Molecular Characterization of Clinical Isolates of Methicillin-Resistant Staphylococcus aureus from Chonburi, Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. S. aureus Phenotypic Characterization and Antimicrobial Susceptibility Testing
2.3. Genomic DNA Purification of S. aureus Isolates
2.4. Real-Time PCR Detection of the mecA Gene
2.5. PCR Detection of Panton–Valentine Leukocidin (pvl) Toxin
2.6. Identification of SCCmec Types
2.7. Polymorphism of the X Region Encoding S. aureus Protein A (spa Typing)
2.8. Multilocus Sequence Typing (MLST) and Clonal Complex (CC)
2.9. Visualization of PCR Products and Analysis of Sequenced PCR Products
2.10. Pulsed-Field Gel Electrophoresis (PFGE)
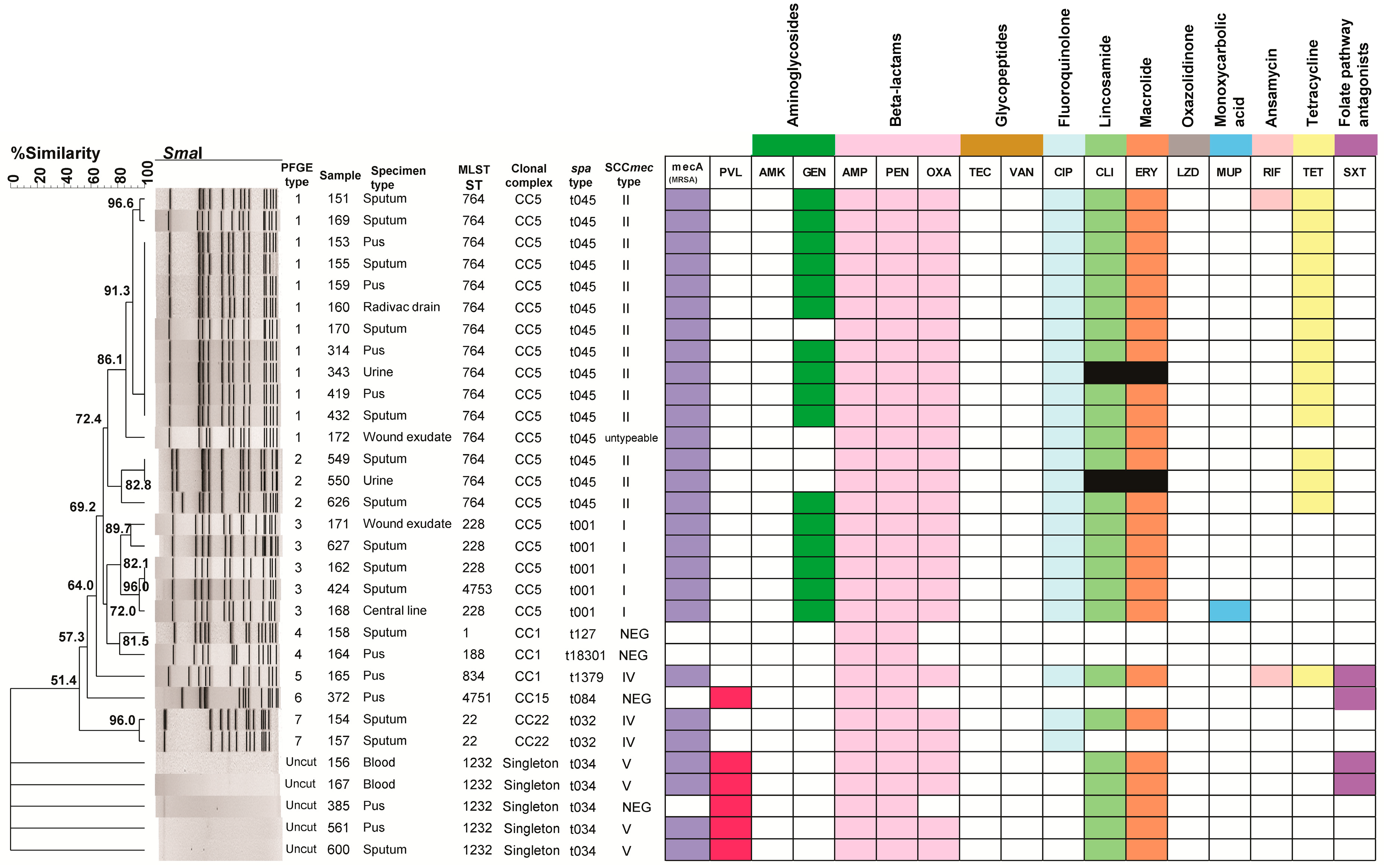
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Goudarzi, H.; Sá Figueiredo, A.M.; Udo, E.E.; Fazeli, M.; Asadzadeh, M.; Seyedjavadi, S.S. Molecular Characterization of Methicillin Resistant Staphylococcus aureus Strains Isolated from Intensive Care Units in Iran: ST22-SCCmec IV/t790 Emerges as the Major Clone. PLoS ONE 2016, 11, e0155529. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [CrossRef]
- Bunnueang, N.; Kongpheng, S.; Yadrak, P.; Rattanachuay, P.; Khianngam, S.; Sukhumungoon, P. Methicillin-Resistant Staphylococcus Aureus: 1-Year Collection and Characterization from Patients in Two Tertiary Hospitals, Southern Thailand. Southeast Asian J. Trop. Med. Public Health 2016, 47, 234–244. [Google Scholar]
- Jaganath, D.; Jorakate, P.; Makprasert, S.; Sangwichian, O.; Akarachotpong, T.; Thamthitiwat, S.; Khemla, S.; DeFries, T.; Baggett, H.C.; Whistler, T.; et al. Staphylococcus aureus Bacteremia Incidence and Methicillin Resistance in Rural Thailand, 2006–2014. Am. J. Trop. Med. Hyg. 2018, 99, 155–163. [Google Scholar] [CrossRef]
- Waitayangkoon, P.; Thongkam, A.; Benjamungkalarak, T.; Rachayon, M.; Thongthaisin, A.; Chatsuwan, T.; Thammahong, A.; Chiewchengchol, D. Hospital epidemiology and antimicrobial susceptibility of isolated methicillin-resistant Staphylococcus aureus: A one-year retrospective study at a tertiary care center in Thailand. Pathog. Glob. Health 2020, 114, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Mendoza, M.; Banga Singh, K.K.; Castanheira, M.; Bell, J.M.; Turnidge, J.D.; Lin, S.S.; Jones, R.N. Regional resistance surveillance program results for 12 Asia-Pacific nations (2011). Antimicrob. Agents Chemother. 2013, 57, 5721–5726. [Google Scholar] [CrossRef] [PubMed]
- Phokhaphan, P.; Tingpej, P.; Apisarnthanarak, A.; Kondo, S. Prevalence and Antibiotic Susceptiblity of Methicillin Resistant Staphylococcus Aureus, Collected at Thammasat University Hospital, Thailand, August 2012—July 2015. Southeast Asian J. Trop. Med. Public Health 2017, 48, 351–359. [Google Scholar] [PubMed]
- Moosavian, M.; Shahin, M.; Navidifar, T.; Torabipour, M. Typing of staphylococcal cassette chromosome mec encoding methicillin resistance in Staphylococcus aureus isolates in Ahvaz, Iran. New Microbes New Infect. 2018, 21, 90–94. [Google Scholar] [CrossRef]
- Funaki, T.; Yasuhara, T.; Kugawa, S.; Yamazaki, Y.; Sugano, E.; Nagakura, Y.; Yoshida, K.; Fukuchi, K. SCCmec typing of PVL-positive community-acquired Staphylococcus aureus (CA-MRSA) at a Japanese hospital. Heliyon 2019, 5, e01415. [Google Scholar] [CrossRef]
- Appelbaum, P.C. Microbiology of Antibiotic Resistance in Staphylococcus aureus. Clin. Infect. Dis. 2007, 45, S165–S170. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Das, B.J.; Pandey, P.; Chetri, S.; Chanda, D.D.; Bhattacharjee, A. An array of multiplex PCR assays for detection of staphylococcal chromosomal cassette mec (SCCmec) types among staphylococcal isolates. J. Microbiol. Methods 2019, 166, 105733. [Google Scholar] [CrossRef]
- Boye, K.; Bartels, M.D.; Andersen, I.S.; Møller, J.A.; Westh, H. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I-V. Clin. Microbiol. Infect. 2007, 13, 725–727. [Google Scholar] [CrossRef] [PubMed]
- McDougal, L.K.; Steward, C.D.; Killgore, G.E.; Chaitram, J.M.; McAllister, S.K.; Tenover, F.C. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: Establishing a national database. J. Clin. Microbiol. 2003, 41, 5113–5120. [Google Scholar] [CrossRef]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Phokhaphan, P.; Tongsima, S.; Ngamphiw, C.; Phornsiricharoenphant, W.; Ruangchai, W.; Disratthakit, A.; Tingpej, P.; Mahasirimongkol, S.; Lulitanond, A.; et al. Molecular characterization of methicillin-resistant Staphylococcus aureus genotype ST764-SCCmec type II in Thailand. Sci. Rep. 2022, 12, 2085. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yan, Z.; He, F.; Chen, J.; Kuang, L.; Liu, X.; Cui, Y.; Wang, X.; Miao, C.; Li, H.; et al. Antibiotic susceptibility and molecular characterization based on whole-genome sequencing of Staphylococcus aureus causing invasive infection in children and women living in Southwest China during 2018–2023. BMC Microbiol. 2025, 25, 47. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, Y.; Kiaei, S.; Kalantar-Neyestanaki, D. Characterization of SCCmec and spa types of methicillin-resistant Staphylococcus aureus isolates from health-care and community-acquired infections in Kerman, Iran. J. Epidemiol. Glob. Health 2017, 7, 263–267. [Google Scholar] [CrossRef]
- Santimaleeworagun, W.; Preechachuawong, P.; Samret, W.; Jitwasinkul, T. The First Report of a Methicillin-Resistant Staphylococcus aureus Isolate Harboring Type IV SCCmec in Thailand. Pathogens 2021, 10, 430. [Google Scholar] [CrossRef]
- Xie, X.; Bao, Y.; Ouyang, N.; Dai, X.; Pan, K.; Chen, B.; Deng, Y.; Wu, X.; Xu, F.; Li, H.; et al. Molecular epidemiology and characteristic of virulence gene of community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus isolates in Sun Yat-sen Memorial hospital, Guangzhou, Southern China. BMC Infect. Dis. 2016, 16, 339. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Garg, A.; Tripathi, P.; Venkatesh, V. Epidemiology of Panton Valentine Leukocidin in clinical Staphylococcus aureus isolates—A prospective study at a tertiary care centre in North India. Clin. Epidemiol. Glob. Health 2022, 15, 101006. [Google Scholar] [CrossRef]
- Ruekit, S.; Srijan, A.; Serichantalergs, O.; Margulieux, K.R.; Mc Gann, P.; Mills, E.G.; Stribling, W.C.; Pimsawat, T.; Kormanee, R.; Nakornchai, S.; et al. Molecular characterization of multidrug-resistant ESKAPEE pathogens from clinical samples in Chonburi, Thailand (2017-2018). BMC Infect. Dis. 2022, 22, 695. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; Institute CaLS: Wayne, PA, USA, 2017. [Google Scholar]
- Mc Gann, P.; Milillo, M.; Kwak, Y.I.; Quintero, R.; Waterman, P.E.; Lesho, E. Rapid and simultaneous detection of the chlorhexidine and mupirocin resistance genes qacA/B and mupA in clinical isolates of methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2013, 77, 270–272. [Google Scholar] [CrossRef]
- National Antimicrobial Resistance Surveillance Thailand: National Prevalence of Bacterial Infection Dashboard. Available online: https://narst.dmsc.moph.go.th/ (accessed on 30 March 2025).
- Ito, T.; Okuma, K.; Ma, X.X.; Yuzawa, H.; Hiramatsu, K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: Genomic island SCC. Drug Resist. Updat. 2003, 6, 41–52. [Google Scholar] [CrossRef]
- Malachowa, N.; DeLeo, F.R. Mobile genetic elements of Staphylococcus aureus. Cell Mol. Life Sci. 2010, 67, 3057–3071. [Google Scholar] [CrossRef]
- Jariyasethpong, T.; Tribuddharat, C.; Dejsirilert, S.; Kerdsin, A.; Tishyadhigama, P.; Rahule, S.; Sawanpanyalert, P.; Yosapol, P.; Aswapokee, N. MRSA carriage in a tertiary governmental hospital in Thailand: Emphasis on prevalence and molecular epidemiology. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Lulitanond, A.; Chanawong, A.; Sribenjalux, P.; Wilailuckana, C.; Kaewkes, W.; Vorachit, M.; Ito, T.; Hiramatsu, K. Preliminary report of SCCmec-types and antimicrobial susceptibilities of methicillin-resistant Staphylococcus aureus isolates from a university hospital in Thailand. Southeast Asian J. Trop. Med. Public Health 2010, 41, 920–927. [Google Scholar] [PubMed]
- Lawung, R.; Chuong, L.V.; Cherdtrakulkiat, R.; Srisarin, A.; Prachayasittikul, V. Revelation of staphylococcal cassette chromosome mec types in methicillin-resistant Staphylococcus aureus isolates from Thailand and Vietnam. J. Microbiol. Methods 2014, 107, 8–12. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wang, Y.; Xiao, T.T.; Ji, J.R.; Shen, P.; Xiao, Y.H. Molecular epidemiology and change trend of methicillin-resistant Staphylococcus aureus from invasive infections of a hospital in Hangzhou from 2012 to 2018. Yi Chuan 2023, 45, 1074–1084. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Y.; Qiao, Z.; Jiang, Z.; Wang, Z.; Xu, H.; Jiao, X.; Li, Q. Prevalence and molecular characteristics of community-associated methicillin-resistant Staphylococcus aureus in the respiratory tracts of Chinese adults with community-acquired pneumonia. J. Infect. Public Health 2023, 16, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Abrudan, M.I.; Shamanna, V.; Prasanna, A.; Underwood, A.; Argimón, S.; Nagaraj, G.; Di Gregorio, S.; Govindan, V.; Vasanth, A.; Dharmavaram, S.; et al. Novel multidrug-resistant sublineages of Staphylococcus aureus clonal complex 22 discovered in India. mSphere 2023, 8, e0018523. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; Osada, M.; Urushibara, N.; Kawaguchiya, M.; Ohashi, N.; Hirose, M.; Ito, M.; Yamada, K.; Tada, K.; Kobayashi, N. Molecular characterization of methicillin-susceptible/resistant Staphylococcus aureus from bloodstream infections in northern Japan: The dominance of CC1-MRSA-IV, the emergence of human-associated ST398 and livestock-associated CC20 and CC97 MSSA. J. Glob. Antimicrob. Resist. 2025, 41, 77–87. [Google Scholar] [CrossRef]
- Sato, T.; Yamaguchi, T.; Aoki, K.; Kajiwara, C.; Kimura, S.; Maeda, T.; Yoshizawa, S.; Sasaki, M.; Murakami, H.; Hisatsune, J.; et al. Whole-genome sequencing analysis of molecular epidemiology and silent transmissions causing meticillin-resistant Staphylococcus aureus bloodstream infections in a university hospital. J. Hosp. Infect. 2023, 139, 141–149. [Google Scholar] [CrossRef]
- Kitti, T.; Seng, R.; Saiprom, N.; Thummeepak, R.; Chantratita, N.; Boonlao, C.; Sitthisak, S. Molecular Characteristics of Methicillin-Resistant Staphylococci Clinical Isolates from a Tertiary Hospital in Northern Thailand. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 8457012. [Google Scholar] [CrossRef] [PubMed]
- Valsesia, G.; Rossi, M.; Bertschy, S.; Pfyffer, G.E. Emergence of SCCmec type IV and SCCmec type V methicillin-resistant Staphylococcus aureus containing the Panton-Valentine leukocidin genes in a large academic teaching hospital in central Switzerland: External invaders or persisting circulators? J. Clin. Microbiol. 2010, 48, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.T.; Cao, R.; Xiao, N.; Li, Z.S.; Wang, R.; Zou, J.M.; Pei, J. Molecular epidemiology and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus isolates in Xiangyang, China. J. Glob. Antimicrob. Resist. 2018, 12, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Harastani, H.H.; Araj, G.F.; Tokajian, S.T. Molecular characteristics of Staphylococcus aureus isolated from a major hospital in Lebanon. Int. J. Infect. Dis. 2014, 19, 33–38. [Google Scholar] [CrossRef]
- Takano, T.; Hung, W.C.; Shibuya, M.; Higuchi, W.; Iwao, Y.; Nishiyama, A.; Reva, I.; Khokhlova, O.E.; Yabe, S.; Ozaki, K.; et al. A new local variant (ST764) of the globally disseminated ST5 lineage of hospital-associated methicillin-resistant Staphylococcus aureus (MRSA) carrying the virulence determinants of community-associated MRSA. Antimicrob. Agents Chemother. 2013, 57, 1589–1595. [Google Scholar] [CrossRef]
- Chen, W.; He, C.; Yang, H.; Shu, W.; Cui, Z.; Tang, R.; Zhang, C.; Liu, Q. Prevalence and molecular characterization of methicillin-resistant Staphylococcus aureus with mupirocin, fusidic acid and/or retapamulin resistance. BMC Microbiol. 2020, 20, 183. [Google Scholar] [CrossRef]
- Gu, F.; He, W.; Xiao, S.; Wang, S.; Li, X.; Zeng, Q.; Ni, Y.; Han, L. Antimicrobial Resistance and Molecular Epidemiology of Staphylococcus aureus Causing Bloodstream Infections at Ruijin Hospital in Shanghai from 2013 to 2018. Sci. Rep. 2020, 10, 6019. [Google Scholar] [CrossRef] [PubMed]
- Chongtrakool, P.; Ito, T.; Ma, X.X.; Kondo, Y.; Trakulsomboon, S.; Tiensasitorn, C.; Jamklang, M.; Chavalit, T.; Song, J.H.; Hiramatsu, K. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: A proposal for a new nomenclature for SCCmec elements. Antimicrob. Agents Chemother. 2006, 50, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Hsueh, P.R.; Chung, D.R.; Ko, K.S.; Kang, C.I.; Peck, K.R.; Yeom, J.S.; Kim, S.W.; Chang, H.H.; Kim, Y.S.; et al. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: An ANSORP study. J. Antimicrob. Chemother. 2011, 66, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Li, V.; Chui, L.; Louie, L.; Simor, A.; Golding, G.R.; Louie, M. Cost-effectiveness and efficacy of spa, SCCmec, and PVL genotyping of methicillin-resistant Staphylococcus aureus as compared to pulsed-field gel Electrophoresis. PLoS ONE 2013, 8, e79149. [Google Scholar] [CrossRef]
- Tsao, F.Y.; Kou, H.W.; Huang, Y.C. Dissemination of methicillin-resistant Staphylococcus aureus sequence type 45 among nursing home residents and staff in Taiwan. Clin. Microbiol. Infect. 2015, 21, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Bens, C.C.; Voss, A.; Klaassen, C.H. Presence of a novel DNA methylation enzyme in methicillin-resistant Staphylococcus aureus isolates associated with pig farming leads to uninterpretable results in standard pulsed-field gel electrophoresis analysis. J. Clin. Microbiol. 2006, 44, 1875–1876. [Google Scholar] [CrossRef] [PubMed]
- Chlebowicz, M.A.; Nganou, K.; Kozytska, S.; Arends, J.P.; Engelmann, S.; Grundmann, H.; Ohlsen, K.; van Dijl, J.M.; Buist, G. Recombination between ccrC genes in a type V (5C2&5) staphylococcal cassette chromosome mec (SCCmec) of Staphylococcus aureus ST398 leads to conversion from methicillin resistance to methicillin susceptibility in vivo. Antimicrob. Agents Chemother. 2010, 54, 783–791. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, L.; Yan, J.; Lin, Y.; Ding, D.; He, L.; Li, Y.; Ying, Y.; Shen, L.; Jiang, Y.; et al. Genomic characterization and outbreak investigations of methicillin-resistant Staphylococcus aureus in a county-level hospital in China. Front. Microbiol. 2024, 15, 1387855. [Google Scholar] [CrossRef]
- Che Hamzah, A.M.; Chew, C.H.; Al-Trad, E.I.; Puah, S.M.; Chua, K.H.; NI, A.R.; Ismail, S.; Maeda, T.; Palittapongarnpim, P.; Yeo, C.C. Whole genome sequencing of methicillin-resistant Staphylococcus aureus clinical isolates from Terengganu, Malaysia, indicates the predominance of the EMRSA-15 (ST22-SCCmec IV) clone. Sci. Rep. 2024, 14, 3485. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, X.; Wang, B.; Shen, L.; Rao, L.; Wang, X.; Zhang, J.; Xiao, Y.; Xu, Y.; Yu, J.; et al. Phenotypic and genomic analysis of the hypervirulent ST22 methicillin-resistant Staphylococcus aureus in China. mSystems 2023, 8, e0124222. [Google Scholar] [CrossRef] [PubMed]

| Number of Resistant Antibiotic Classes | Antibiotic Classes | MSSA | MRSA | PVL Positive |
|---|---|---|---|---|
| 1 | β-Lactam (AMP and PEN only) | 2 | - | - |
| 2 | β-Lactam (AMP and PEN only), Folate pathway antagonists | 1 | - | 1 |
| β-Lactam, Fluoroquinolones | - | 1 | - | |
| 3 | β-lactam (AMP and PEN only), Lincosamides, Macrolides | 1 | - | 1 |
| β-Lactam, Fluoroquinolones, Tetracyclines | - | 1 | - | |
| β-Lactam, Lincosamides, Macrolides | - | 2 | 2 | |
| 4 | β-Lactam, Lincosamides, Macrolides, Folate pathway antagonists | - | 2 | 2 |
| β-Lactam, Fluoroquinolones, Aminoglycosides, Tetracyclines | - | 1 | - | |
| β-Lactam, Fluoroquinolones, Lincosamides, Macrolides | - | 2 | - | |
| 5 | β-Lactam, Fluoroquinolones, Lincosamides, Macrolides, Tetracyclines | - | 2 | - |
| β-Lactam, Fluoroquinolones, Lincosamides, Macrolides, Aminoglycosides | - | 4 | - | |
| 6 | β-Lactam, Fluoroquinolones, Lincosamides, Macrolides, Aminoglycosides, Tetracyclines | - | 9 | - |
| β-Lactam, Fluoroquinolones, Lincosamides, Macrolides, Aminoglycosides, Monoxycarbolic acid | - | 1 | - | |
| 7 | β-Lactam, Fluoroquinolones, Lincosamides, Macrolides, Aminoglycosides, Ansamycins, Tetracyclines | - | 1 | - |
| β-Lactam, Fluoroquinolones, Lincosamides, Macrolides, Ansamycins, Tetracyclines, Folate pathway antagonists | - | 1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wassanarungroj, P.; Nobthai, P.; Ruekit, S.; Srijan, A.; Sukhchat, P.; Serichantalergs, O.; Crawford, J.M.; Swierczewski, B.E.; Chaudhury, S.; Lertsethtakarn, P. Molecular Characterization of Clinical Isolates of Methicillin-Resistant Staphylococcus aureus from Chonburi, Thailand. Pathogens 2025, 14, 406. https://doi.org/10.3390/pathogens14050406
Wassanarungroj P, Nobthai P, Ruekit S, Srijan A, Sukhchat P, Serichantalergs O, Crawford JM, Swierczewski BE, Chaudhury S, Lertsethtakarn P. Molecular Characterization of Clinical Isolates of Methicillin-Resistant Staphylococcus aureus from Chonburi, Thailand. Pathogens. 2025; 14(5):406. https://doi.org/10.3390/pathogens14050406
Chicago/Turabian StyleWassanarungroj, Patcharawalai, Panida Nobthai, Sirigade Ruekit, Apichai Srijan, Prawet Sukhchat, Oralak Serichantalergs, John M. Crawford, Brett E. Swierczewski, Sidhartha Chaudhury, and Paphavee Lertsethtakarn. 2025. "Molecular Characterization of Clinical Isolates of Methicillin-Resistant Staphylococcus aureus from Chonburi, Thailand" Pathogens 14, no. 5: 406. https://doi.org/10.3390/pathogens14050406
APA StyleWassanarungroj, P., Nobthai, P., Ruekit, S., Srijan, A., Sukhchat, P., Serichantalergs, O., Crawford, J. M., Swierczewski, B. E., Chaudhury, S., & Lertsethtakarn, P. (2025). Molecular Characterization of Clinical Isolates of Methicillin-Resistant Staphylococcus aureus from Chonburi, Thailand. Pathogens, 14(5), 406. https://doi.org/10.3390/pathogens14050406






