Abstract
Salmonellosis remains one of the most frequent foodborne zoonosis, constituting a worldwide major public health concern. The most frequent sources of human infections are food products of animal origin, being pork meat one of the most relevant. Currently, particular pig food production well-adapted and persistent Salmonella enterica serotypes (e.g., Salmonella Typhimurium, Salmonella 1,4,[5],12:i:-, Salmonella Derby and Salmonella Rissen) are frequently reported associated with human infections in diverse industrialized countries. The dissemination of those clinically-relevant Salmonella serotypes/clones has been related to the intensification of pig production chain and to an increase in the international trade of pigs and pork meat. Those changes that occurred over the years along the food chain may act as food chain drivers leading to new problems and challenges, compromising the successful control of Salmonella. Among those, the emergence of antibiotic resistance in non-typhoidal Salmonella associated with antimicrobials use in the pig production chain is of special concern for public health. The transmission of pig-related multidrug-resistant Salmonella serotypes, clones and/or genetic elements carrying clinically-relevant antibiotic resistance genes, frequently associated with metal tolerance genes, from pigs and pork meat to humans, has been reported and highlights the contribution of different drivers to the antibiotic resistance burden. Gathered data strengthen the need for global mandatory interventions and strategies for effective Salmonella control and surveillance across the pig production chain. The purpose of this review was to provide an overview of the role of pig and pork meat in human salmonellosis at a global scale, highlighting the main factors contributing to the persistence and dissemination of clinically-relevant pig-related Salmonella serotypes and clones.
1. Introduction
Salmonella enterica infections are a worldwide major public health concern, specifically human salmonellosis caused by non-typhoidal Salmonella (NTS) [,]. Salmonellosis is typically characterized by a self-limiting gastroenteritis syndrome, with diarrhea as its the main symptom; however, fever, vomiting and abdominal pain can also occur [,]. Despite being uncommon, more severe invasive Salmonella infections, as bacteraemia and/or other extra-intestinal infections, can occur and affect particular high-risk groups (infants, young children, older people or immunocompromised patients) [,]. In these cases, the use of antimicrobial agents is required, being of concern the emergence of Salmonella resistant to antibiotics, especially those considered by the World Health Organization (WHO) as “highest priority critically important antimicrobials” such as fluoroquinolones and extended-spectrum cephalosporins that can compromise the effective treatment of infections [,,].
In industrialized countries, the main reservoir of NTS is the gastrointestinal tract of warm-blooded animals, in particular food-producing animals, which lead to foodstuffs contamination [,]. Therefore, the ingestion of contaminated food, particularly foods of animal origin, is recognized as the most relevant source of transmission of NTS to humans, with a high global impact in human health []. NTS causes an estimated 93.8 million cases of human illnesses and 155.000 deaths each year worldwide [,]. In the United States of America (USA), the 2015 report from the Centers for Disease Control and Prevention (CDC) showed that Salmonella was the second foodborne pathogen responsible for outbreaks (34%), being the first associated with outbreak illnesses (39%), hospitalizations (64%) and deaths (60%) []. Additionally, the European Food Safety Authority (EFSA) reported that salmonellosis has been the second most common zoonosis (91,662 confirmed salmonellosis cases in 2017) and the most frequent cause of foodborne outbreaks (24,4% of all cases in 2017) in the European Union (EU), in spite of a decreasing number of cases since 2008, with a stabilizing trend between the years 2013–2017 []. Salmonellosis has been mostly associated with the consumption of poultry products, including eggs and egg products, at a global level [,,,,,,]. However, pork meat has been considered one of the major food products of animal origin responsible for Salmonella transmission to humans in diverse countries, including industrialized ones [,,,]. In the EU, pork meat has been a common source of human salmonellosis cases (varying from 2% to 13%), after eggs and egg products [,,,]. Additionally, in the USA (2015 data), pork meat was the second source attributed to Salmonella outbreaks (5%) and the meat product mostly associated with the largest number of illnesses (16%), hospitalizations (2%) and deaths (11%) [].
Although different serotypes have been associated with salmonellosis, the major ones responsible for human infections in diverse industrialized countries include Salmonella Enteritidis, Salmonella Typhimurium and its monophasic variant—Salmonella 1,4,[5],12:i:- [,,,,,]. At a global level, S. Enteritidis is commonly associated with poultry and products thereof, being considered a poultry-related serotype [,,]. In contrast, S. Typhimurium has a wider host range, including pigs [,,,]. Nevertheless, in the last decades, a changing trend in Salmonella serotypes associated with foodborne salmonellosis has been observed, with the worldwide expansion of previously less common serotypes (e.g., S. 1,4,[5],12:i:-, Salmonella Derby and Salmonella Rissen). Those are currently well-known serotypes associated with pig production chain and frequently multidrug-resistant [,,,,,,,,,,,,,,].
In the EU, the successful implementation of mandatory Salmonella control programmes along poultry/egg production chain was responsible for the reduction of the prevalence of Salmonella serotypes considered relevant for public health, particularly S. Enteritidis [,,]. In contrast to poultry production, where Salmonella control programmes are harmonized, for pig production each EU member state applies a specific national monitoring programme [,,]. In addition, the intensive food production/farming and increased globalization of food supply (live animals and foodstuffs), with pork meat being one of the most consumed and traded meat products (pork exports increased in value by 18.2% from 2015 to 2016) [,], may trigger new problems regarding salmonellosis control. This review will provide evidence of the relevant role of pig production and pork meat in salmonellosis on a global scale.
2. Non-Typhoidal Salmonella in Pigs and Pig Production Chain
The colonization of pig populations with NTS is frequent and normally results in asymptomatic healthy carriers. This pig colonization can occur throughout all stages of pig production chain by horizontal (through external agents in the environment, e.g., rodents, birds, people, trucks, pets, other foodstuffs) and vertical transmission (e.g., from sow to piglet and from pig to pig at herd to slaughter). Additionally, a designated circular transmission (combination of vertical and horizontal transmission), which is a permanent cycle of contamination on a farm (e.g., environmental contamination through pig shedding and pig contamination through farm environment) can also determine pig colonization [,,,,]. The presence of Salmonella in those healthy pig carriers (e.g., tonsils, gut and gut-associated lymphoid tissue) is suggested to be the main risk factor for the spread and transmission of these bacteria across pig production chain to humans: in pre-harvest (holding period of pig on the farm), in the harvest stage (during slaughter and further processing of meat carcasses) and in the post-harvest stage (during final preparation of pork meat and products thereof) [,,,,,].
In fact, diverse studies aiming to detect Salmonella in the pig production chain, including pigs and pork meat, have been performed in high or low-income countries with diverse results. In the EU, data from the last EFSA reports revealed Salmonella-positive samples in fresh pork meat (2.4%-2016 and 1.6%-2017) and products thereof (1.9%-2016), with an overall Salmonella prevalence in pigs of 6.7% at the herd (ranging from 0%-63% between the different member states, 2016) and of 3.5% (2016) and 12.7% (2017) at slaughter [,]. A noteworthy, high prevalence of Salmonella in fecal samples (30.5%), rectal swabs (24%) and carcass swabs (9.6%) of slaughter pigs was reported by a United Kingdom (UK) study []. In contrast, other EU countries (Finland, Sweden and Norway), with special guarantees concerning Salmonella on pig carcasses (according to Regulation (EC) No 853/2004) reported a lower incidence of Salmonella in pig carcasses samples (0.02%) [,]. With the objective to establish the main targets for Salmonella reduction in breeding herds of pigs (in line with the Regulation (EC) No 2160/2003) [], a baseline survey was performed in EU (2008) due to the lack of information about Salmonella control in holdings of breeding pig []. In this survey, high levels of Salmonella-positive holdings with breeding pigs (31.8%) and breeding holdings (28.7%) were observed. These data have shown that breeding pigs may be a relevant source of Salmonella dissemination along the pig production chain (e.g., to slaughter pigs through trade and movement of live animals and contamination of holding, transport, lairage and slaughter facilities), leading to pork meat contamination and consequently to human infections []. In fact, high levels of Salmonella contamination occurred in slaughterhouses by diverse routes (e.g., pork meat carcasses cross-contamination, slaughter environment and equipment, meat handlers), as reported in diverse studies [,,]. Another EU survey based on the analysis of quantitative microbiological risk assessment of Salmonella in slaughter and breeder pigs showed that an 80–90% reduction of Salmonella prevalence in lymph nodes should result in a comparable reduction in the number of human cases attributable to pork meat products []. Consequently, several interventions have been proposed in order to prevent or reduce Salmonella contamination, persistence and dissemination across pig production (at farm and slaughterhouse). These included the use of uncontaminated feed, isolation of newly purchased animals before introducing them into herd, regular veterinary checks, vaccination, prevention of environmental contamination at the farm, transport, lairage and slaughter, implementation of high standards of hygiene (cleaning and disinfection) and the promising alternative approach bacteriophage use [,,,,,]. Starting 2006, in all EU member states, the report of data regarding Salmonella monitoring and surveillance became mandatory under the Commission Regulation (EC) No 2073/2005 on microbiological criteria in foodstuffs, including for Salmonella in pig carcasses at dressing and before chilling stage. Furthermore, competent authorities must verify the correct implementation of the process hygiene criteria for Salmonella on pig carcasses by food business operators and, if it is not complied, business operators might implement corrective actions (e.g., improvements of hygiene slaughter, biosecurity measures in the farms and revision of process controls) with the specific instructions of authorities []. In 2014, legislation was revised, with Regulation (EC) No 217/2014 proposing the reduction of Salmonella acceptable number in pig carcasses, from “c = 5 out of n = 50”—10% to “c = 3 out of n = 50”—6% (n-number of units comprising the sample; c- detection number of samples with Salmonella), in order to strengthen the process hygiene criterion [].
In the USA, another high-income country, data from 2015 showed a remarkable higher prevalence of Salmonella in pig fecal samples (50%-sows and 35%-market swine) than in other animal samples (25%-chickens, 9%-turkeys, 22%-dairy and 8%-beef) []. Moreover, in developing countries (some with an expansion of food-animal industry), Salmonella was detected at high levels in pig samples (animal, carcasses and meat), ranging from 17–39% in South America [,], to 14–40% in Africa [,] and 29–100% in Asia [,]. Those high levels possibly reflect the different pig production practices and the absence of control measures. Overall, these data point out the need of implementing effective global measures for Salmonella control, highlighting the need for its detection at all stages of pig production chain, including in primary production [,,]. This is particularly urgent in developing countries, which currently seem to present a severely underserved monitoring surveillance program [].
3. Major Pig-Related Salmonella Serotypes Associated with Human Infections
In recent years, Salmonella transmission from pigs to humans through the pork food chain has been evidenced, namely through the study of serotypes prevalence in different matrices as well as food-borne outbreaks associated with consumption of pork products.
Worldwide data concerning the prevalence of Salmonella serotypes in humans, pigs and products thereof have contributed to establishing their epidemiological correlation, with particular serotypes overlapping between humans, pig and pork meat [,,,,,,,,]. For instance, in EU, an association between Salmonella serotypes causing human infections and those occurring in pig and pork meat was observed (Figure 1), reinforcing the major role of pork meat in the transmission of Salmonella to humans. The most frequent serotypes in pig and pork meat have been S. Typhimurium (pig: 54.7%-2014, 56.9%-2015, 29.5%-2016 and 20.6%-2017; pork meat: 27.8%-2014, 23%-2015, 30.7%-2016 and 27%-2017), S. 1,4,[5],12:i:- (pig: 8.4%-2014, 8.6%-2015, 34.1%-2016 and 37.4%-2017; pork meat: 18%-2014, 22.3%-2015, 24.3%-2016 and 22%-2017), and S. Derby (pigs: 17.5%-2014, 13.7%-2015 and 19.2%-2016; pork meat: 24.4%-2014, 22.9%-2015 and 17%-2016) [,,,]. These three serotypes are also among the major ones associated with human salmonellosis (second-, third- and fifth-ranked, respectively, in 2014-2016) [,,]. From the 2008 EU baseline survey, S. Derby was the most frequent serotype found in both breeding (29.6%) and production holdings (28.5%) and S. Typhimurium was the second most detected (breeding holdings-25.4% and production holdings-20.1%) []. It is also of note the emergence of S. Rissen in pig sources (pigs: 1.5%-2014, 2.8%-2015 and 1.2%-2016; pork meat: 4.9%-2014, 5.1%-2015 and 5.9%-2016) in the EU (the fifth most common serotype since 2014 in pig sources) in spite of its low association with human infections (Figure 1) [,,]. The EU baseline survey reported a high incidence of S. Rissen in breeding and production holdings, particularly in Portugal (40% and 22.4%, respectively) and Spain (25% and 29.7%, respectively), being the first or second most frequently reported serotype in those settings [,]. In fact, in Portugal, S. Rissen was the fourth most frequently serotype detected in human clinical isolates between 2002 and 2016 [,]. Although S. Enteritidis (number one in human infections) is typically associated with eggs and poultry meat, it is important to point out that in the last years, in the EU this serotype was also common in both pig and pork meat samples (varying from 1% and 3.5%) [,,,]. Moreover, Salmonella Infantis, another typically poultry-related serotype causing human infections (top 4), was detected in pigs and particularly in pork meat (varying from 3.9% to 8.8%) [,,,]. Therewithal, both S. Enteritidis and S. Infantis serotypes have been recovered from pigs/pork and products thereof in other non-EU regions and associated with human salmonellosis [,,,,].
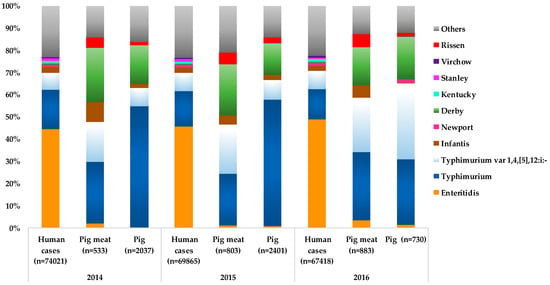
Figure 1.
Distribution of the major serotypes of non-typhoidal Salmonella associated with human cases (salmonellosis), pig and pig meat in EU, 2014 to 2016 [,,]. S. Rissen was included for being one of the five most frequent Salmonella serotypes recovered from pig meat and pig animal in EU, 2014 to 2016 [,,]. The percentages were calculated based on the total number of serotyped isolates (represented by the numbers in brackets) per human salmonellosis cases, pig meat or pig animal.
4. Dissemination of Pig-Associated Salmonella Serotypes and Clones
Besides worldwide data concerning Salmonella serotypes prevalence in humans and pig sources, the contribution of pork meat for human salmonellosis has been also evidenced throughout the spread of certain pig-associated strains and clones. Several examples of outbreaks associated with pig-related Salmonella serotypes have been described involving diverse countries (Table 1). For instance, S. 1,4,[5],12:i:- strains causing human infections, including some particular major clones, were identified in diverse European countries and associated with the consumption of different pork products (Table 1). Moreover, since 2015, several notifications were reported by the Rapid Alert System for Food and Feed (RASFF) due to the presence of Salmonella in pork products from several European countries, including alerts of suspected multi-country foodborne outbreaks (e.g., S. Typhimurium ST19 in Denmark associated with chilled sliced salami from Spain) (Table 1). This scenario alerts for the relevant role of pig/pork meat international trade on the dissemination of clinically-relevant pig-related Salmonella serotypes/clones, highlighting the need for global effective surveillance and detection programmes at all stages of pig production [,].

Table 1.
Salmonella outbreaks linked to pork meat products (2004–2018).
Regarding S. Typhimurium, diverse examples of pork products-related outbreaks have been reported in the last years, highlighting the importance of this serotype in the pig production chain (Table 1). Moreover, during the last decade, S. Typhimurium has been associated with clinically-relevant multi-drug resistant (MDR) clones, being of note the globally disseminated S. Typhimurium DT104 phagetype clone/sequence type (ST by MLST) 19, already reported associated with pig production [,,,,,]. In the same way, MDR S. Typhimurium OXA-30-producing/ST19 associated with swine production samples was also reported in Portugal and other European countries [,,,]. More recently, S. Typhimurium European clone/ST34 with a MDR-profile (ASSuT; Ampicillin-A, Streptomycin-S, sulphonamides-Su and Tetracycline-T) and Pulsed-Field Gel Eletrophoresis (PFGE)-types similar to S. 1,4,[5],12:i:- European clone, were reported in Europe, particularly among piggeries, abattoirs, pork meat as well as human infections [,,,].
In the EU, S. 1,4,[5],12:i:- is considered an emergent serotype that causes human infections [,,], with a remarkable increase in the incidence in pig and pork, surpassing even in the last years S. Typhimurium [,,,]. In fact, recent European surveys have demonstrated a high prevalence of S. 1,4,[5],12:i:- in pigs, carcass and environmental samples (14% to 43%) [,]. Furthermore, several studies have already demonstrated the same clonal relatedness between S. 1,4,[5],12:i:- isolates from human clinical and pigs and/or products thereof [,,,,], which evidence that pigs are the main animal reservoir of this emerging serotype in European countries [,,,]. Interestingly, a remarkable increase of this serotype in human clinical cases was observed in Portugal, from third- (4.5%, between 2000 and 2012) [] to first-ranked serotype (36.6%, in 2014 to 2016) [], surpassing S. Enteritidis and S. Typhimurium. In addition to S. Typhimurium, S. 1,4,[5],12:i:- was, in recent years, the other major serotype responsible for large outbreaks associated with diverse pork products (Table 1). Moreover, two predominant MDR clones of S. 1,4,[5],12:i:-, the European clone/ST34 and the Spanish clone/ST19, have been recognized as responsible for most human infections through pork products. The European clone, frequently belonging to DT120 and DT193 phage types has been circulating in several regions of Europe [,,,] and more recently in America [,], Asia [] and even Australia []. Meanwhile, the Spanish clone, with most of the isolates belonging to DT104/U302 phage types, was originally identified in Spain and further reported since 2002 in the Iberian Peninsula [,,,,]. The maintenance and dissemination of these MDR clones in Europe could be explained by common pig breeding lines and by the intense commercial trade of pigs and products thereof between countries []. Additionally, a third less frequent MDR clone of S. 1,4,[5],12:i:-, Southern-European clone/ST19, was reported in Portugal [,] and sporadically in Italy and Spain [].
S. Derby and S. Rissen have been other predominant serotypes in both pig and pork meat in Europe [,,], and in USA [], despite being less implicated in human salmonellosis. Nevertheless, S. Derby has been reported at global level with identical MDR (mainly SSuT) and/or PFGE profiles in isolates from human clinical cases, pigs and products thereof, demonstrating their potential role in human infections [,,,,,,,,,]. In Southern European countries, S. Rissen is considered a clinically-relevant serotype, being frequently detected the same strains in humans, pigs and products thereof [,,,,,,,,]. Moreover, S. Rissen strains detected among humans and across pig production chain, particularly belonging to the successful MDR clone ST469, have been reported in geographic distant countries [,,,,,,,,,]. In particular, the circulation of a specific MDR (ASSuTTm, Trimethoprim-Tm) S. Rissen clone between the Iberian countries can be explained by the intensive trade of pigs and products thereof []. Additionally, some S. Rissen isolates recovered from human, pig and pork isolates in Denmark showed similar PFGE profiles with isolates from imported pigs or pork meat from Spain and Germany, as well as with isolates from human clinical cases of people who travelled to Thailand [] (Table 1). These data enhance the contribution of live animals and international food trade to the spread of this S. Rissen clone, besides human travel to developing countries.
The enhanced ability to colonize food animals and to persist along the food chain of pig-related Salmonella serotypes and clones associated with human infections is a topic of great concern [,,]. Specific adaptive features, such as colonization/virulence determinants, have been pointed out as an advantage for the maintenance and spread of these serotypes/clones in diverse environments and hosts (pig/human) []. Recent studies have found the presence of several virulence genes, associated with an enhanced adaptation to the food-animal host, in S. Typhimurium [,], in specific clones of S. 1,4,[5],12:i:- strains circulating in Europe [,,,] and in S. Rissen, including in isolates belonging to the ST469 [,]. Those virulence genes encode for proteins that improve colonization (e.g., clpB), adhesion (e.g., csgA, fimA/C, pefA, stbD, marT), intestinal invasion (e.g., invA, invE, spvC), survival in host tissues (e.g., sopA, avrA, sseI, mig5) and biofilm formation (e.g., bss) [,,,,]. Interestingly, S. Typhimurium DT193 and S. 1,4,[5],12:i:- were associated with long-term survival in pig faeces comparing with other serotypes (S. Derby and S. Bredeney), due to their increased adaptation to acid fecal pH and organic acid supplementation of feed []. Additionally, a UK study demonstrated that SPI-23 present in S. Derby strains, which contain genes (e.g., potR) that encode Type III effector proteins, contributes for the host intestinal cells invasion in pigs []. More recently, the presence of this SPI-23 was also reported in French pork isolates of S. Derby ST39 and ST40, helping to explain the host pig specificity of those epidemic strains [].
Moreover, those emergent pig-related Salmonella serotypes/clones were usually enriched with antimicrobial resistance genes, in most cases located in the mobile genetic elements that also carry virulence genes. For example, resistance plasmids of S. 1,4,[5],12:i:- isolates (from pigs and humans) circulating in Europe, carry several virulence genes, namely spvC±mig5 genes in IncA/C and IncR plasmids, associated with the Spanish and Southern European clones, respectively []. Furthermore, several genes associated with tolerance to metals and/or biocides (e.g., copper), widely used in food-animal production, were found in pig-related Salmonella serotypes/clones (e.g., S. Rissen MDR clone, European clone of S. 1,4,[5],12:i:- and S. Typhimurium), which might also be an additional advantage for their maintenance and spread in the food production environment and hosts (pig/human) [,,].
5. Antimicrobial Resistance in Salmonella and the Pork Linkage
Antibiotic resistance is considered by several relevant public health entities one of the major threats to human health and a relevant concern for food safety, particularly if involves pathogenic bacteria transmitted to humans through food-chain [,]. The emergence and spread of Salmonella isolates presenting resistance to several antibiotics, especially to “Highest Priority Critically Important Antimicrobials” (fluoroquinolones and 3rd and higher generations cephalosporins) [], is of concern since they are crucial to the successful treatment of NTS invasive infections [,]. The adverse consequences of resistance to critically important antibiotics in humans include an increase in the severity of infections and in the frequency of treatment failures, as well as the requirement of last-line antibiotics use (e.g., carbapenems, colistin) [,].
The common practice of antibiotic use in intensive food-animal production has been considered the main driver for the selection and transmission of antibiotic-resistant foodborne bacteria, including Salmonella, to humans [,,,,,,]. This scenario is aggravated particularly in pig production, which has been associated with a higher antimicrobial consumption, compared with other animal-food production systems, at global level [], including in the EU []. In 2010, the annual average of antimicrobial consumption per kilogram of animal produced was 172 mg·kg−1 in pigs, higher than the 148 mg·kg−1 and 45 mg·kg−1 consumption in chicken and cattle, respectively []. Although there is still controversy about the contribution of food-animal reservoirs and food vehicles in the transmission of antibiotic-resistant bacteria with an impact in human health, there is accumulating evidence linking the pig production with antimicrobial resistance in NTS that will be discussed in the next sections.
5.1. Association between Antibiotic Use in Pig Production and Resistance in Salmonella
The first evidence of this linkage is the association between the amount and pattern of antimicrobial agents used in the pig production and the occurrence of resistant NTS in pigs, pork meat and/or humans. One illustrative case in pig production includes a study showing that the administration of tetracycline to pigs colonized with tetracycline-resistant S. Typhimurium DT104 was associated with higher pig shedding of this resistant strain compared with untreated pigs []. Other study reported that enrofloxacin, used for treatment of pigs, induced the selection of S. Typhimurium with decreased susceptibility to ciprofloxacin []. Furthermore, a Danish surveillance study performed after the ban of antibiotics as growth promoters showed higher levels of tetracycline resistance in S. Typhimurium isolates recovered from pig and human clinical cases, potentially caused by an increased usage of tetracycline in pigs [].
5.2. Correlation of Antimicrobial Resistance Rates between Salmonella from Pigs and Humans
The correlation between antibiotic resistance rates among Salmonella from pigs, pork meat and humans obtained from systematic surveillance data evidences the impact of pig production practices on NTS antibiotic resistance. The 2016 EFSA report showed a high prevalence of MDR Salmonella in humans (29.3%), pigs (58.7%) and pork meat (40.4%), including the most used antibiotics in pig production (tetracyclines, penicillin’s, sulphonamides and colistin) [,]. In fact, high levels of resistance to A-27.8%, Su-32.4% and T-28.1% and MDR-29.3% were observed in Salmonella from human isolates, as well as from pigs (A-45.3%; Su-52.6%; T-53.5%; MDR-43.9%) and pork meat (A-44.7%; Su-48.5%; T-49.1%; MDR-40.4%). Furthermore, the ASuT phenotype was the most frequent MDR profile observed in pig and pork meat (82.3% and 80%, respectively), with the majority of the isolates belonging to the pig-related serotype S. 1,4,[5],12:i:- (66.4%-pig and 69.6%-pork meat) []. Indeed, high levels of MDR were observed in pig-related serotypes, namely S. 1,4,[5],12:i:- (MDR = 81.1%-humans; 82.3%-pigs; 73.8%-pork meat), S. Typhimurium (44.4%-humans; 52.4%-pigs; 54.5%-pork meat), S. Derby (23.8%-humans; 20.3%-pigs; 10.4%-pork meat) and S. Rissen (33.3%-pigs; 52.8%-pork meat). Additionally, this report highlights that those pig-related serotypes were the major contributors to the observed prevalence of resistance in Salmonella in both pig and pork meat samples. From 2014 to 2015 MDR in humans increased more than 10% in both S. Typhimurium and S. 1,4,[5],12:i:- serotypes []. In the USA, data from the National Antimicrobial Resistance Monitoring System (NARMS) showed an increase of the ASSuT phenotype in S. 1,4,[5],12:i:- from human (from 43% in 2014 to 60% in 2015), and swine isolates (65% in 2015) [].
Data about MDR phenotypes are of concern due to the possible role of diverse antibiotics in the co-selection of Salmonella strains resistant to clinically-relevant ones, such as fluoroquinolones (e.g., ciprofloxacin-Cp), extended-spectrum cephalosporins (e.g., ceftazidime-Caz, cefotaxime-Ctx) and colistin (Col) [,,,]. In the last EU report, relatively low levels of Salmonella resistance to Cp (13,3%), Caz (0.9%), Ctx (0.9%), and Col (11.4%) were observed [,]. Moreover, the highest levels of Col resistance in humans (excluding the S. Enteritidis serotype which presents intrinsic resistance) were more common in the pig-related serotypes S. 1,4,[5],12:i:- (2.4%) and S. Typhimurium (1.5%) [,]. Additionally, data from 2015 in USA revealed low levels of decreased susceptibility to Cp (5.8%) among humans, being the highest levels detected in retail pork samples (5.3%) comparing with other retail meat (0% in poultry and beef) [], which suggest an important contribution of pig production chain to the human disease burden.
In contrast, high rates of antibiotic resistance were reported among humans and pig/pork products in middle-income countries. For instance, high rates of Salmonella with resistance to Cp (15–48%), ceftriaxone-Cro (38%) and Col (36%) were observed in humans [,,]. High levels of resistance to clinically-relevant antibiotics were also observed in pigs or pork meat, in particular resistance to Cp (10–49.4%) [,,] and Col (7–21%) [,], being in most cases higher than those detected in poultry samples (Cp-0–29%, Col-3.8%) [,,]. Moreover, different Chinese studies reported higher rates of acquired Col resistance mechanisms (Mobile Colistin Resistance—MCR) in pigs (10–14.8%) than in poultry samples (0.8–7.5%) [,,]. Indeed, it was already suggested that pig production has the highest impact on colistin resistance in humans because of its extensive use in this system contrasting with other food-producing animals [,]. Overall, the high incidence of resistance to clinically-relevant antibiotics observed in Salmonella from pig production highlights the potential role of pork products in its spread and may be the consequence of the high, inappropriate or uncontrolled use of antibiotics in farming practices in middle-income countries [,,,,,]. In fact, it was estimated that the global consumption of antimicrobials in livestock will increase by 67% between 2010 and 2030, with an expected increase in pigs of 124%, particularly in Asia, in order to respond to the increasing demand of pork meat, one of the most consumed and traded meat products [,,]. Hence, international trade of live pigs (piglets, weaner, grower or breeder pigs) and pork meat can contribute for the worldwide spread of antibiotic-resistant Salmonella, with a consequent impact on human health.
5.3. Transmission of Antimicrobial Resistant Salmonella from Pork Meat to Humans
The presence of the same mobile genetic elements carrying clinically-relevant antibiotic resistance genes and/or antibiotic-resistant Salmonella serotypes/clones in pig and human isolates is an additional evidence of transmission of antimicrobial resistant Salmonella from pork meat to humans. In the last decades, clinically-relevant antibiotic resistance genes such as those coding for extended-spectrum β-lactamases (ESBLs), acquired AmpC β-lactamases (qAmpC), plasmid-mediated quinolone resistance (PMQR) and more recently plasmid-mediated colistin resistance (MCR), have been globally reported in a wide range of Salmonella serotypes associated with pig and pork products (Table 2). Additionally, some examples that illustrate the linkage and transmission of MDR Salmonella from pigs/pork products to humans are shown in Table 2. For instance, as examples of strains/clones shared between pork and human were S. Typhimurium with blaCTX-M-1, blaCMY-2 or oqxAB±aac(6′)-Ib-cr, Salmonella Virchow with blaCTX-M-15, and S. Typhimurium, S. 1,4,[5],12:i:- or S. Bovismorbificans carrying mcr-1 gene (Table 2).

Table 2.
Salmonella serotypes/clones carrying clinically-relevant antibiotic resistance genes recovered from pig and products thereof.
The most prevalent genes coding for resistance to extended-spectrum cephalosporins were those coding for CTX-M enzymes (e.g., CTX-M-1, CTX-M-14, CTX-M-15) followed by qAmpC CMY-2, both reported in pigs/pork products and humans, and increasingly associated with pig-related serotypes (e.g., S. Typhimurium and S. 1,4,[5],12:i:-) (Table 2) [,]. Plasmid-mediated quinolone resistance (PMQR) mechanisms have been also widely reported in diverse NTS serotypes from both pig/pork products and human isolates [,,]. The most frequent PMQR were QNR proteins (e.g., QnrB19, QnrS1/S2) found in diverse regions and serotypes (Table 2). Also the aminoglycoside acetyltransferase AAC(6′)-Ib-cr, commonly associated with the efflux pump OqxAB, was widely reported among relevant pig-related NTS serotypes (S. Typhimurium, S. Derby and S. Rissen), particularly in Asian countries (Table 2). Finally, pig/pork seems to be an important vehicle of NTS carrying the emergent plasmid-mediated colistin resistance mechanism, (mostly MCR-1) (Table 2), also predominantly in pig-related NTS serotypes causing human infections [,,,].
All these data demonstrate that pig/pork products might be an important vehicle for the transmission and dissemination of NTS carrying acquired clinically-relevant resistance genes to humans through the food chain. The most frequent and clinically-relevant antibiotic resistance genes and their associated genetic elements (such as specific plasmids) reported in pigs/pork products were also found in NTS human isolates (Table 2). In fact, transmission of the most commonly reported genes in both sources has been associated with several epidemic plasmid families, such as IncN (blaCTX-M-1, -27, -65), IncFIB (blaCTX-M-14, -27, -65, blaCMY-2), IncA/C (blaCTX-M-27, blaCMY-2), IncI1 (blaCTX-M-1, blaCMY-2), IncHI2 (blaCTX-M-1, -14, -15, oqxAB±aac(6′)-Ib-cr, mcr-1), IncI2 or IncX4 (mcr-1) (Table 2) [,,,]. More worrying is the presence, in the same Salmonella strains, of different clinically-relevant antibiotic resistance genes (e.g., mcr-1+blaCTX-M-1, mcr-1+oqxAB+aac(6′)-Ib-cr) co-located in the same genetic elements [,] (Table 2), worsening the possibility of clinical treatment failure of invasive NTS infections.
The acquisition of resistance mechanisms to antibiotics commonly used in food-animal production (e.g., ampicillin, sulphonamides, tetracyclines) is also relevant for their potential role in the co-selection of pig-related MDR Salmonella clones in pig production and further transmission to humans. Several studies have provided evidence of successful transmission of MDR Salmonella clones from diverse serotypes from pork to humans [,,,,,]. For example, we recently reported mcr-1 located on epidemic plasmids (IncX4 and IncHI2) in clinically-relevant MDR S. 1,4,[5],12:i:-/ST34 []. Additionally, in China, diverse studies reported the clonal spread of mcr-1+oqxAB+aac(6′)-Ib-cr in S. Typhimurium/ST34 in pigs presenting resistance to multiple antibiotics (e.g., A, S, Su, T) [,]. Moreover, some of these successful clones presented acquired metal tolerance genes, an additional feature that might be contributing for the survival and persistence of these strains in metal contaminated environments, such as the pig production setting [,,,]. For example, sil±pco genes encoding for copper/silver tolerance were often found in the emergent European clone of S. 1,4,[5],12:i:- and S. Typhimurium, as well as S. Rissen/ST469 MDR clone [,,]. In fact, copper is one of the most used metal compounds in pig setting (e.g., as supplements in animal feed), suggesting that in these environments higher selective pressures could contribute for the co-selection of MDR NTS clones [,,,,,,], with consequences for food safety and human health.
The data presented here show that pig production setting can be a relevant reservoir of successful and worldwide emergent MDR pig-related Salmonella serotypes/clones, enriched with different adaptive features (e.g., metal/biocides tolerance genes) besides genes conferring resistance to critical antibiotics, which might spread to humans through the food chain. Moreover, the increasing trend of intensive pig production systems and the globalization of pork products pose a major challenge for the spread of antimicrobial resistance in zoonotic bacteria such as NTS, with a consequent impact on human health. Therefore, it is crucial to restrain the use of antimicrobial agents in pig production and to improve biosecurity measures (e.g., high standards of hygiene, regular veterinary checks, vaccination), aiming to minimize selection and spread of MDR clones along all stages of pig production chain.
6. Conclusions
Pork products are among the most frequent foodstuffs implicated in human salmonellosis, with pig and pork meat reported worldwide as important sources of NTS resistant to clinically-relevant antibiotics, representing a major threat to the treatment of invasive infections. Furthermore, the high incidence of resistance to clinically-relevant antibiotics reported in diverse countries together with the increasing demand for pork meat and the global trade of pig/pork products raised the current public health concern. Hence, the continuous control and monitoring of Salmonella, particularly targeting specific MDR pig-related serotypes/clones, along the food chain (from primary production to consumption) is critical to minimize their introduction in the food-animal production and further transmission to humans. Therefore, a global integrated surveillance (“One Health” approach), and the implementation of more effective measures are critically needed, including the improvement of biosecurity measures at farms (e.g., providing uncontaminated feed, isolation of new purchased animals, high standards of hygiene, regular veterinary checks, vaccination), during slaughtering/processing (e.g., prevent external sources of contamination during transport, lairage and slaughter, cross-contamination with equipment and workers and hygiene practices such as cleaning and disinfection) and retail/consumer level (e.g., avoiding cross-contamination, using safe cooking temperature). Surveillance of antibiotic resistance levels in NTS throughout the pig food chain is crucial to ensure public health, not only through the detection of new food safety risks involving foodstuffs such as pork meat but also to avoid salmonellosis treatment failures.
Author Contributions
Conceptualization and review design, J.C. and P.A.; Manuscript writing, J.C.; Review and editing of the manuscript, J.M., L.P. and P.A.; Manuscript critical revision for important intellectual content and final approval for submission, L.P. and P.A.
Funding
This work was supported by the Applied Molecular Biosciences Unit- UCIBIO which is financed by national funds from FCT/MCTES (UID/Multi/04378/2019) and by the QREN project NORTE-01-0145-FEDER-000011–Qualidade e Segurança Alimentar—uma abordagem (nano) tecnológica. J.C. was supported by a Ph.D. fellowship from FCT (grant number SFRH/BD/93091/2013) and J.M. was supported by a post-doc fellowship from project NORTE-01-0145- FEDER-000024.
Acknowledgments
The authors would like to thank to the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Food- and Water-borne Infections Study Group (EFWISG).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Crump, J.A.; Sjölund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [PubMed]
- Arya, G.; Holtslander, R.; Robertson, J.; Yoshida, C.; Harris, J.; Parmley, J.; Nichani, A.; Johnson, R.; Poppe, C. Epidemiology, Pathogenesis, Genoserotyping, Antimicrobial Resistance, and Prevention and Control of Non-Typhoidal Salmonella Serovars. Curr. Clin. Microbiol. Rep. 2017, 4, 43–53. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Critically Important Antimicrobials for Human Medicine—5th Revision 2016—Ranking of Medically Important Antimicrobials for Risk Management of Antimicrobial Resistance Due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/handle/10665/255027/9789241512220-eng.pdf;jsessionid=72B265EF2D6C6CBC2136A8634744F0CB?sequence=1 (accessed on 13 March 2018).
- Centers for Disease Control and Prevention (CDC). Surveillance for Foodborne Disease Outbreaks, United States, 2015, Annual Report; US Department of Health and Human Services, CDC: Atlanta, Georgia, 2017. Available online: https://www.cdc.gov/foodsafety/pdfs/2015FoodBorneOutbreaks_508.pdf (accessed on 13 March 2018).
- European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar]
- European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, 5077. [Google Scholar]
- European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016, 14, 4634. [Google Scholar]
- European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015, 13, 4329. [Google Scholar]
- Hugas, M.; Beloeil, P. Controlling Salmonella along the food chain in the European Union—Progress over the last ten years. Eurosurveillance 2014, 19, 20804. [Google Scholar] [CrossRef]
- Glass, K.; Fearnley, E.; Hocking, H.; Raupach, J.; Veitch, M.; Ford, L.; Kirk, M.D. Bayesian Source Attribution of Salmonellosis in South Australia. Risk Anal. 2016, 36, 561–570. [Google Scholar] [CrossRef]
- Bonardi, S. Salmonella in the pork production chain and its impact on human health in the European Union. Epidemiol. Infect. 2017, 145, 1513–1526. [Google Scholar] [CrossRef]
- Wong, D.M.A.L.F.; Hald, T.; van der Wolf, P.; Swanenburg, M. Epidemiology and control measures for Salmonella in pigs and pork. Livest. Prod. Sci. 2002, 76, 215–222. [Google Scholar] [CrossRef]
- Vidic, B.; Savic, S.; Prica, N.; Suvajdzic, L. Epizootiology and Control Measures for Salmonella in Pigs. Procedia Food Sci. 2015, 5, 312–315. [Google Scholar] [CrossRef]
- Communicable Diseases Branch. OzFoodNet—Enhancing Foodborne Disease Surveillance across Australia Third Quarter Summary, July—September, 2017 NSW; Health Protection NSW: Sydney, Australia, 2018. Available online: https://www.health.nsw.gov.au/Infectious/foodborne/Publications/NSW-3rd-quarterly-report-2017.pdf (accessed on 13 March 2018).
- Government of Canada. National Enteric Surveillance Program Annual Summary 2014; Public Health Agency of Canada: Guelph, ON, Canada, 2016. Available online: http://publications.gc.ca/collections/collection_2016/aspc-phac/HP37-15-2014-eng.pdf (accessed on 13 March 2018).
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Wong, D.M.A.L.F.; Jensen, A.B.; Wegener, H.C.; Aarestrup, F.M. Global Monitoring of Salmonella Serovar Distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: Results of Quality Assured Laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Ke, B.; Deng, X.; Liang, J.; Ran, L.; Lu, L.; He, D.; Huang, Q.; Ke, C.; Li, Z.; et al. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal Salmonella from human patients in Guangdong Province, China, 2009–2012. BMC Infect. Dis. 2015, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Sekizuka, T.; Tamamura, Y.; Tanaka, K.; Barco, L.; Izumiya, H.; Kusumoto, M.; Hinenoya, A.; Yamasaki, S.; Iwata, T.; et al. Phylogenetic Characterization of Salmonella enterica Serovar Typhimurium and Its Monophasic Variant Isolated from Food Animals in Japan Revealed Replacement of Major Epidemic Clones in the Last 4 Decades. J. Clin. Microbiol. 2018, 56, e01758-17. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, S.; Alpigiani, I.; Bruini, I.; Barilli, E.; Brindani, F.; Morganti, M.; Cavallini, P.; Bolzoni, L.; Pongolini, S. Detection of Salmonella enterica in pigs at slaughter and comparison with human isolates in Italy. Int. J. Food Microbiol. 2016, 218, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kerouanton, A.; Rose, V.; Weill, F.-X.; Granier, S.A.; Denis, M. Genetic Diversity and Antimicrobial Resistance Profiles of Salmonella enterica Serotype Derby Isolated from Pigs, Pork, and Humans in France. Foodborne Pathog. Dis. 2013, 10, 977–984. [Google Scholar] [CrossRef] [PubMed]
- García-Fierro, R.; Montero, I.; Bances, M.; González-Hevia, M.Á.; Rodicio, M.R. Antimicrobial Drug Resistance and Molecular Typing of Salmonella enterica Serovar Rissen from Different Sources. Microb. Drug Resist. 2016, 22, 211–217. [Google Scholar] [CrossRef]
- Piras, F.; Brown, D.J.; Meloni, D.; Mureddu, A.; Mazzette, R. Investigation of Salmonella enterica in Sardinian slaughter pigs: Prevalence, serotype and genotype characterization. Int. J. Food Microbiol. 2011, 151, 201–209. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet 2015 Surveillance Report (Final Data); U.S. Department of Health and Human Services, CDC: Atlanta, Georgia, 2017. Available online: https://www.cdc.gov/foodnet/pdfs/FoodNet-Annual-Report-2015-508c.pdf (accessed on 13 March 2018).
- Mourão, J.; Machado, J.; Novais, C.; Antunes, P.; Peixe, L. Characterization of the emerging clinically-relevant multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- (monophasic variant of S. Typhimurium) clones. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2249–2257. [Google Scholar] [CrossRef]
- Mourão, J.; Marçal, S.; Ramos, P.; Campos, J.; Machado, J.; Peixe, L.; Novais, C.; Antunes, P. Tolerance to multiple metal stressors in emerging non-typhoidal MDR Salmonella serotypes: A relevant role for copper in anaerobic conditions. J. Antimicrob. Chemother. 2016, 71, 2147–2157. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2015. EFSA J. 2017, 15, 4694. [Google Scholar]
- Hendriksen, R.S.; Bangtrakulnonth, A.; Pulsrikarn, C.; Pornreongwong, S.; Hasman, H.; Song, S.W.; Aarestrup, F.M. Antimicrobial Resistance and Molecular Epidemiology of Salmonella Rissen from Animals, Food Products, and Patients in Thailand and Denmark. Foodborne Pathog. Dis. 2008, 5, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Arnott, A.; Wang, Q.; Bachmann, N.; Sadsad, R.; Biswas, C.; Sotomayor, C.; Howard, P.; Rockett, R.; Wiklendt, A.; Iredell, J.R.; et al. Multidrug-Resistant Salmonella enterica 4,[5],12:i:- Sequence Type 34, New South Wales, Australia, 2016–2017. Emerg. Infect. Dis. 2018, 24, 751–753. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the Control of Salmonella and Other Specified Food-Borne Zoonotic Agents. Off. J. Eur. Union 2003, L 325, 1–15. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32003R2160 (accessed on 10 May 2018).
- Stiftung, H. Meat Atlas: Facts and Figures about the Animals We Eat; Heinrich-Böll-Stiftung: Berlin, Germany; Brussels, Belgium, 2014; Available online: https://www.boell.de/sites/default/files/meat_atlas2014_kommentierbar.pdf?dimension1=ds_fleischatlas_2014 (accessed on 15 May 2018).
- World’s Top Exports. Available online: www.worldstopexports.com/pork-exports-by-country (accessed on 15 May 2018).
- European Food Safety Authority (EFSA). Analysis of the baseline survey on the prevalence of Salmonella in holdings with breeding pigs in the EU, 2008—Part A: Salmonella prevalence estimates. EFSA J. 2009, 7, 1377. [Google Scholar]
- European Food Safety Authority (EFSA). Analysis of the baseline survey on the prevalence of Salmonella in holdings with breeding pigs, in the EU, 2008—Part B: Factors associated with Salmonella pen positivity. EFSA J. 2011, 9, 2329. [Google Scholar]
- Food Control Consultants Ltd Consortium (FCC Consortium). Analysis of the Costs and Benefits of Setting a Target for the Reduction of Salmonella in Breeding Pigs for European Commission Health and Consumers Directorate-General SANCO/2008/E2/056, Final Report. 2011. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/biosafety_food-borne-disease_salmonella_breeding-pigs_salm-cost-benefit.pdf (accessed on 10 May 2018).
- Andres, V.M.; Davies, R.H. Biosecurity Measures to Control Salmonella and Other Infectious Agents in Pig Farms: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 317–335. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on biological hazards (BIOHAZ) related to “Risk assessment and mitigation options of Salmonella in pig production”. EFSA J. 2006, 4, 341. [Google Scholar] [CrossRef]
- Department for Environment, Food and Rural Affairs (DEFRA) of Public Health England. Zoonoses Report UK 2013. 2015. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/447771/pb13987-zoonoses-report-2013.pdf (accessed on 10 May 2018).
- Commission Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying down Specific Hygiene Rules for Food of Animal Origin. Off. J. Eur. Union 2004, L 139, 55–205. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32004R0853 (accessed on 10 May 2018).
- Gomes-Neves, E.; Antunes, P.; Tavares, A.; Themudo, P.; Cardoso, M.F.; Gärtner, F.; Costa, J.M.; Peixe, L. Salmonella cross-contamination in swine abattoirs in Portugal: Carcasses, meat and meat handlers. Int. J. Food Microbiol. 2012, 157, 82–87. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on a Quantitative Microbiological Risk Assessment of Salmonella in slaughter and breeder pigs. EFSA J. 2010, 8, 1547. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Cao, Z.; Wang, L.; Li, X.; Li, S.; Xu, Y. Bacteriophages as antimicrobial agents against major pathogens in swine: A review. J. Anim. Sci. Biotechnol. 2015, 6, 39. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 50, 1–26.
- Commission Regulation (EC) No 217/2014 of 7 March 2014 amending Regulation (EC) No 2073/2005 as regards Salmonella in pig carcases. Off. J. Eur. Union 2014, 69, 93–94. Available online: https://publications.europa.eu/en/publication-detail/-/publication/2a5f9172-a699-11e3-8438-01aa75ed71a1/language-en (accessed on 10 May 2018).
- U.S. Food and Drug Administration (FDA). Available online: www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm059103.htm (accessed on 2 June 2018).
- Kich, J.D.; Coldebella, A.; Morés, N.; Nogueira, M.G.; Cardoso, M.; Fratamico, P.M.; Call, J.E.; Fedorka-Cray, P.; Luchansky, J.B. Prevalence, distribution, and molecular characterization of Salmonella recovered from swine finishing herds and a slaughter facility in Santa Catarina, Brazil. Int. J. Food Microbiol. 2011, 151, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Montes de Oca, S.; Talavera-Rojas, M.; Soriano-Vargas, E.; Barba-León, J.; Vázquez-Navarrete, J.; Acosta-Dibarrat, J.; Salgado-Miranda, C. Phenotypic and genotypic profile of clinical and animal multidrug-resistant Salmonella enterica isolates from Mexico. J. Appl. Microbiol. 2018, 124, 67–74. [Google Scholar] [CrossRef]
- Kikuvi, G.M.; Ombui, J.N.; Mitema, E.S. Serotypes and antimicrobial resistance profiles of Salmonella isolates from pigs at slaughter in Kenya. J. Infect. Dev. Ctries. 2010, 4, 243–248. [Google Scholar] [CrossRef]
- Akoachere, J.-F.T.K.; Tanih, N.F.; Ndip, L.M.; Ndip, R.N. Phenotypic characterization of Salmonella Typhimurium isolates from food-animals and abattoir drains in Buea, Cameroon. J. Health Popul. Nutr. 2009, 27, 612–618. [Google Scholar]
- Ma, S.; Lei, C.; Kong, L.; Jiang, W.; Liu, B.; Men, S.; Yang, Y.; Cheng, G.; Chen, Y.; Wang, H. Prevalence, Antimicrobial Resistance, and Relatedness of Salmonella Isolated from Chickens and Pigs on Farms, Abattoirs, and Markets in Sichuan Province, China. Foodborne Pathog. Dis. 2017, 14, 667–677. [Google Scholar] [CrossRef]
- Trongjit, S.; Angkititrakul, S.; Tuttle, R.E.; Poungseree, J.; Padungtod, P.; Chuanchuen, R. Prevalence and antimicrobial resistance in Salmonella enterica isolated from broiler chickens, pigs and meat products in Thailand-Cambodia border provinces. Microbiol. Immunol. 2017, 61, 23–33. [Google Scholar] [CrossRef]
- Rahmat, S.; Cheong, C.B.; Hamid, M.S.R.B.A. Challenges of Developing Countries in Complying Quality and Enhancing Standards in Food Industries. Procedia Soc. Behav. Sci. 2016, 224, 445–451. [Google Scholar] [CrossRef]
- Marder, E.P.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Hurd, S.; Jervis, R.; Lathrop, S.; Muse, A.; Ryan, P.; Smith, K.; et al. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2006–2017. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.P.H.; Phuong, T.L.T.; Huu, H.N.; Thuy, L.; Mather, A.E.; Park, S.E.; Marks, F.; Thwaites, G.E.; Van Vinh Chau, N.; Thompson, C.N.; et al. Invasive Non-typhoidal Salmonella Infections in Asia: Clinical Observations, Disease Outcome and Dominant Serovars from an Infectious Disease Hospital in Vietnam. PLoS Negl. Trop. Dis. 2016, 10, e0004857. [Google Scholar]
- Flockhart, L.; Pintar, K.; Cook, A.; McEwen, S.; Friendship, R.; Kelton, D.; Pollari, F. Distribution of Salmonella in Humans, Production Animal Operations and a Watershed in a FoodNet Canada Sentinel Site. Zoonoses Public Health 2017, 64, 41–52. [Google Scholar] [CrossRef]
- Silveira, L.; Marques, A.; Machado, J. Infecҫões por Salmonella Enterica no Período Entre 2000–2012; Boletim Epidemiológico; Instituto Nacional de Saúde Dr Ricardo Jorge (INSA): Lisbon, Portugal, 2013; Available online: http://repositorio.insa.pt/bitstream/10400.18/1680/1/observações%20Nº%20Especial%201%202013_artigo6.pdf (accessed on 10 May 2018).
- Silveira, L.; Pista, Â.; Machado, J. Caracterização Fenotípica e Genotípica de Isolados de Salmonella Enterica Recebidos no INSA Entre 2014 e 2016; Boletim Epidemiológico; Instituto Nacional de Saúde Dr Ricardo Jorge (INSA): Linbon, Portugal, 2017; Available online: http://repositorio.insa.pt/bitstream/10400.18/5592/1/Boletim_Epidemiologico_Observacoes_N22_2018_artigo11.pdf (accessed on 10 May 2018).
- Tamang, M.D.; Gurung, M.; Nam, H.-M.; Moon, D.C.; Kim, S.-R.; Jang, G.-C.; Jung, D.-Y.; Jung, S.-C.; Park, Y.-H.; Lim, S.-K. Prevalence and characterization of Salmonella in pigs from conventional and organic farms and first report of S. serovar 1,4,[5],12:i:- from Korea. Vet. Microbiol. 2015, 178, 119–124. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Zhang, B.; Sun, S.; Chang, W. Characterization of Integrons and Resistance Genes in Salmonella Isolates from Farm Animals in Shandong Province, China. Front. Microbiol. 2017, 8, 1300. [Google Scholar] [CrossRef] [PubMed]
- Dahshan, H.; Chuma, T.; Shahada, F.; Akiba, M.; Fujimoto, H.; Akasaka, K.; Kamimura, Y.; Okamoto, K. Characterization of antibiotic resistance and the emergence of AmpC-producing Salmonella Infantis from pigs. J. Vet. Med. Sci. 2010, 72, 1437–1442. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Available online: http://www.cdc.gov/salmonella/outbreaks.html (accessed on 5 April 2018).
- Australian Government Department of Health (AGDH). Available online: www.health.gov.au/ (accessed on 5 April 2018).
- Antunes, P.; Mourão, J.; Pestana, N.; Peixe, L. Leakage of emerging clinically relevant multidrug-resistant Salmonella clones from pig farms. J. Antimicrob. Chemother. 2011, 66, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Machado, J.; Peixe, L. Characterization of antimicrobial resistance and class 1 and 2 integrons in Salmonella enterica isolates from different sources in Portugal. J. Antimicrob. Chemother. 2006, 58, 297–304. [Google Scholar] [CrossRef]
- Butaye, P.; Michael, G.B.; Schwarz, S.; Barrett, T.J.; Brisabois, A.; White, D.G. The clonal spread of multidrug-resistant non-typhi Salmonella serotypes. Microbes Infect. 2006, 8, 1891–1897. [Google Scholar] [CrossRef]
- Gomes-Neves, E.; Antunes, P.; Manageiro, V.; Gärtner, F.; Caniça, M.; da Costa, J.M.C.; Peixe, L. Clinically relevant multidrug resistant Salmonella enterica in swine and meat handlers at the abattoir. Vet. Microbiol. 2014, 168, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Threlfall, E.J. Epidemic Salmonella Typhimurium DT 104—A truly international multiresistant clone. J. Antimicrob. Chemother. 2000, 46, 7–10. [Google Scholar] [CrossRef]
- Antunes, P.; Coque, T.M.; Peixe, L. Emergence of an IncIγ plasmid encoding CMY-2 ß-lactamase associated with the international ST19 OXA-30-producing ß-lactamase Salmonella Typhimurium multidrug-resistant clone. J. Antimicrob. Chemother. 2010, 65, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Machado, J.; Sousa, J.C.; Peixe, L. Dissemination amongst humans and food products of animal origin of a Salmonella Typhimurium clone expressing an integron-borne OXA-30 beta-lactamase. J. Antimicrob. Chemother. 2004, 54, 429–434. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Montero, I.; Bances, M.; Rodicio, R.; Rodicio, M.R. Incidence and Genetic Bases of Nitrofurantoin Resistance in Clinical Isolates of Two Successful Multidrug-Resistant Clones of Salmonella enterica Serovar Typhimurium: Pandemic “DT 104” and pUO-StVR2. Microb. Drug Resist. 2017, 23, 405–412. [Google Scholar] [CrossRef]
- Lucarelli, C.; Dionisi, A.M.; Torpdahl, M.; Villa, L.; Graziani, C.; Hopkins, K.; Threlfall, J.; Caprioli, A.; Luzzi, I. Evidence for a Second Genomic Island Conferring Multidrug Resistance in a Clonal Group of Strains of Salmonella enterica Serovar Typhimurium and its Monophasic Variant Circulating in Italy, Denmark, and the United Kingdom. J. Clin. Microbiol. 2010, 48, 2103–2109. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Kirchner, M.; Guerra, B.; Granier, S.A.; Lucarelli, C.; Porrero, M.C.; Jakubczak, A.; Threlfall, E.J.; Mevius, D.J. Multiresistant Salmonella enterica serovar 4,[5],12:i:- in Europe: A new pandemic strain? Euro Surveill. 2010, 15, 19580. [Google Scholar]
- Hauser, E.; Tietze, E.; Helmuth, R.; Junker, E.; Blank, K.; Prager, R.; Rabsch, W.; Appel, B.; Fruth, A.; Malorny, B. Pork Contaminated with Salmonella enterica Serovar 4,[5],12:i:-, an Emerging Health Risk for Humans. Appl. Environ. Microbiol. 2010, 76, 4601–4610. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on monitoring and assessment of the public health risk of “Salmonella Typhimurium-like” strains. EFSA J. 2010, 8, 1826. [Google Scholar] [CrossRef]
- Pesciaroli, M.; Cucco, L.; De Luca, S.; Massacci, F.R.; Maresca, C.; Medici, L.; Paniccià, M.; Scoccia, E.; Staffolani, M.; Pezzotti, G.; et al. Association between pigs with high caecal Salmonella loads and carcass contamination. Int. J. Food Microbiol. 2017, 242, 82–86. [Google Scholar] [CrossRef]
- Carnevali, C.; Morganti, M.; Scaltriti, E.; Bolzoni, L.; Pongolini, S.; Casadei, G. Occurance of MCR-1 colistin-resitant Salmonella isolates recovered from human and animals in Italy, 2012–2015. Antimicrob. Agents Chemother. 2016, 60, 7532–7534. [Google Scholar]
- Lucarelli, C.; Dionisi, A.M.; Filetici, E.; Owczarek, S.; Luzzi, I.; Villa, L. Nucleotide sequence of the chromosomal region conferring multidrug resistance (R-type ASSuT) in Salmonella Typhimurium and monophasic Salmonella Typhimurium strains. J. Antimicrob. Chemother. 2012, 67, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Mandilara, G.; Lambiri, M.; Polemis, M.; Passiotou, M.; Vatopoulos, A. Phenotypic and molecular characterisation of multiresistant monophasic Salmonella Typhimurium (1,4,[5],12:i:-) in Greece, 2006 to 2011. Eurosurveillance 2013, 18, 20496. [Google Scholar] [PubMed]
- Mulvey, M.R.; Finley, R.; Allen, V.; Ang, L.; Bekal, S.; El Bailey, S.; Haldane, D.; Hoang, L.; Horsman, G.; Louie, M.; et al. Emergence of multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- involving human cases in Canada: Results from the Canadian Integrated Program on Antimicrobial Resistance Surveillance (CIPARS), 2003–2010. J. Antimicrob. Chemother. 2013, 68, 1982–1986. [Google Scholar] [CrossRef] [PubMed]
- Moreno Switt, A.I.; Soyer, Y.; Warnick, L.D.; Wiedmann, M. Emergence, Distribution, and Molecular and Phenotypic Characteristics of Salmonella enterica Serotype 4,5,12:i:-. Foodborne Pathog. Dis. 2009, 6, 407–415. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Malorny, B.; Hauser, E.; Mendoza, M.C.; Rodicio, M.R. Genetic Types, Gene Repertoire, and Evolution of Isolates of the Salmonella enterica Serovar 4,5,12:i:- Spanish Clone Assigned to Different Phage Types. J. Clin. Microbiol. 2013, 51, 973–978. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Guerra, B.; Bances, M.; Mendoza, M.C.; Rodicio, M.R. IncA/C plasmids mediate antimicrobial resistance linked to virulence genes in the Spanish clone of the emerging Salmonella enterica serotype 4,[5],12:i:-. J. Antimicrob. Chemother. 2011, 66, 543–549. [Google Scholar] [CrossRef]
- Antunes, P.; Machado, J.; Peixe, L. Dissemination of sul3-containing elements linked to class 1 integrons with an unusual 3′ conserved sequence region among Salmonella isolates. Antimicrob. Agents Chemother. 2007, 51, 1545–1548. [Google Scholar] [CrossRef]
- García, P.; Hopkins, K.L.; García, V.; Beutlich, J.; Mendoza, M.C.; Threlfall, J.; Mevius, D.; Helmuth, R.; Rodicio, M.R.; Guerra, B. Diversity of Plasmids Encoding Virulence and Resistance Functions in Salmonella enterica subsp. enterica Serovar Typhimurium Monophasic Variant 4,[5],12:i:- Strains Circulating in Europe. PLoS ONE 2014, 9, e89635. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Available online: https://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/UCM498134.pdf (accessed on 2 June 2018).
- Kérouanton, A.; Hirchaud, E.; Rose, V.; Esnault, E.; Naquin, D.; Denis, M. First Complete Genome Sequence of a Salmonella enterica subsp. enterica Serovar Derby Strain Associated with Pork in France. Genome Announc. 2015, 3, e00853-15. [Google Scholar]
- Vo, A.T.T.; van Duijkeren, E.; Fluit, A.C.; Wannet, W.J.B.; Verbruggen, A.J.; Maas, H.M.E.; Gaastra, W. Antibiotic resistance, integrons and Salmonella genomic island 1 among non-typhoidal Salmonella serovars in The Netherlands. Int. J. Antimicrob. Agents 2006, 28, 172–179. [Google Scholar] [CrossRef]
- Valdezate, S.; Vidal, A.; Herrera-León, S.; Pozo, J.; Rubio, P.; Usera, M.A.; Carvajal, A.; Echeita, M.A. Salmonella Derby Clonal Spread from Pork. Emerg. Infect. Dis. 2005, 11, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ren, X.; Feng, Z.; Fu, Y.; Hong, Y.; Shen, Z.; Zhang, L.; Liao, M.; Xu, X.; Zhang, J. Phenotypic Characteristics and Genetic Diversity of Salmonella enterica Serotype Derby Isolated from Human Patients and Foods of Animal Origin. Foodborne Pathog. Dis. 2017, 14, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Pornsukarom, S.; Patchanee, P.; Erdman, M.; Cray, P.F.; Wittum, T.; Lee, J.; Gebreyes, W.A. Comparative phenotypic and genotypic analyses of Salmonella Rissen that originated from food animals in Thailand and United States. Zoonoses Public Health 2015, 62, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.H.A.M.; de Jonge, R.; van Overbeek, W.M.; Bouw, E.; Pielaat, A.; Smid, J.H.; Malorny, B.; Junker, E.; Löfström, C.; Pedersen, K.; et al. A quantitative approach towards a better understanding of the dynamics of Salmonella spp. in a pork slaughter-line. Int. J. Food Microbiol. 2012, 153, 45–52. [Google Scholar] [CrossRef]
- Luzzi, I.; Galetta, P.; Massari, M.; Rizzo, C.; Dionisi, A.M.; Filetici, E.; Cawthorne, A.; Tozzi, A.; Argentieri, M.; Bilei, S.; et al. An Easter outbreak of Salmonella Typhimurium DT 104A associated with traditional pork salami in Italy. Eurosurveillance 2007, 12, 11–12. [Google Scholar] [CrossRef]
- Torpdahl, M.; Sorensen, G.; Ethelberg, S.; Sandø, G.; Gammelgård, K.; Porsbo, L.J. A regional outbreak of S. Typhimurium in Denmark and identification of the source using MLVA typing. Eurosurveillance 2006, 11, 134–136. [Google Scholar] [CrossRef]
- Bruun, T.; Sørensen, G.; Forshell, L.P.; Jensen, T.; Nygard, K.; Kapperud, G.; Lindstedt, B.A.; Berglund, T.; Wingstrand, A.; Petersen, R.F.; et al. An outbreak of Salmonella Typhimurium infections in Denmark, Norway and Sweden, 2008. Eurosurveillance 2009, 14, 19147. [Google Scholar]
- Kuhn, K.; Torpdahl, M.; Frank, C.; Sigsgaard, K.; Ethelberg, S. An outbreak of Salmonella Typhimurium traced back to salami, Denmark, April to June 2010. Eurosurveillance 2011, 16, 19863. [Google Scholar]
- Wójcik, O.P.; Kjelsø, C.; Kuhn, K.G.; Müller, L.; Jensen, T.; Kjeldsen, M.K.; Ethelberg, S. Salmonella Typhimurium outbreak associated with smoked pork tenderloin in Denmark, January to March 2011. Scand. J. Infect. Dis. 2012, 44, 903–908. [Google Scholar] [CrossRef]
- Arnedo-Pena, A.; Sabater-Vidal, S.; Herrera-León, S.; Bellido-Blasco, J.B.; Silvestre-Silvestre, E.; Meseguer-Ferrer, N.; Yague-Muñoz, A.; Gil-Fortuño, M.; Romeu-García, A.; Moreno-Muñoz, R. An outbreak of monophasic and biphasic Salmonella Typhimurium, and Salmonella Derby associated with the consumption of dried pork sausage in Castellon (Spain). Enferm. Infecc. Microbiol. Clin. 2016, 34, 544–550. [Google Scholar] [CrossRef]
- Australian Government Department of Health (AGDH). OzFoodNet: Foodborne Disease in Australia Annual Reports of the OzFoodNet Network. Available online: http://www.health.gov.au (accessed on 1 June 2018).
- RASFF (Rapid Alert System for Food and Feed). Available online: https://ec.europa.eu/food/safety/rasff_en (accessed on 1 June 2018).
- Mossong, J.; Marques, P.; Ragimbeau, C.; Huberty-Krau, P.; Losch, S.; Meyer, G.; Moris, G.; Strottner, C.; Rabsch, W.; Schneider, F. Outbreaks of monophasic Salmonella enterica serovar 4,[5],12:i:- in Luxembourg, 2006. Eurosurveillance 2007, 12, E11–E12. [Google Scholar] [CrossRef]
- Bone, A.; Noel, H.; Le Hello, S.; Pihier, N.; Danan, C.; Raguenaud, M.E.; Salah, S.; Bellali, H.; Vaillant, V.; Weill, F.X.; et al. Nationwide outbreak of Salmonella enterica serotype 4,12:i:- infections in France, linked to dried pork sausage, March–May 2010. Eurosurveillance 2010, 15, 1–3. [Google Scholar]
- Gossner, C.M.; van Cauteren, D.; Le Hello, S.; Weill, F.X.; Terrien, E.; Tessier, S.; Janin, C.; Brisabois, A.; Dusch, V.; Vaillant, V.; et al. Nationwide outbreak of Salmonella enterica serotype 4,[5],12:i:- infection associated with consumption of dried pork sausage, France, November to December 2011. Eurosurveillance 2012, 17, 20071. [Google Scholar] [CrossRef]
- Morganti, M.; Bolzoni, L.; Scaltriti, E.; Casadei, G.; Carra, E.; Rossi, L.; Gherardi, P.; Faccini, F.; Arrigoni, N.; Sacchi, A.R.; et al. Rise and fall of outbreak-specific clone inside endemic pulsotype of Salmonella 4,[5],12:i:-; insights from high-resolution molecular surveillance in Emilia-Romagna, Italy, 2012 to 2015. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Hernández Arricibita, E.; Santamaria Zuazua, R.; Ramos López, G.; Herrera-León, S.; Kárkamo Zuñeda, J.A.; Muniozguren Agirre, N. Brote de infecciones por Salmonella enterica serovar Typhimurium asociado al consumo de chorizo en Bizkaia. Enferm. Infecc. Microbiol. Clin. 2016, 34, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Trost, E.; Bender, J.; Fuchs, S.; Malorny, B.; Rabsch, W.; Prager, R.; Tietze, E.; Flieger, A. Evaluation of WGS based approaches for investigating a food-borne outbreak caused by Salmonella enterica serovar Derby in Germany. Food Microbiol. 2018, 71, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Gilsdorf, A.; Jansen, A.; Alpers, K.; Dieckmann, H.; van Treeck, U.; Hauri, A.M.; Fell, G.; Littmann, M.; Rautenberg, P.; Prager, R.; et al. A nationwide outbreak of Salmonella Bovismorbificans PT24, Germany, December 2004–March 2005. Eurosurveillance 2005, 10, E050324.1. [Google Scholar] [CrossRef]
- Brandwagt, D.; van den Wijngaard, C.; Tulen, A.D.; Mulder, A.C.; Hofhuis, A.; Jacobs, R.; Heck, M.; Verbruggen, A.; van den Kerkhof, H.; Slegers-Fitz-James, I.; et al. Outbreak of Salmonella Bovismorbificans associated with the consumption of uncooked ham products, the Netherlands, 2016 to 2017. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Jansen, A.; Frank, C.; Prager, R.; Oppermann, H.; Stark, K. Nation-wide outbreak of Salmonella Give in Germany, 2004. Z. Gastroenterol. 2005, 43, 707–713. [Google Scholar] [CrossRef]
- Horváth, J.K.; Mengel, M.; Krisztalovics, K.; Nogrady, N.; Pászti, J.; Lenglet, A.; Takkinen, J. Investigation into an unusual increase of human cases of Salmonella Goldcoast infection in Hungary in 2009. Eurosurveillance 2013, 18, 20422. [Google Scholar] [CrossRef] [PubMed]
- Scavia, G.; Ciaravino, G.; Luzzi, I.; Lenglet, A.; Ricci, A.; Barco, L.; Pavan, A.; Zaffanella, F.; Dionisi, A.M. A multistate epidemic outbreak of Salmonella Goldcoast infection in humans, June 2009 to March 2010: The investigation in Italy. Eurosurveillance 2013, 18, 20424. [Google Scholar] [CrossRef] [PubMed]
- Wegener, H.C.; Baggesen, D.L. Investigation of an outbreak of human salmonellosis caused by Salmonella enterica ssp. enterica serovar Infantis by use of pulsed field gel electrophoresis. Int. J. Food Microbiol. 1996, 32, 125–131. [Google Scholar] [CrossRef]
- Chironna, M.; Tafuri, S.; Gallone, M.S.; Sallustio, A.; Martinelli, D.; Prato, R.; Germinario, C. Outbreak of Salmonella Infantis gastroenteritis among people who had eaten at a hash house in southern Italy. Public Health 2014, 128, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.; Harries, M.; Prager, R.; Hofig, A.; Ahrens, B.; Hoffmann, L.; Rabsch, W.; Mertens, E.; Rimek, D. A prolonged outbreak of Salmonella Infantis associated with pork products in central Germany, April–October 2013. Epidemiol. Infect. 2016, 144, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Noël, H.; Dominguez, M.; Weill, F.X.; Brisabois, A.; Duchazeaubeneix, C.; Kerouanton, A.; Delmas, G.; Pihier, N.; Couturier, E. Outbreak of Salmonella enterica serotype Manhattan infection associated with meat products, France, 2005. Eurosurveillance 2006, 11, 270–273. [Google Scholar] [CrossRef]
- Schielke, A.; Rabsch, W.; Prager, R.; Simon, S.; Fruth, A.; Helling, R.; Schnabel, M.; Siffczyk, C.; Wieczorek, S.; Schroeder, S.; et al. Two consecutive large outbreaks of Salmonella Muenchen linked to pig farming in Germany, 2013 to 2014: Is something missing in our regulatory framework? Eurosurveillance 2017, 22, 30528. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, S.; Dierick, K.; Heylen, K.; De Baere, T.; Pochet, B.; Robesyn, E.; Lokietek, S.; Van Meervenne, E.; Imberechts, H.; De Zutter, L.; et al. Lessons learned from the management of a national outbreak of Salmonella Ohio linked to pork meat processing and distribution. J. Food Prot. 2010, 73, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Fabrega, A.; Vila, J. Salmonella enterica Serovar Typhimurium Skills to Succeed in the Host: Virulence and Regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef]
- Chaudhuri, R.R.; Morgan, E.; Peters, S.E.; Pleasance, S.J.; Hudson, D.L.; Davies, H.M.; Wang, J.; van Diemen, P.M.; Buckley, A.M.; Bowen, A.J.; et al. Comprehensive Assignment of Roles for Salmonella Typhimurium Genes in Intestinal Colonization of Food-Producing Animals. PLoS Genet. 2013, 9, e1003456. [Google Scholar] [CrossRef] [PubMed]
- Garaizar, J.; Porwollik, S.; Echeita, A.; Rementeria, A.; Herrera, S.; Wong, R.M.-Y.; Frye, J.; Usera, M.A.; McClelland, M. DNA Microarray-Based Typing of an Atypical Monophasic Salmonella enterica Serovar. J. Clin. Microbiol. 2002, 40, 2074–2078. [Google Scholar] [CrossRef]
- Patchanee, P.; Eiamsam-ang, T.; Vanaseang, J.; Boonkhot, P.; Tadee, P. Determination of regional relationships among Salmonella spp. isolated from retail pork circulating in the Chiang Mai municipality area using a WGS data approach. Int. J. Food Microbiol. 2017, 254, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wannaprasat, W.; Padungtod, P.; Chuanchuen, R. Class 1 integrons and virulence genes in Salmonella enterica isolates from pork and humans. Int. J. Antimicrob. Agents 2011, 37, 457–461. [Google Scholar] [CrossRef]
- Del Cerro, A.; Soto, S.; Mendoza, M. Virulence and antimicrobial-resistance gene profiles determined by PCR-based procedures for Salmonella isolated from samples of animal origin. Food Microbiol. 2003, 20, 431–438. [Google Scholar] [CrossRef]
- Guerra, B.; Laconcha, I.; Soto, S.M.; Gonzãlez-Hevia, M.Ã.; Mendoza, M.C. Molecular characterisation of emergent multiresistant Salmonella enterica serotype [4,5,12:i:-] organisms causing human salmonellosis. FEMS Microbiol. Lett. 2000, 190, 341–347. [Google Scholar] [CrossRef]
- Rajtak, U.; Boland, F.; Leonard, N.; Bolton, D.; Fanning, S. Roles of Diet and the Acid Tolerance Response in Survival of Common Salmonella Serotypes in Feces of Finishing Pigs. Appl. Environ. Microbiol. 2012, 78, 110–119. [Google Scholar] [CrossRef]
- Hayward, M.R.; AbuOun, M.; La Ragione, R.M.; Tchórzewska, M.A.; Cooley, W.A.; Everest, D.J.; Petrovska, L.; Jansen, V.A.A.; Woodward, M.J. SPI-23 of S. Derby: Role in Adherence and Invasion of Porcine Tissues. PLoS ONE 2014, 9, e107857. [Google Scholar] [CrossRef] [PubMed]
- Sévellec, Y.; Vignaud, M.-L.; Granier, S.A.; Lailler, R.; Feurer, C.; Le Hello, S.; Mistou, M.-Y.; Cadel-Six, S. Polyphyletic Nature of Salmonella enterica Serotype Derby and Lineage-Specific Host-Association Revealed by Genome-Wide Analysis. Front. Microbiol. 2018, 9, 891. [Google Scholar] [CrossRef]
- Mourão, J.; Novais, C.; Machado, J.; Peixe, L.; Antunes, P. Metal tolerance in emerging clinically relevant multidrug-resistant Salmonella enterica serotype 4,[5],12:i:- clones circulating in Europe. Int. J. Antimicrob. Agents 2015, 45, 610–616. [Google Scholar] [CrossRef]
- Petrovska, L.; Mather, A.E.; AbuOun, M.; Branchu, P.; Harris, S.R.; Connor, T.; Hopkins, K.L.; Underwood, A.; Lettini, A.A.; Page, A.; et al. Microevolution of Monophasic Salmonella Typhimurium during Epidemic, United Kingdom, 2005–2010. Emerg. Infect. Dis. 2016, 22, 617–624. [Google Scholar] [CrossRef]
- Aarestrup, F.M. The livestock reservoir for antimicrobial resistance: A personal view on changing patterns of risks, effects of interventions and the way forward. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140085. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals; World Health Organization: Geneva, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/handle/10665/258970/9789241550130-eng.pdf?sequence=1 (accessed on 25 May 2018).
- Barton, M.D. Impact of antibiotic use in the swine industry. Curr. Opin. Microbiol. 2014, 19, 9–15. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the public health risks of bacterial strains producing extended-spectrum β-lactamases and/or AmpC β-lactamases in food and food-producing animals. EFSA J. 2011, 9, 2322. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Panel on Biological Hazards (BIOHAZ). Joint Opinion on antimicrobial resistance (AMR) focused on zoonotic infections. EFSA J. 2009, 7, 1372. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Sales of Veterinary Antimicrobial Agents in 30 European Countries in 2015. 2017. Available online: https://www.ema.europa.eu/documents/report/seventh-esvac-report-sales-veterinary-antimicrobial-agents-30-european-countries-2015_en.pdf (accessed on 25 May 2018).
- Delsol, A.A.; Anjum, M.; Woodward, M.J.; Sunderland, J.; Roe, J.M. The effect of chlortetracycline treatment and its subsequent withdrawal on multi-resistant Salmonella enterica serovar Typhimurium DT104 and commensal Escherichia coli in the pig. J. Appl. Microbiol. 2003, 95, 1226–1234. [Google Scholar] [CrossRef]
- Wiuff, C.; Lykkesfeldt, J.; Svendsen, O.; Aarestrup, F. The effects of oral and intramuscular administration and dose escalation of enrofloxacin on the selection of quinolone resistance among Salmonella and coliforms in pigs. Res. Vet. Sci. 2003, 75, 185–193. [Google Scholar] [CrossRef]
- Emborg, H.-D.; Vigre, H.; Jensen, V.F.; Vieira, A.R.P.; Baggesen, D.L.; Aarestrup, F.M. Tetracycline Consumption and Occurrence of Tetracycline Resistance in Salmonella Typhimurium Phage Types from Danish Pigs. Microb. Drug Resist. 2007, 13, 289–294. [Google Scholar] [CrossRef] [PubMed]
- The National Antimicrobial Resistance Monitoring System (NARMS). NARMS 2015 Integrated Report. 2017. Available online: https://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/UCM581468.pdf (accessed on 2 June 2018).
- European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance in Europe Resistance of Salmonella and E. coli in Food, Animals and Humans, Country by Country. Available online: https://www.efsa.europa.eu/en/interactive_pages/AMR_Report_2015 (accessed on 2 June 2018).
- U.S. Food and Drug Administration (FDA)—NARMS Now: Integrated Data. Available online: https://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm416741.htm (accessed on 2 June 2018).
- Lee, H.-Y.; Su, L.-H.; Tsai, M.-H.; Kim, S.-W.; Chang, H.-H.; Jung, S.-I.; Park, K.-H.; Perera, J.; Carlos, C.; Tan, B.H.; et al. High Rate of Reduced Susceptibility to Ciprofloxacin and Ceftriaxone among Nontyphoid Salmonella Clinical Isolates in Asia. Antimicrob. Agents Chemother. 2009, 53, 2696–2699. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.-S.; Chen, Y.-T.; Wang, Y.-W.; Liu, Y.-Y.; Kuo, H.-C.; Tu, Y.-H.; Lin, A.-C.; Liao, Y.-S.; Hong, Y.-P. Dissemination of mcr-1-Carrying Plasmids among Colistin-Resistant Salmonella Strains from Humans and Food-Producing Animals in Taiwan. Antimicrob. Agents Chemother. 2017, 61, e00338-17. [Google Scholar] [CrossRef]
- Van, T.T.H.; Nguyen, H.N.K.; Smooker, P.M.; Coloe, P.J. The antibiotic resistance characteristics of non-typhoidal Salmonella enterica isolated from food-producing animals, retail meat and humans in South East Asia. Int. J. Food Microbiol. 2012, 154, 98–106. [Google Scholar] [CrossRef]
- Bai, L.; Lan, R.; Zhang, X.; Cui, S.; Xu, J.; Guo, Y.; Li, F.; Zhang, D. Prevalence of Salmonella Isolates from Chicken and Pig Slaughterhouses and Emergence of Ciprofloxacin and Cefotaxime Co-Resistant S. enterica Serovar Indiana in Henan, China. PLoS ONE 2015, 10, e0144532. [Google Scholar] [CrossRef]
- Ellerbroek, L.; Narapati, D.; Tai, N.P.; Poosaran, N.; Pinthong, R.; Sirimalaisuwan, A.; Tshering, P.; Fries, R.; Zessin, K.-H.; Baumann, M.; et al. Antibiotic Resistance in Salmonella Isolates from Imported Chicken Carcasses in Bhutan and from Pig Carcasses in Vietnam. J. Food Prot. 2010, 73, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lai, J.; Wang, Y.; Liu, S.; Li, Y.; Liu, K.; Shen, J.; Wu, C. Prevalence and characterization of Salmonella species isolated from pigs, ducks and chickens in Sichuan Province, China. Int. J. Food Microbiol. 2013, 163, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.S.; Fragoso de Araújo, J.; de Moura Gomes, V.T.; Reis Costa, A.T.; dos Prazeres Rodrigues, D.; Porfida Ferreira, T.S.; de Lima Filsner, P.H.N.; Felizardo, M.R.; Micke Moreno, A. Colistin Resistance in Escherichia coli and Salmonella enterica Strains Isolated from Swine in Brazil. Sci. World J. 2012, 2012, 109795. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Zhang, A.-Y.; Ma, S.-Z.; Kong, L.-H.; Li, Y.-X.; Liu, J.-X.; Davis, M.A.; Guo, X.-Y.; Liu, B.-H.; Lei, C.-W.; et al. Co-occurrence of mcr-1 and ESBL on a single plasmid in Salmonella enterica. J. Antimicrob. Chemother. 2016, 71, 2336–2338. [Google Scholar] [CrossRef]
- Li, X.-P.; Fang, L.-X.; Song, J.-Q.; Xia, J.; Huo, W.; Fang, J.-T.; Liao, X.-P.; Liu, Y.-H.; Feng, Y.; Sun, J. Clonal spread of mcr-1 in PMQR-carrying ST34 Salmonella isolates from animals in China. Sci. Rep. 2016, 6, 38511. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Wang, J.; Gao, Y.; Liu, Y.; Doi, Y.; Wu, R.; Zeng, Z.; Liang, Z.; Liu, J.-H. mcr-1-Harboring Salmonella enterica Serovar Typhimurium Sequence Type 34 in Pigs, China. Emerg. Infect. Dis. 2017, 23, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Beaudry, F.; Thériault, W.; Letellier, A. Colistin in Pig Production: Chemistry, Mechanism of Antibacterial Action, Microbial Resistance Emergence, and One Health Perspectives. Front. Microbiol. 2016, 7, 1789. [Google Scholar] [CrossRef]
- Poirel, L.; Nordmann, P. Emerging plasmid-encoded colistin resistance: The animal world as the culprit? J. Antimicrob. Chemother. 2016, 71, 2326–2327. [Google Scholar] [CrossRef]
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zeng, X.; Li, X.-P.; Liao, X.-P.; Liu, Y.-H.; Lin, J. Plasmid-mediated colistin resistance in animals: Current status and future directions. Anim. Heal. Res. Rev. 2017, 18, 136–152. [Google Scholar] [CrossRef] [PubMed]
- McGlone, J. The Future of Pork Production in the World: Towards Sustainable, Welfare-Positive Systems. Animals 2013, 3, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef]
- Jacoby, G.A. Plasmid-Mediated Quinolone Resistance. In Antimicrobial Drug Resistance; Springer International Publishing: Cham, Switzerland, 2017; pp. 265–268. [Google Scholar]
- Veldman, K.; Cavaco, L.M.; Mevius, D.; Battisti, A.; Franco, A.; Botteldoorn, N.; Bruneau, M.; Perrin-Guyomard, A.; Cerny, T.; De Frutos Escobar, C.; et al. International collaborative study on the occurrence of plasmid-mediated quinolone resistance in Salmonella enterica and Escherichia coli isolated from animals, humans, food and the environment in 13 European countries. J. Antimicrob. Chemother. 2011, 66, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Cristino, L.; Peixe, L.; Antunes, P. MCR-1 in multidrug-resistant and copper-tolerant clinically relevant Salmonella 1,4,[5],12:i:- and S. Rissen clones in Portugal, 2011 to 2015. Eurosurveillance 2016, 21, 30270. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.; Card, R.M.; Nunez, J.; Pomba, C.; Mendonça, N.; Anjum, M.F.; Da Silva, G.J. Detection of an mcr-1-encoding plasmid mediating colistin resistance in Salmonella enterica from retail meat in Portugal. J. Antimicrob. Chemother. 2016, 71, 2338–2340. [Google Scholar] [CrossRef] [PubMed]
- Barco, L.; Ramon, E.; Cortini, E.; Longo, A.; Dalla Pozza, M.C.; Lettini, A.A.; Dionisi, A.M.; Olsen, J.E.; Ricci, A. Molecular Characterization of Salmonella enterica Serovar 4,[5],12:i:- DT193 ASSuT Strains from Two Outbreaks in Italy. Foodborne Pathog. Dis. 2014, 11, 138–144. [Google Scholar] [CrossRef]
- Gallati, C.; Stephan, R.; Hächler, H.; Malorny, B.; Schroeter, A.; Nüesch-Inderbinen, M. Characterization of Salmonella enterica Subsp. enterica Serovar 4,[5],12:i:- Clones Isolated from Human and Other Sources in Switzerland Between 2007 and 2011. Foodborne Pathog. Dis. 2013, 10, 549–554. [Google Scholar]
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Assessment of the Antibiotic Resistance Effects of Biocides. 2009. Available online: http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_021.pdf (accessed on 1 April 2018).
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef]
- Yu, Z.; Gunn, L.; Wall, P.; Fanning, S. Antimicrobial resistance and its association with tolerance to heavy metals in agriculture production. Food Microbiol. 2017, 64, 23–32. [Google Scholar] [CrossRef]
- De Jong, A.; Smet, A.; Ludwig, C.; Stephan, B.; De Graef, E.; Vanrobaeys, M.; Haesebrouck, F. Antimicrobial susceptibility of Salmonella isolates from healthy pigs and chickens (2008–2011). Vet. Microbiol. 2014, 171, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Barownick, W.; Helmuth, R.; Mendoza, M.C.; Rodicio, M.R.; Schroeter, A.; Guerra, B. Extended-spectrum {beta}-lactamases and AmpC {beta}-lactamases in ceftiofur-resistant Salmonella enterica isolates from food and livestock obtained in Germany during 2003–07. J. Antimicrob. Chemother. 2009, 64, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-H.; Lin, X.-Y.; Xu, L.; Gu, X.-X.; Yang, L.; Li, W.; Ren, S.-Q.; Liu, Y.-H.; Zeng, Z.-L.; Jiang, H.-X. CTX-M-27 Producing Salmonella enterica Serotypes Typhimurium and Indiana Are Prevalent among Food-Producing Animals in China. Front. Microbiol. 2016, 7, 436. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, R.; Quinn, T.; Drudy, D.; Walsh, C.; Whyte, P.; Mattar, S.; Fanning, S. Antimicrobial resistance in nontyphoidal Salmonella from food sources in Colombia: Evidence for an unusual plasmid-localized class 1 integron in serotypes Typhimurium and Anatum. Microb. Drug Resist. 2006, 12, 269–277. [Google Scholar] [CrossRef]
- Zaidi, M.B.; Leon, V.; Canche, C.; Perez, C.; Zhao, S.; Hubert, S.K.; Abbott, J.; Blickenstaff, K.; McDermott, P.F. Rapid and widespread dissemination of multidrug-resistant blaCMY-2 Salmonella Typhimurium in Mexico. J. Antimicrob. Chemother. 2007, 60, 398–401. [Google Scholar] [CrossRef]
- Glenn, L.M.; Lindsey, R.L.; Folster, J.P.; Pecic, G.; Boerlin, P.; Gilmour, M.W.; Harbottle, H.; Zhao, S.; McDermott, P.F.; Fedorka-Cray, P.J.; et al. Antimicrobial resistance genes in multidrug-resistant Salmonella enterica isolated from animals, retail meats, and humans in the United States and Canada. Microb. Drug Resist. 2013, 19, 175–184. [Google Scholar] [CrossRef]
- Lee, K.-E.; Lim, S.-I.; Choi, H.-W.; Lim, S.-K.; Song, J.-Y.; An, D.-J. Plasmid-mediated AmpC β-lactamase (CMY-2) gene in Salmonella Typhimurium isolated from diarrheic pigs in South Korea. BMC Res. Notes 2014, 7, 329. [Google Scholar] [CrossRef]
- Lin, D.; Chen, K.; Chan, E.W.-C.; Chen, S. Increasing prevalence of ciprofloxacin-resistant food-borne Salmonella strains harboring multiple PMQR elements but not target gene mutations. Sci. Rep. 2015, 5, 14754. [Google Scholar] [CrossRef]
- Li, L.; Liao, X.; Yang, Y.; Sun, J.; Li, L.; Liu, B.; Yang, S.; Ma, J.; Li, X.; Zhang, Q.; et al. Spread of oqxAB in Salmonella enterica serotype Typhimurium predominantly by IncHI2 plasmids. J. Antimicrob. Chemother. 2013, 68, 2263–2268. [Google Scholar] [CrossRef]
- Quesada, A.; Ugarte-Ruiz, M.; Iglesias, M.R.; Porrero, M.C.; Martínez, R.; Florez-Cuadrado, D.; Campos, M.J.; García, M.; Píriz, S.; Sáez, J.L.; et al. Detection of plasmid mediated colistin resistance (MCR-1) in Escherichia coli and Salmonella enterica isolated from poultry and swine in Spain. Res. Vet. Sci. 2016, 105, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Baloch, Z.; Zou, M.; Dong, Y.; Peng, Z.; Hu, Y.; Xu, J.; Yasmeen, N.; Li, F.; Fanning, S. Complete Genomic Analysis of a Salmonella enterica Serovar Typhimurium Isolate Cultured from Ready-to-Eat Pork in China Carrying One Large Plasmid Containing mcr-1. Front. Microbiol. 2018, 9, 616. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Cristino, L.; Ribeiro, S.; Mourão, J.; Peixe, L.A.; Antunes, P. MCR-1 dispersion in clinically relevant clones of multidrug-resistant and copper-tolerant Salmonella from Portugal. In Proceedings of the 27th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Vienna, Austria, 22–25 April 2017; Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjy75L79LjfAhVNSRUIHbiDC5gQFjAAegQIAhAC&url=https%3A%2F%2Fwww.escmid.org%2Fescmid_publications%2Fescmid_elibrary%2Fmaterial%2F%3Fmid%3D52654&usg=AOvVaw1Ywvlmi8Ktp9Vguytd3P_m (accessed on 1 February 2018).
- Anjum, M.F.; Duggett, N.A.; AbuOun, M.; Randall, L.; Nunez-Garcia, J.; Ellis, R.J.; Rogers, J.; Horton, R.; Brena, C.; Williamson, S.; et al. Colistin resistance in Salmonella and Escherichia coli isolates from a pig farm in Great Britain. J. Antimicrob. Chemother. 2016, 71, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Zhang, J.; Zhang, C.; Li, R.; Chan, E.W.-C.; Wu, C.; Wu, C.; Chen, S. Distinct mechanisms of acquisition of mcr-1–bearing plasmid by Salmonella strains recovered from animals and food samples. Sci. Rep. 2017, 7, 13199. [Google Scholar] [CrossRef]
- Freire Martín, I.; AbuOun, M.; Reichel, R.; La Ragione, R.M.; Woodward, M.J. Sequence analysis of a CTX-M-1 IncI1 plasmid found in Salmonella 4,5,12:i:-, Escherichia coli and Klebsiella pneumoniae on a UK pig farm. J. Antimicrob. Chemother. 2014, 69, 2098–2101. [Google Scholar] [CrossRef]
- Rodríguez, I.; Jahn, S.; Schroeter, A.; Malorny, B.; Helmuth, R.; Guerra, B. Extended-spectrum β-lactamases in German isolates belonging to the emerging monophasic Salmonella enterica subsp. enterica serovar Typhimurium 4,[5],12:i:- European clone. J. Antimicrob. Chemother. 2012, 67, 505–508. [Google Scholar]
- Clemente, L.; Manageiro, V.; Ferreira, E.; Jones-Dias, D.; Correia, I.; Themudo, P.; Albuquerque, T.; Caniça, M. Occurrence of extended-spectrum β-lactamases among isolates of Salmonella enterica subsp. enterica from food-producing animals and food products, in Portugal. Int. J. Food Microbiol. 2013, 167, 221–228. [Google Scholar] [CrossRef]
- Tyson, G.H.; Tate, H.P.; Zhao, S.; Li, C.; Dessai, U.; Simmons, M.; McDermott, P.F. Identification of Plasmid-Mediated Quinolone Resistance in Salmonella Isolated from Swine Ceca and Retail Pork Chops in the United States. Antimicrob. Agents Chemother. 2017, 61, e01318-17. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef]
- Garcia-Graells, C.; De Keersmaecker, S.C.J.; Vanneste, K.; Pochet, B.; Vermeersch, K.; Roosens, N.; Dierick, K.; Botteldoorn, N. Detection of Plasmid-Mediated Colistin Resistance, mcr-1 and mcr-2 Genes, in Salmonella spp. Isolated from Food at Retail in Belgium from 2012 to 2015. Foodborne Pathog. Dis. 2018, 15. [Google Scholar] [CrossRef]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance 2017, 22, 30589. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.Y.; Chen, S. First detection of oqxAB in Salmonella spp. isolated from food. Antimicrob. Agents Chemother. 2013, 57, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Webb, H.E.; Granier, S.A.; Marault, M.; Millemann, Y.; den Bakker, H.C.; Nightingale, K.K.; Bugarel, M.; Ison, S.A.; Scott, H.M.; Loneragan, G.H. Dissemination of the mcr-1 colistin resistance gene. Lancet. Infect. Dis. 2016, 16, 144–145. [Google Scholar] [CrossRef]
- Riaño, I.; Moreno, M.A.; Teshager, T.; Sáenz, Y.; Domínguez, L.; Torres, C. Detection and characterization of extended-spectrum beta-lactamases in Salmonella enterica strains of healthy food animals in Spain. J. Antimicrob. Chemother. 2006, 58, 844–847. [Google Scholar] [CrossRef]
- Luk-In, S.; Pulsrikarn, C.; Bangtrakulnonth, A.; Chatsuwan, T.; Kulwichit, W. Occurrence of a novel class 1 integron harboring qnrVC4 in Salmonella Rissen. Diagn. Microbiol. Infect. Dis. 2017, 88, 282–286. [Google Scholar] [CrossRef]
- Keelara, S.; Thakur, S. Dissemination of plasmid-encoded AmpC β-lactamases in antimicrobial resistant Salmonella serotypes originating from humans, pigs and the swine environment. Vet. Microbiol. 2014, 173, 76–83. [Google Scholar] [CrossRef] [PubMed]
- El Garch, F.; de Jong, A.; Bertrand, X.; Hocquet, D.; Sauget, M. mcr-1- like detection in commensal Escherichia coli and Salmonella spp. from food-producing animals at slaughter in Europe. Vet. Microbiol. 2018, 213, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Wasyl, D.; Hoszowski, A.; Zając, M. Prevalence and characterisation of quinolone resistance mechanisms in Salmonella spp. Vet. Microbiol. 2014, 171, 307–314. [Google Scholar] [CrossRef]
- Winokur, P.L.; Brueggemann, A.; De Salvo, D.L.; Hoffmann, L.; Apley, M.D.; Uhlenhopp, E.K.; Pfaller, M.A.; Doern, G.V. Animal and human multidrug-resistant, cephalosporin-resistant Salmonella isolates expressing a plasmid-mediated CMY-2 AmpC beta-lactamase. Antimicrob. Agents Chemother. 2000, 44, 2777–2783. [Google Scholar] [CrossRef]
- Andrysiak, A.K.; Olson, A.B.; Tracz, D.M.; Dore, K.; Irwin, R.; Ng, L.-K.; Gilmour, M.W.; Canadian Integrated Program for Antimicrobial Resistance Surveillance Collaborative. Genetic characterization of clinical and agri-food isolates of multi drug resistant Salmonella enterica serovar Heidelberg from Canada. BMC Microbiol. 2008, 8, 89. [Google Scholar] [CrossRef]
- Jiang, H.-X.; Song, L.; Liu, J.; Zhang, X.-H.; Ren, Y.-N.; Zhang, W.-H.; Zhang, J.-Y.; Liu, Y.-H.; Webber, M.A.; Ogbolu, D.O.; et al. Multiple transmissible genes encoding fluoroquinolone and third-generation cephalosporin resistance co-located in non-typhoidal Salmonella isolated from food-producing animals in China. Int. J. Antimicrob. Agents 2014, 43, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Rodríguez, I.; Schmoger, S.; Friese, A.; Roesler, U.; Helmuth, R.; Guerra, B. Salmonella enterica subsp. enterica producing VIM-1 carbapenemase isolated from livestock farms. J. Antimicrob. Chemother. 2013, 68, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Clemente, L.; Manageiro, V.; Jones-Dias, D.; Correia, I.; Themudo, P.; Albuquerque, T.; Geraldes, M.; Matos, F.; Almendra, C.; Ferreira, E.; et al. Antimicrobial susceptibility and oxymino-β-lactam resistance mechanisms in Salmonella enterica and Escherichia coli isolates from different animal sources. Res. Microbiol. 2015, 166, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Kylla, H.; Kumar Dutta, T.; Roychoudhury, P.; Subudhi, P.K. Salmonella enterica serovar Miami Possessing Both Virulence and Extended-Spectrum β-Lactamase Resistant Genes Isolated from Diarrhoeic Piglets of North East India (Mizoram). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1451–1456. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).