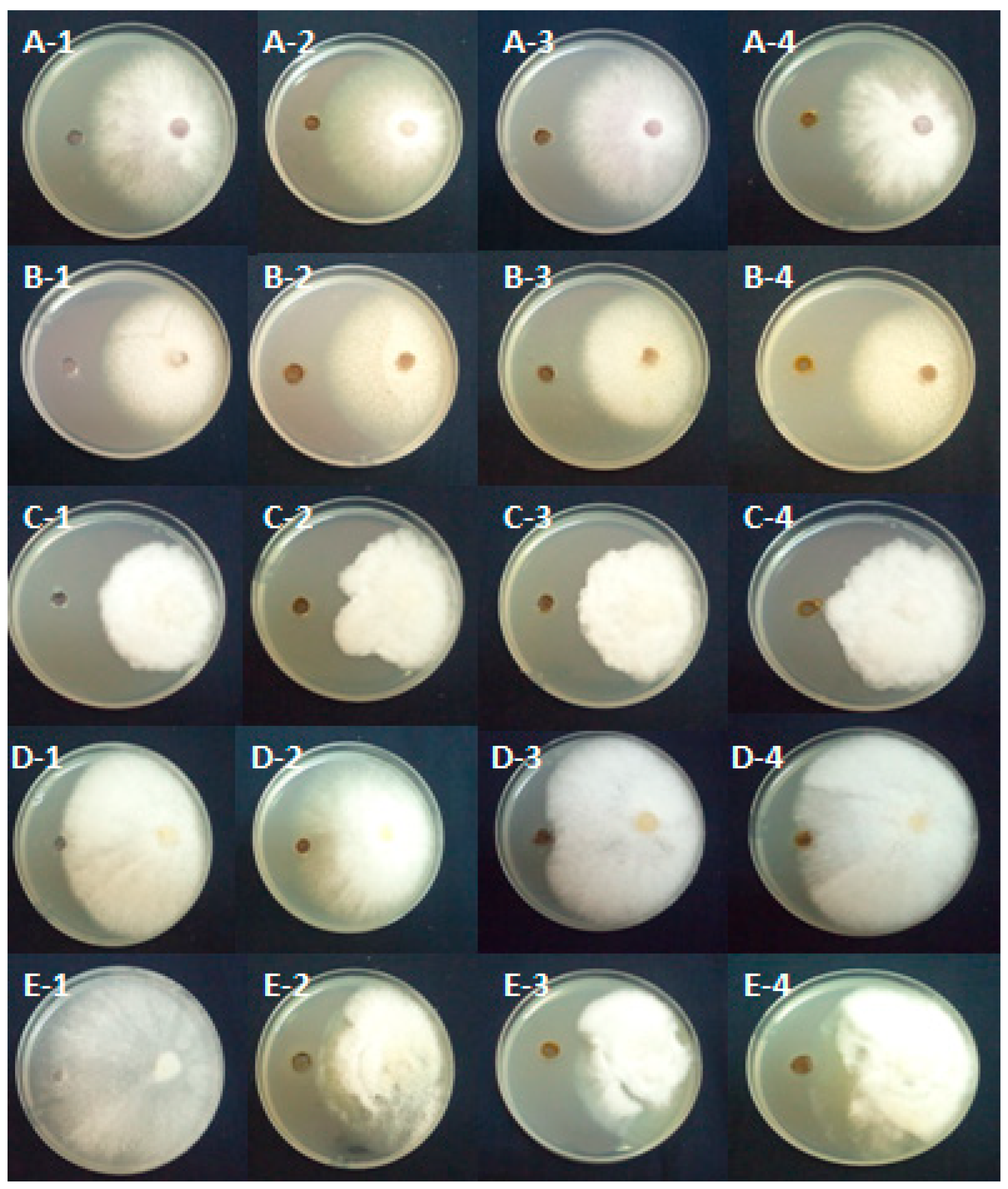
3.2. Effect of Asphodelus microcephalus Aqueous Extracts on the Mycelial Growth of Pathogens Associated with Apple and Peach Plants Decline
3.3. Effect of Asphodelus microcephalus Methanolic Extracts on the Mycelial Growth of Pathogens Associated with Apple and Peach Plants Decline
3.4. Effect of Asphodelus microcephalus In Vivo on Inoculated Peach Seedlings
3.5. Effect of Asphodelus microcephalus In Vivo on Inoculated Apple Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, B.; Zhan, J.; Chen, C. Evolution of a Development Model for Fruit Industry against Background of an Aging Population: Intensive or Extensive Adjustment? Sustainability 2018, 10, 49. [Google Scholar] [CrossRef]
- Mazzola, M.; Andrews, P.K.; Reganold, J.P.; Lévesque, C.A. Frequency, virulence, and metalaxyl sensitivity of Pythium spp. isolated from apple roots under conventional and organic production systems. Plant Dis. 2002, 86, 669–675. [Google Scholar]
- McLeod, A.; Botha, W.J.; Meitz, J.C.; Spies, C.F.J.; Tewoldemedhin, Y.T.; Mostert, L. Morphological and phylogenetic analyses of Pythium species in South Africa. Mycol. Res. 2009, 113, 933–951. [Google Scholar]
- Mannai, S.; Benfradj, N.; Boughalleb-M’Hamdi, N. Occurrence of Globisporangium and Phytopythium species associated with apple and peach seedlings decline in Tunisian nurseries. J. Plant Pathol. 2024, 106, 643–655. [Google Scholar] [CrossRef]
- Zondo, P.T.; Denman, S.; Labuschagne, I.F. Effect of season and aggressiveness of isolates on the response of two apple rootstocks to Phytophthora cactorum infection. Australas. Plant Pathol. 2007, 36, 240–244. [Google Scholar]
- Tewoldemedhin, Y.T.; Mazzola, M.; Spies, C.F.J.; McLeod, A. Characterization of fungi (Fusarium and Rhizoctonia) and oomycetes (Phytophthora and Pythium) associated with apple orchards in South Africa. Eur. J. Plant. Pathol. 2011, 130, 215–229. [Google Scholar]
- Mannai, S.; Benfradj, N.; Horrigue-Raouani, N.; Boughalleb-M’Hamdi, N. Prevalence of Fusarium Species Associated with Peach Decline in Tunisian Nurseries. Microbiol. Res. J. Int. 2018, 23, 1–16. [Google Scholar]
- Mannai, S.; Horrigue-Raouani, N.; Boughalleb-M’Hamdi, N. Characterization of Fusarium species associated with apple decline in Tunisian nurseries. J. Biol. Stud. 2018, 1, 14–34. [Google Scholar]
- Cook, R.J.; Baker, K.F. The Nature and Practice of Biological Control of Plant Pathogens; American Phytopathological Society: St Paul, MN, USA, 1983; 539p. [Google Scholar]
- Watson, R.T.; Albritton, S.; Anderson, O.; Lee-Bapty, S. Methyl Bromide: Its Atmospheric Science, Technology and Economics. In Montreal Protocol Assessment Supplement; United Nations Environment Programme: Nairobi, Kenya, 1992; p. 234. [Google Scholar]
- El-Hamalawi, Z.A.; Menge, J.A.; Adams, C.J. Methods of fosetyl-Al application and phosporous level in avocado tissue needed to control stem canker caused by Phytophthora citricola. Plant Dis. 1995, 79, 770–778. [Google Scholar]
- Whipps, J.M.; Lumsden, R.D. Biological control of Pythium species. Biocontrol. Sci. Technol. 1991, 1, 75–90. [Google Scholar]
- Mannai, S.; Benfradj, N.; Horrigue-Raouani, N.; Boughalleb-M’Hamdi, N. Antifungal activity and growth promotion of three types of compost extracts against Fusarium oxysporum and Fusarium solani associated with peach seedling decline in nurseries. J. Crop. Prot. 2018, 3, 349–363. [Google Scholar]
- Maynard, A.A. Effect of annual amendments of compost on nitrate leaching in nursery stock. Compost. Sci. Util. 1994, 2, 54–55. [Google Scholar]
- Mannai, S.; Benfradj, N.; Karoui, A.; Ben Salem, I.; Fathallah, A.; M’Hamdi, M.; Boughalleb-M’Hamdi, N. Analysis of chemical composition and in vitro and in vivo antifungal activity of Raphanus raphanistrum extracts against Fusarium and Pythiaceae, affecting apple and peach seedlings. Molecules 2021, 26, 2479. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Feeney, P.P. Allelochemics: Chemical Interactions between Species. Science 1971, 171, 757–770. [Google Scholar] [PubMed]
- Abu-Darwish, S.M.; Ateyyat, M. The pharmacological and pesticidal actions of naturally occurring 1, 8-dihydroxyanthraquinones derivatives. World J. Agric. Sci. 2008, 4, 495–505. [Google Scholar]
- García-Sosa, K.; Villarreal-Álvarez, N.; Lübben, P.; Peña-Rodríguez, L.M. Chrysophanol, an Antimicrobial Anthraquinone from the Root Extract of Colubrina greggii. J. Mex. Chem. Soc. 2006, 50, 76–78. [Google Scholar]
- Malmir, M.; Serrano, R.; Caniça, M.; Silva-Lima, B.; Silva, O. A Comprehensive Review on the Medicinal Plants from the Genus Asphodelus. Plants 2018, 7, 20. [Google Scholar] [CrossRef]
- Salhi, N.; Ayesh, S.; Saghir, M.; Terzi, V.; Brahmi, I.; Ghedairi, N.; Bissati, S. Antifungal Activity of Aqueous Extracts of Some Dominant Algerian Medicinal Plants. Biomed Res. Int. Artic. 2017, 2017, 7526291. [Google Scholar]
- Babar, H.B.; Tanveer, A.; Tahir, M.; Aziz, A.; Haqahmad, A.; Nadeem, M.A.; Javaid, M.M. Allelopathic potential of wild onion (Asphodelus tenuifolius) on the germination and seedling growth of chickpea (Cicer arietinum). Weed Biol. Manag. 2009, 9, 146–151. [Google Scholar]
- Abu-Romman, S. Differential Allelopathic Expression of different plant parts of Achillea biebersteinii. Acta Biol. Hung. 2016, 67, 159–168. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Fais, A.; Pintus, F.; Santos-Buelga, C.; Gonzalez-Paramas, A.M.; Piras, V.; Orru, G.; Mameli, A.; Tramontano, E.; Frau, A. Broad-range potential of Asphodelus microcarpus leaves extract for drug development. BMC Microbiol. 2017, 17, 159. [Google Scholar]
- Malmir, M.; Serrano, R.; Lima, K.; Duarte, M.P.; Moreira da Silva, I.; Silva Lima, B.; Caniça, M.; Silva, O. Monographic Quality Parameters and Genotoxicity Assessment of Asphodelus bento-rainhae and Asphodelus macrocarpus Root Tubers as Herbal Medicines. Plants 2022, 11, 3173. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Campanella, P.; Cocco, A.; Arena, C.; Malagnino, G.; Sovereto, D.; Aiuto, R.; Laino, L.; Laneve, E.; Dioguardi, A.; et al. Possible uses of plants of the genus Asphodelus in oral medicine. Biomedicines 2019, 7, 67. [Google Scholar] [CrossRef]
- Javaid, A.; Bashir, A. Radish extracts as natural fungicides for management of Fusarium oxysporum f. sp. lycopersici, the cause of tomato wilt. Pak. J. Bot. 2015, 47, 321–324. [Google Scholar]
- Oumzil, H.; Ghoulami, S.; Rhajaoui, M.; Ilidrissi, A.; Fkih-Tetouani, S.; Faid, M.; Benjouad, A. Antibacterial and antifungal activity of essential oils of Mentha suaveolens. Phytother. Res. 2002, 16, 727–731. [Google Scholar]
- Mazzola, M.; Zhao, X. Brassica juncea seed meal particle size influences chemistry but not soil biology-based suppression of individual agents inciting apple replant disease. Plant Soil 2010, 337, 313–324. [Google Scholar] [CrossRef]
- Santini, A.; Biancalani, F.; Biancalani, F.; Barzanti, G.P.; Capretti, P. Pathogenicity of four Phytophthora species on wild cherry and Italian alder seedlings. J. Phytopathol. 2006, 154, 163–167. [Google Scholar]
- Zöngür, A.; Buzpinar, M.A. AI-assisted antifungal discovery of Aspergillus parasiticus and Aspergillus flavus: Investigating the potential of Asphodelus aestivus, Beta vulgaris, and Morus alba plant leaf extracts. Model Earth Syst. Environ. 2023, 9, 2745–2756. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar]
- Inderjit; Mallik, A.U. Can Kalmia angustifolia interference to black spruce (Picea mariana) be explained by allelopathy? For. Ecol. Manag. 2002, 160, 75–84. [Google Scholar]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Batish, R.D.; Lavanya, K.; Singh, H.P.; Kohli, R.K. Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul. 2007, 51, 119–128. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Gautam, S.S. Allelopathic Interaction of Wild Onion Asphodelus tenuifolius Cav on Germination and Growth of Sorghum sorghum bicolor L. and Maize Zea mays L. Master’s Thesis, Agra University, Agra, India, 2013. [Google Scholar]
- Chung, I.M.; Seigler, D.; Miller, D.A.; Kyung, S.H. Autotoxic compounds from fresh alfalfa leaf extracts: Identification and biological activity. J. Chem. Ecol. 2000, 26, 315–327. [Google Scholar]
- Scrivanti, L.R. Allelopathic potential of Bothriochloa laguroides var. laguroides (DC.) Herter (Poaceae: Andropogoneae). Flora 2010, 205, 302–305. [Google Scholar] [CrossRef]
- Baziramakenga, R.; Leroux, G.D.; Simard, R.R.; Nadeau, P. Allelopathic effects of phenolic acids on nucleic acid and protein levels in soybean seedlings. Canad. J. Bot. 1997, 75, 445–450. [Google Scholar] [CrossRef]
- Mersie, W.; Singh, M. Phenolic acids affect photosynthesis and protein synthesis by isolated leaf cells of velvet-leaf. J. Chem. Ecol. 1993, 19, 1293–1301. [Google Scholar] [CrossRef]
- Yang, C.M.; Chang, I.F.; Lin, S.J.; Chou, C.H. Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: II. Stimulation of consumption-orientation. Bot. Bull. Acad. Sin. 2004, 45, 119–125. [Google Scholar]
- Yang, C.M.; Lee, C.N.; Chou, C.H. Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: I. Inhibition of supply-orientation. Bot. Bull. Acad. Sin. 2002, 43, 299–304. [Google Scholar]


| Species | Isolates | Origins | Sampling Years | GenBank Accession Number |
|---|---|---|---|---|
| F. oxysporum | Fo22 | peach | 2013 | MF993097 |
| F. solani | F48 | peach | 2012 | MF993094 |
| Pythium ultimum | P42 | peach | 2013 | MF993110 |
| Po2 | apple | 2012 | MH260594 | |
| Phytopythium mercuriale | Po26 | apple | 2013 | MF993112 |
| Phytophthora citrophthora | P39 | peach | 2013 | ND |
| Organs | Methanolic Extract Yield (%) |
|---|---|
| Fruits | 6.33 |
| Leaves | 37.80 |
| Roots | 27.35 |
| Pathogens | Doses (%) | Fruits | Leaves | Roots | p-Value *** |
|---|---|---|---|---|---|
| Fusarium solani | 5 | 100.00 ± 0.00 aA* | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | |
| 10 | 100.00 ± 0.00 a**A | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | ||
| 15 | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | ||
| p-value | |||||
| Fusarium oxysporum | 5 | 72.73 ± 0.06 aA | 100.00 ± 0.00 aB | 100.00 ± 0.00 aB | p ≤ 0.05 |
| 10 | 100.00 ± 0.00 bA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | ||
| 15 | 100.00 ± 0.00 bA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | ||
| p-value | p ≤ 0.05 | ||||
| Phytophthora citrophthora | 5 | 51.35 ± 2.79 aA | 100.00 ± 0.00 aB | 69.19 ± 2.07 aA | p ≤ 0.05 |
| 10 | 100.00 ± 0.00 bB | 100.00 ± 0.00 aB | 73.51 ± 1.08 bA | p ≤ 0.05 | |
| 15 | 100.00 ± 0.00 bB | 100.00 ± 0.00 aB | 77.84 ± 2.07 cA | p ≤ 0.05 | |
| p-value | p ≤ 0.05 | ||||
| Pythium ultimum | 5 | 75.00 ± 1.57 aA | 100.00 ± 0.00 aB | 100.00 ± 0.00 aB | p ≤ 0.05 |
| 10 | 100.00 ± 0.00 bA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | ||
| 15 | 100.00 ± 0.00 bA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | ||
| p-value | p ≤ 0.05 | ||||
| Phytopythium mercuriale | 5 | 80.19 ± 1.09 aA | 100.00 ± 0.00 aB | 100.00 ± 0.00 aB | p ≤ 0.05 |
| 10 | 100.00 ± 0.00 bA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | ||
| 15 | 100.00 ± 0.00 bA | 100.00 ± 0.00 aA | 100.00 ± 0.00 aA | ||
| p-value | p ≤ 0.05 |
| Fusarium oxysporum | F. solani | Phytophthora citrophthora | Pythium ultimum | Phytopythium mercuriale | p-Value *** | |
|---|---|---|---|---|---|---|
| PPDF | 0.00 ± 0.00 A*a** | 0.00 ± 0.00 Aa | 19.03 ± 7.94 Bb | 9.09 ± 2.46 Aa | 20.22 ± 5.10 Bb | p ≤ 0.001 |
| PPDL | 3.72 ± 1.57 Bb | 0.00 ± 0.00 Aa | 26.39 ± 1.82 Db | 23.83 ± 2.46 Db | 10.75 ± 2.03 Ca | p ≤ 0.001 |
| PPDR | 3.72 ± 1.57 Ab | 0.00 ± 0.00 Aa | 5.36 ± 5.84 Aa | 14.00 ± 10.42 Ba | 4.67 ± 2.03 Aa | p ≤ 0.05 |
| p-value *** | p ≤ 0.05 | p ≥ 0.05 | p ≤ 0.05 | p ≤ 0.05 | p ≤ 0.05 |
| Pathogens | Parameters | Treatment | Fruits | Leaves | Roots | p-Value *** |
|---|---|---|---|---|---|---|
| F. oxysporum | Root Browning Index | 1W | 2.00 ± 0.00 a*A** | 1.67 ± 0.58 aA | 2.67 ± 0.58 bA | p ≥ 0.05 |
| 8W | 1.67 ± 0.58 aA | 1.67 ± 0.58 aA | 2.00 ± 0.00 abA | p ≥ 0.05 | ||
| NIC | 2.00 ± 0.00 a | 2.00 ± 0.00 a | 2.00 ± 0.00 a | nd | ||
| IC | 1.33 ± 0.43 a | 1.33 ± 0.43 a | 1.33 ± 0.43 a | nd | ||
| p-value | p ≥ 0.05 | p ≥ 0.05 | p ≤ 0.05 | |||
| Disease Index | 1W | 1.67 ± 0.58 aA | 1.33 ± 0.58 abA | 2.33 ± 0.58 aA | p ≥ 0.05 | |
| 8W | 2.33 ± 0.58 aB | 0.67 ± 0.58 bA | 3.33 ± 0.58 aB | p ≤ 0.05 | ||
| NIC | 2.33 ± 0.58 a | 2.33 ± 0.58 a | 2.33 ± 0.58 a | nd | ||
| IC | 2.67 ± 0.83 a | 2.67 ± 0.83 a | 2.67 ± 0.83 a | nd | ||
| p-value | p ≥ 0.05 | p ≤ 0.05 | p ≥ 0.05 | |||
| Height (cm) | 1W | 65.00 ± 2.00 aA | 68.50 ± 8.41 bA | 66.50 ± 0.87 aA | p ≥ 0.05 | |
| 8W | 52.83 ± 2.25 aB | 39.33 ± 2.52 aA | 50.00 ± 3.97 aB | p ≤ 0.05 | ||
| NIC | 62.83 ± 6.79 a | 62.83 ± 6.79 ab | 62.83 ± 6.79 a | nd | ||
| IC | 59.17 ± 13.83 a | 59.17 ± 13.83 ab | 59.17 ± 13.83 a | nd | ||
| p-value | p ≥ 0.05 | p ≤ 0.05 | p ≥ 0.05 | |||
| Root Weight (g) | 1W | 9.53 ± 1.14 bA | 8.74 ± 2.81 bA | 7.50 ± 1.38 bA | p ≥ 0.05 | |
| 8W | 8.73 ± 0.91 bB | 5.90 ± 0.33 abA | 5.04 ± 1.31 abA | p ≤ 0.05 | ||
| NIC | 7.38 ± 2.09 b | 7.38 ± 2.09 b | 7.38 ± 2.09 b | nd | ||
| IC | 2.88 ± 1.52 a | 2.88 ± 1.52 a | 2.88 ± 1.52 a | nd | ||
| p-value | p ≤ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| F. solani | Root browning | 1W | 2.00 ± 0.00 aA | 2.67 ± 0.58 bAB | 3.33 ± 0.58 bB | p ≥ 0.05 |
| 8W | 1.33 ± 0.58 aA | 1.67 ± 0.58 abA | 1.33 ± 0.58 aA | p ≥ 0.05 | ||
| NIC | 1.33 ± 0.58 a | 1.33 ± 0.58 a | 1.33 ± 0.58 a | nd | ||
| IC | 2.00 ± 0.58 a | 2.00 ± 0.58 ab | 2.00 ± 0.00 a | nd | ||
| p-value | p ≥ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| Disease Index | 1W | 3.00 ± 1.00 aA | 3.67 ± 0.58 cA | 3.67 ± 0.58 cA | p ≥ 0.05 | |
| 8W | 1.33 ± 0.58 aA | 0.67 ± 0.58 aA | 0.67 ± 0.58 aA | p ≥ 0.05 | ||
| NIC | 1.67 ± 0.58 a | 1.67 ± 0.58 ab | 1.67 ± 0.58 ab | nd | ||
| IC | 2.33 ± 0.58 a | 2.33 ± 0.58 b | 2.33 ± 0.58 b | nd | ||
| p-value | p ≥ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| Height (cm) | 1W | 72.00 ± 3.50 cB | 70.50 ± 3.00 bB | 58.17 ± 0.76 cA | p ≤ 0.001 | |
| 8W | 45.83 ± 0.29 aC | 41.50 ± 2.29 aB | 36.33 ± 2.75 aA | p ≤ 0.05 | ||
| NIC | 62.83 ± 6.79 b | 62.83 ± 6.79 b | 62.83 ± 6.79 c | nd | ||
| IC | 48.00 ± 4.58 a | 48.00 ± 4.58 a | 48.00 ± 4.58 b | nd | ||
| p-value | p ≤ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| Root Weight (g) | 1W | 5.21 ± 1.66 abA | 4.58 ± 0.58 aA | 4.10 ± 0.86 aA | p ≥ 0.05 | |
| 8W | 6.15 ± 0.20 bA | 9.52 ± 0.93 bB | 7.59 ± 0.91 bA | p ≤ 0.05 | ||
| NIC | 7.38 ± 2.09 b | 7.38 ± 2.09 b | 7.38 ± 2.09 b | nd | ||
| IC | 2.61 ± 0.78 a | 2.61 ± 0.78 a | 2.61 ± 0.78 a | nd | ||
| p-value | p ≤ 0.05 | p ≤ 0.05 | p ≤ 0.05 |
| Pathogens | Parameters | Treatment | Fruits | Leaves | Roots | p-Value *** |
|---|---|---|---|---|---|---|
| P. ultimum | Root Browning Index | 1W | 2.33 ± 0.58 a*A** | 2.00 ± 0.00 a | 2.33 ± 0.58 a | p ≥ 0.05 |
| 8W | 1.67 ± 0.58 a | 2.67 ± 0.58 a | 2.33 ± 0.58 a | p ≥ 0.05 | ||
| NIC | 2.00 ± 0.00 a | 2.00 ± 0.00 a | 2.00 ± 0.00 a | nd | ||
| IC | 2.00 ± 0.00 a | 2.00 ± 0.00 a | 2.00 ± 0.00 a | nd | ||
| p-value | p ≥ 0.05 | p ≥ 0.05 | P ≥ 0.05 | |||
| Disease Index | 1W | 3.00 ± 1.00 a | 2.33 ± 0.58 ab | 3.00 ± 0.00 b | p ≥ 0.05 | |
| 8W | 1.33 ± 0.58 aA | 3.00 ± 0.00 bC | 2.00 ± 0.00 aB | p ≤ 0.05 | ||
| NIC | 2.33 ± 0.58 a | 2.33 ± 0.58 ab | 2.33 ± 0.58 ab | nd | ||
| IC | 1.67 ± 0.58 a | 1.67 ± 0.83 a | 1.67 ± 0.83 a | nd | ||
| p-value | p ≥ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| Height (cm) | 1W | 68.17 ± 3.25 b | 48.00 ± 17.68 a | 60.33 ± 2.02 b | p ≥ 0.05 | |
| 8W | 37.38 ± 7.29 a | 48.50 ± 3.77 a | 37.67 ± 4.75 a | p ≥ 0.05 | ||
| NIC | 62.83 ± 6.79 b | 62.83 ± 6.79 a | 62.83 ± 6.79 b | nd | ||
| IC | 61.17 ± 4.75 b | 61.17 ± 4.75 a | 61.17 ± 4.75 b | nd | ||
| p-value | p ≤ 0.001 | p ≥ 0.05 | p ≤ 0.001 | |||
| Root Weight (g) | 1W | 4.17 ± 0.04 aA | 3.79 ± 2.00 aA | 7.13 ± 0.60 bB | p ≤ 0.05 | |
| 8W | 7.69 ± 0.77 bB | 4.27 ± 0.53 aA | 3.28 ± 0.44 aA | p ≤ 0.001 | ||
| NIC | 7.38 ± 2.09 b | 7.38 ± 2.09 a | 7.38 ± 2.09 b | nd | ||
| IC | 3.71 ± 0.26 a | 3.71 ± 0.26 a | 3.71 ± 0.26 a | nd | ||
| p-value | p ≤ 0.05 | p≥ 0.05 | p ≤ 0.05 | |||
| Ph. citrophthora | Root Browning Index | 1W | 3.00 ± 1.00 aA | 3.33 ± 0.58 bA | 4.00 ± 0.00 bA | p ≥ 0.05 |
| 8W | 1.33 ± 0.58 aA | 1.67 ± 0.58 aA | 2.33 ± 0.58 aA | p ≥ 0.05 | ||
| NIC | 2.00 ± 0.00 a | 2.00 ± 0.00 a | 2.00 ± 0.00 a | nd | ||
| IC | 2.33 ± 0.58 a | 2.33 ± 0.58 a | 2.33 ± 0.58 a | nd | ||
| p-value | p ≥ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| Disease Index | 1W | 2.33 ± 0.58 bA | 2.00 ± 0.00 aA | 2.67 ± 0.58 aA | p ≥ 0.05 | |
| 8W | 0.67 ± 0.58 aA | 1.33 ± 0.58 aA | 1.67 ± 0.58 aA | p ≥ 0.05 | ||
| NIC | 2.33 ± 0.58 b | 2.33 ± 0.58 a | 2.33 ± 0.58 a | nd | ||
| IC | 2.33 ± 0.58 b | 2.33 ± 0.58 a | 2.33 ± 0.58 a | nd | ||
| p-value | p ≤ 0.05 | p ≥ 0.05 | p ≥ 0.05 | |||
| High (cm) | 1W | 69.83 ± 6.01 bA | 58.83 ± 2.25 bA | 62.17 ± 19.04 bA | p ≥ 0.05 | |
| 8W | 68.67 ± 4.25 bB | 29.67 ± 2.75 aA | 27.67 ± 1.61 aA | p ≤ 0.001 | ||
| NIC | 62.83 ± 6.79 a | 62.83 ± 6.79 b | 62.83 ± 6.79 b | nd | ||
| IC | 52.50 ± 7.26 a | 52.50 ± 7.26 b | 52.50 ± 7.26 b | nd | ||
| p-value | p ≤ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| Root Weight (g) | 1W | 9.11 ± 3.16 bA | 9.92 ± 1.23 cA | 7.10 ± 1.08 bA | p ≥ 0.05 | |
| 8W | 9.01 ± 0.97 bC | 4.47 ± 0.51 aB | 1.94 ± 0.38 aA | p ≤ 0.001 | ||
| NIC | 7.38 ± 2.09 b | 7.38 ± 2.09 b | 7.38 ± 2.09 b | nd | ||
| IC | 3.25 ± 0.36 a | 3.25 ± 0.36 a | 3.25 ± 0.36 a | nd | ||
| p-value | p ≤ 0.05 | p ≤ 0.05 | p ≤ 0.05 |
| Pathogens | Parameters | Treatment | Fruits | Leaves | Roots | p-Value *** |
|---|---|---|---|---|---|---|
| Pythium ultimum | Root Browning Index | 1W | 2.00 ± 0.00 ab*A** | 2.00 ± 0.00 abA | 2.33 ± 0.58 abA | p ≥ 0.05 |
| 8W | 2.00 ± 0.00 abA | 1.67 ± 0.58 abA | 2.00 ± 0.00 abA | p ≥ 0.05 | ||
| NIC | 1.33 ± 0.58 a | 1.33 ± 0.58 a | 1.33 ± 0.58 a | nd | ||
| IC | 2.67 ± 0.58 b | 2.67 ± 0.58 b | 2.67 ± 0.58 b | nd | ||
| p-value | p ≤ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| Disease Index | 1W | 2.33 ± 0.58 aA | 2.33 ± 0.58 aA | 1.67 ± 0.58 aA | p ≥ 0.05 | |
| 8W | 2.00 ± 0.00 aA | 1.67 ± 0.58 aA | 1.33 ± 0.58 aA | p ≥ 0.05 | ||
| NIC | 1.33 ± 0.58 a | 1.33 ± 0.58 a | 1.33 ± 0.58 a | nd | ||
| IC | 1.67 ± 0.58 a | 1.67 ± 0.58 a | 1.67 ± 0.58 a | nd | ||
| p-value | p ≥ 0.05 | p ≥ 0.05 | p ≥ 0.05 | |||
| Height (cm) | 1W | 30.00 ± 0.50 aA | 32.83 ± 3.01 aA | 33.90 ± 7.35 aA | p ≥ 0.05 | |
| 8W | 52.00 ± 2.00 bA | 53.50 ± 8.00 bA | 61.00 ± 8.00 bA | p ≥ 0.05 | ||
| NIC | 90.33 ± 11.02 c | 90.33 ± 11.02 c | 90.33 ± 11.02 c | nd | ||
| IC | 62.00 ± 6.00 b | 62.00 ± 6.00 b | 62.00 ± 6.00 b | nd | ||
| p-value | p ≤ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| Root Weight (g) | 1W | 3.74 ± 2.44 aA | 5.48 ± 1.18 aA | 5.81 ± 2.15 aA | p ≥ 0.05 | |
| 8W | 13.94 ± 3.30 bA | 16.18 ± 6.20 bA | 13.39 ± 4.67 bA | p ≥ 0.05 | ||
| NIC | 11.81 ± 1.32 b | 11.81 ± 1.32 ab | 11.81 ± 1.32 ab | nd | ||
| IC | 7.05 ± 0.06 a | 7.05 ± 0.06 a | 7.05 ± 0.06 a | nd | ||
| p-value | p ≤ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| Phytopythium mercuriale | Root Browning Index | 1W | 2.33 ± 0.58 aB | 2.33 ± 0.58 aB | 1.00 ± 0.00 aA | p ≤ 0.05 |
| 8W | 1.67 ± 0.58 aA | 1.33 ± 0.58 aA | 1.00 ± 0.00 aA | p ≥ 0.05 | ||
| NIC | 1.33 ± 0.58 a | 1.33 ± 0.58 a | 1.33 ± 0.58 a | nd | ||
| IC | 2.67 ± 0.58 a | 2.67 ± 0.58 a | 2.67 ± 0.58 b | nd | ||
| p-value | p ≥ 0.05 | p ≥ 0.05 | p ≤ 0.05 | |||
| Disease Index | 1W | 2.33 ± 0.58 aA | 2.00 ± 0.00 aA | 1.33 ± 0.58 aA | p ≥ 0.05 | |
| 8W | 1.67 ± 0.58 aA | 1.33 ± 0.58 aA | 1.33 ± 0.58 aA | p ≥ 0.05 | ||
| NIC | 1.33 ± 0.58 a | 1.33 ± 0.58 a | 1.33 ± 0.58 a | nd | ||
| IC | 1.33 ± 0.58 a | 1.33 ± 0.58 a | 1.33 ± 0.58 a | nd | ||
| p-value | p ≥ 0.05 | p ≥ 0.05 | p ≥ 0.05 | |||
| Height (cm) | 1W | 38.83 ± 5.13 aAB | 44.50 ± 5.27 aB | 32.67 ± 1.76 aA | p ≤ 0.05 | |
| 8W | 42.33 ± 8.02 aA | 58.33 ± 0.58 bA | 59.00 ± 12.00 bA | p ≥ 0.05 | ||
| NIC | 90.33 ± 11.02 b | 90.33 ± 11.02 c | 90.33 ± 11.02 c | nd | ||
| IC | 80.67 ± 2.08 b | 80.67 ± 2.08 c | 80.67 ± 2.08 c | nd | ||
| p-value | p ≤ 0.05 | p ≤ 0.05 | p ≤ 0.05 | |||
| Root Weight (g) | 1W | 5.22 ± 0.99 aA | 10.04 ± 1.36 aB | 8.54 ± 1.89 aB | p ≤ 0.05 | |
| 8W | 6.86 ± 2.71 aA | 13.71 ± 3.10 aA | 10.97 ± 3.34 aA | p ≥ 0.05 | ||
| NIC | 11.81 ± 1.32 b | 11.81 ± 1.32 a | 11.81 ± 1.32 a | nd | ||
| IC | 11.64 ± 0.45 b | 11.64 ± 0.45 a | 11.64 ± 0.45 a | nd | ||
| p-value | p ≤ 0.05 | p ≥ 0.05 | p ≥ 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannai, S.; Boughalleb-M’Hamdi, N. Antimicrobial Evaluation of Asphodelus microcephalus Extracts and Fine Powder of Dried Organs Against Fusarium and Oomycetes Responsible for Apple and Peach Decline Disease. Pathogens 2025, 14, 401. https://doi.org/10.3390/pathogens14050401
Mannai S, Boughalleb-M’Hamdi N. Antimicrobial Evaluation of Asphodelus microcephalus Extracts and Fine Powder of Dried Organs Against Fusarium and Oomycetes Responsible for Apple and Peach Decline Disease. Pathogens. 2025; 14(5):401. https://doi.org/10.3390/pathogens14050401
Chicago/Turabian StyleMannai, Sabrine, and Naima Boughalleb-M’Hamdi. 2025. "Antimicrobial Evaluation of Asphodelus microcephalus Extracts and Fine Powder of Dried Organs Against Fusarium and Oomycetes Responsible for Apple and Peach Decline Disease" Pathogens 14, no. 5: 401. https://doi.org/10.3390/pathogens14050401
APA StyleMannai, S., & Boughalleb-M’Hamdi, N. (2025). Antimicrobial Evaluation of Asphodelus microcephalus Extracts and Fine Powder of Dried Organs Against Fusarium and Oomycetes Responsible for Apple and Peach Decline Disease. Pathogens, 14(5), 401. https://doi.org/10.3390/pathogens14050401






