Protective Effects of Evening Primrose Oil against Cyclophosphamide-Induced Biochemical, Histopathological, and Genotoxic Alterations in Mice
Abstract
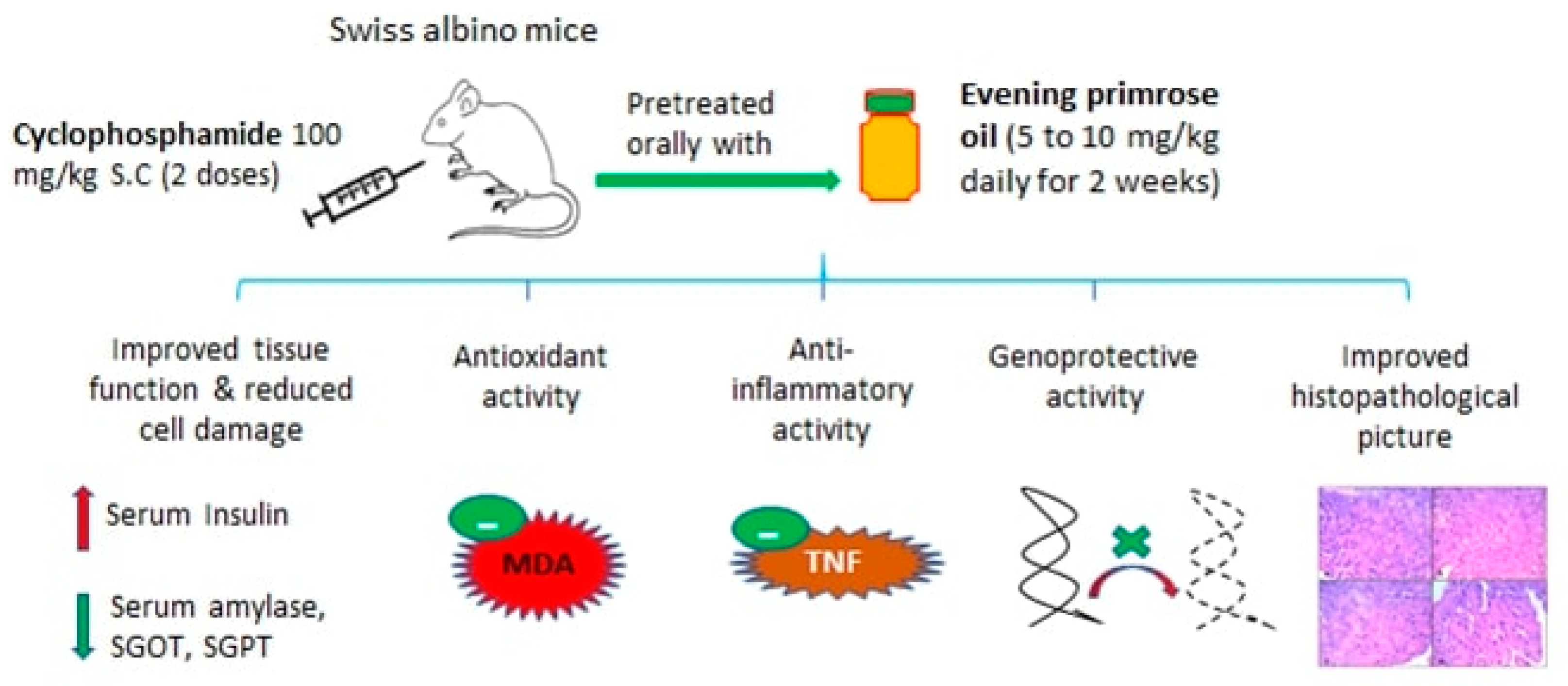
:1. Introduction
2. Results
2.1. GC-MS Analysis Results
2.2. Serum Biochemical Analysis
2.3. Tissue Biochemical Analysis
2.4. Histopathological Examination and Analysis
2.5. DNA Laddering
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Experimental Animals
4.3. Experimental Design
4.4. Sample Collection and Preparation
4.5. Measurement of Biochemical Parameters
4.5.1. Determination of Serum Level of Liver Enzymes and Pancreatic Amylase
4.5.2. Determination of Fasting Serum Insulin Level
4.5.3. Measurement of Tissue Homogenate Level of TNF-α and MDA
4.6. Histopathological Examination
4.7. DNA Laddering
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Habibi, E.; Shokrzadeh, M.; Chabra, A.; Naghshvar, F.; Keshavarz-Maleki, R.; Ahmadi, A. Protective effects of Origanum vulgare ethanol extract against cyclophosphamide-induced liver toxicity in mice. Pharm. Biol. 2015, 53, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Q.; Yung Chan, S.; Chuen Li, S.; Zhou, S.; Duan, W.; Zhu, Y.-Z. Metabolism and transport of oxazaphosphorines and the clinical implications. Drug Metab. Rev. 2005, 37, 611–703. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, R.; Khan, S.N.; Shaeib, F.; Kohan-Ghadr, H.-R.; Aldhaheri, S.R.; Najafi, T.; Thakur, M.; Morris, R.; Abu-Soud, H.M. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic. Biol. Med. 2017, 110, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.T.; Hamzeh, M.; Beklar, S.Y.; Hosseinimehr, S.J. Anti-apoptotic and antioxidant effect of cerium oxide nanoparticles on cyclophosphamide-induced hepatotoxicity. Erciyes Med. J. 2018, 40, 148–154. [Google Scholar] [CrossRef]
- Hamzeh, M.; Hosseinimehr, S.J.; Khalatbary, A.R.; Mohammadi, H.R.; Dashti, A.; Amiri, F.T. Atorvastatin mitigates cyclophosphamide-induced hepatotoxicity via suppression of oxidative stress and apoptosis in rat model. Res. Pharm. Sci. 2018, 13, 440. [Google Scholar]
- Munir, R.; Semmar, N.; Farman, M.; Ahmad, N.S. An updated review on pharmacological activities and phytochemical constituents of evening primrose (genus Oenothera). Asian Pac. J. Trop. Biomed. 2017, 7, 1046–1054. [Google Scholar] [CrossRef]
- Edwards, S.; Rocha, I.C.; Williamson, E.M.; Heinrich, M. Evening Primrose (Oil). In Phytopharmacy: An Evidence-Based Guide to Herbal Medical Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 144–148. [Google Scholar]
- SafaaHussain, M.; Abdulridha, M.K.; Khudhair, M.S. Anti-inflammatory, Anti-oxidant, and Vasodilating Effect of Evening Primrose Oil in Type 2 Diabetic Patients. Int. J. Pharm. Sci. Rev. Res 2016, 39, 173–178. [Google Scholar]
- Granica, S.; Czerwińska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa Hudziok obtained after seeds cultivation. J. Agric. Food Chem. 2013, 61, 801–810. [Google Scholar] [CrossRef]
- Jamilian, M.; Karamali, M.; Taghizadeh, M.; Sharifi, N.; Jafari, Z.; Memarzadeh, M.R.; Mahlouji, M.; Asemi, Z. Vitamin D and evening primrose oil administration improve glycemia and lipid profiles in women with gestational diabetes. Lipids 2016, 51, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, J.R.; Wyse, D.L.; Sheaffer, C.C.; Kauppi, K.L.; Fulcher, R.G.; Ehlke, N.J.; Biesboer, D.D.; Bey, R.F. Antioxidant and antimicrobial activity of seed from plants of the Mississippi river basin. J. Med. Plants Res. 2008, 2, 081–093. [Google Scholar]
- Rock, E.; DeMichele, A. Nutritional approaches to late toxicities of adjuvant chemotherapy in breast cancer survivors. J. Nutr. 2003, 133, 3785S–3793S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, G.B.; Slattery, J.T.; Bouvier, M.E.; Ren, S.; Batchelder, A.L.; Kalhorn, T.F.; Schoch, H.G.; Anasetti, C.; Gooley, T. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood 2003, 101, 2043–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, S.R.; Cader, R.A.; Mohd, R.; Yen, K.W.; Ghafor, H.A. Low-dose cyclophosphamide-induced acute hepatotoxicity. Am. J. Case Rep. 2013, 14, 345. [Google Scholar]
- Sharma, P.K.; Misra, A.K.; Singh, V.; Gupta, A.; Saroha, S.; Singh, S. Cyclophosphamide and epirubicin-induced diabetes mellitus in breast cancer: A rare occurrence. J. Pharmacol. Pharmacother. 2016, 7, 146. [Google Scholar]
- Salvador, V.B.; Singh, M.; Witek, P.; Peress, G. Cyclophosphamide and Doxorubicin-induced acute pancreatitis in a patient with breast cancer. Br. J. Med Pract. 2014, 7, 727. [Google Scholar]
- Shokrzadeh, M.; Ahmadi, A.; Naghshvar, F.; Chabra, A.; Jafarinejhad, M. Prophylactic efficacy of melatonin on cyclophosphamide-induced liver toxicity in mice. Biomed Res. Int. 2014, 2014, 470425. [Google Scholar] [CrossRef]
- Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFκB/MAPK pathway. Chem. Biol. Interact. 2015, 231, 98–107. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of Nrf2 and PPARγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmacother. 2017, 86, 297–306. [Google Scholar] [CrossRef]
- Ohtani, T.; Nakamura, T.; Toda, K.; Furukawa, F. Cyclophosphamide enhances TNF-alpha-induced apoptotic cell death in murine vascular endothelial cell. FEBS Lett. 2006, 580, 1597–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Lee, I.C.; Ko, J.W.; Moon, C.; Kim, S.H.; Shin, I.S.; Seo, Y.W.; Kim, H.C.; Kim, J.C. Diallyl disulfide prevents cyclophosphamide-induced hemorrhagic cystitis in rats through the inhibition of oxidative damage, MAPKs, and NF-κB pathways. Biomol. Ther. 2015, 23, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, B.A.; Harmon, B.V.; Cameron, D.P.; Allan, D.J. Nicotinamide prevents the development of diabetes in the cyclophosphamide-induced NOD mouse model by reducing beta-cell apoptosis. J. Pathol. 2000, 191, 86–92. [Google Scholar] [CrossRef]
- Reddy, S.; Bradley, J.; Ginn, S.; Pathipati, P.; Ross, J.M. Immunohistochemical study of caspase-3-expressing cells within the pancreas of non-obese diabetic mice during cyclophosphamide-accelerated diabetes. Histochem. Cell Biol. 2003, 119, 451–461. [Google Scholar] [CrossRef]
- Knorr, R.; Hamburger, M. Quantitative analysis of anti-inflammatory and radical scavenging triterpenoid esters in evening primrose oil. J. Agric. Food Chem. 2004, 52, 3319–3324. [Google Scholar] [CrossRef]
- Kanbur, M.; Eraslan, G.; Sarıca, Z.S.; Aslan, Ö. The effects of evening primrose oil on lipid peroxidation induced by subacute aflatoxin exposure in mice. Food Chem. Toxicol. 2011, 49, 1960–1964. [Google Scholar] [CrossRef]
- Mohamed, H. Prophyloctic Effect of the Evening Primrose Oil on Genotoxicity of the Anticancer Drug Ifosfamide in Mice. Egypt. J. Zool. 2012, 174, 1–32. [Google Scholar]
- Soeken, K.; Miller, S.; Ernst, E. Herbal medicines for the treatment of rheumatoid arthritis: A systematic review. Rheumatology 2003, 42, 652–659. [Google Scholar] [CrossRef] [Green Version]
- Chung, B.Y.; Park, S.Y.; Jung, M.J.; Kim, H.O.; Park, C.W. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: A randomized, double-blinded, placebo-controlled clinical study. Ann. Dermatol. 2018, 30, 409–416. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Garcia-Gimenez, M.D.; Angel-Martin, M.; Perez-Camino, M.C.; Fernandez Arche, A. Long-chain fatty alcohols from evening primrose oil inhibit the inflammatory response in murine peritoneal macrophages. J. Ethnopharmacol. 2014, 151, 131–136. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Fernandez-Arche, A.; Angel-Martin, M.; Garcia-Gimenez, M.D. The sterols isolated from Evening Primrose oil modulate the release of proinflammatory mediators. Phytomedicine 2012, 19, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Abdulridha, M.; Hussain, M.; Khudhair, M. Study Effect of Evening Primrose Oil Supplement on Type 2 Diabetes Mellitus—Associated Metabolic Parameters. UK J. Pharm. Biosci. 2017, 5, 17–23. [Google Scholar] [CrossRef]
- Jack, A.M.; Keegan, A.; Cotter, M.A.; Cameron, N.E. Effects of diabetes and evening primrose oil treatment on responses of aorta, corpus cavernosum and mesenteric vasculature in rats. Life Sci. 2002, 71, 1863–1877. [Google Scholar] [CrossRef]
- Sujatha, S.; Anand, S.; Sangeetha, K.; Shilpa, K.; Lakshmi, J.; Balakrishnan, A.; Lakshmi, B. Biological evaluation of (3β)-STIGMAST-5-EN-3-OL as potent anti-diabetic agent in regulating glucose transport using in vitro model. Int. J. Diabetes Mellit. 2010, 2, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.; Jha, S.; Murthy, P.; Manik, S.A. Isolation and characterization of stigmast-5-en-3β-ol (β-sitosterol) from the leaves of Hygrophila spinosa T. Anders. Int. J. Pharma Sci. Res. 2010, 1, 95–100. [Google Scholar]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem. Biol. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef]
- Kurahashi, M.; Inomata, K. Amylase secretion by parotid glands and pancreas of diabetic rats during feeding. Am. J. Physiol. Gastrointest. Liver Physiol. 1988, 254, G878–G882. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
- Dixon, J.B.; Bhathal, P.S.; Hughes, N.R.; O’Brien, P.E. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology 2004, 39, 1647–1654. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds cyclophosphamide and evening primrose oil are available from the authors. |




| Peak | Retention Time | Name | Area% | Molecular Weight | Molecular Formula |
|---|---|---|---|---|---|
| 1 | 4.08 | Decane | 1.39 | 142 | C10H22 |
| 2 | 6.63 | 1-Hexadecanol, 2-methyl- | 1.13 | 256 | C17H36O |
| 3 | 9.36 | Dodecane | 1.62 | 170 | C12H26 |
| 4 | 10.10 | Thiophene, tetrahydro-, 1,1-dioxide | 1.52 | 120 | C4H8O2S |
| 5 | 12.10 | 2,4-Dodecadienal, (E,E)- | 0.88 | 180 | C12H20O |
| 6 | 12.70 | Deca-2,4-Dienal | 1.25 | 152 | C10H16O |
| 7 | 14.43 | 1-Dodecene | 0.77 | 168 | C12H24 |
| 8 | 14.63 | (3β,5α)-Cholestanol | 2.52 | 389 | C27H48O |
| 9 | 17.54 | 2-(1,1-dimethylethyl)-5-(2-propenyl)-1,4-benzenediol | 5.38 | 206 | C13H18O2 |
| 10 | 18.11 | Ergost-5-en-3-ol(3β24R) | 17.10 | 401 | C28H48O |
| 11 | 19.22 | 7-Hexadecene, (Z)- | 1.24 | 224 | C16H32 |
| 12 | 19.38 | Hexadecane | 1.63 | 226 | C16H34 |
| 13 | 20.30 | Caryophyllene | 8.23 | 204 | C15H24 |
| 14 | 21.73 | 3-Nonanol, 2-methyl- | 0.77 | 158 | C10H22O |
| 15 | 23.67 | Nonadecane | 1.63 | 268 | C19H40 |
| 16 | 27.08 | Stigmast-5-en-3-ol, (3β)- | 39.81 | 415 | C29H50O |
| 17 | 27.44 | 1-Eicosanol | 2.05 | 298 | C20H42O |
| 18 | 27.73 | Gibberellic acid | 3.92 | 346 | C19H22O6 |
| 19 | 30.20 | Linoleic acid ethyl ester | 4.51 | 308 | C20H36O2 |
| 20 | 31.85 | 8,11,14-Eicosatrienoic acid, (Z,Z,Z)- | 2.12 | 306 | C20H34O2 |
| Σ99.47 |
| Groups | SGPT u/L | SGOT u/L | Amylase u/L | Insulin ng/mL |
|---|---|---|---|---|
| Control | 9.87 ± 0.44 | 35.6 ± 3.22 | 29.7 ± 1.2 | 10.43 ± 0.3 |
| CP-intoxicated | 24.6 ± 1.7 a | 68.6 ± 1.7 a | 55.2 ± 1.8 a | 5.5 ± 0.26 a |
| CP + EPO (5 mg/kg) | 14.6 ± 1.26 ab | 49.12 ± 1.96 ab | 44.1 ± 1.98 ab | 7.1 ± 0.22 ab |
| CP + EPO (10 mg/kg) | 11.87 ± 0.6 b | 41.1 ± 0.55 bc | 33.6 ± 2.7 bc | 10.7 ± 0.69 bc |
| Groups | Liver TNF-α | Pancreas TNF-α | Liver MDA | Pancreas MDA |
|---|---|---|---|---|
| Control | 53.6 ± 5.9 | 25.6 ± 5.8 | 2.2 ± 0.21 | 0.52 ± 0.15 |
| CP-intoxicated | 132.3 ± 6.8 a | 151.3 ± 6.9 a | 13.3 ± 0.57 a | 1.36 ± 0.06 a |
| CP + EPO (5 mg/kg) | 83.3 ± 6.7 ab | 78.3 ± 6.6 ab | 6 ± 0.78 ab | 0.59 ± 0.08 b |
| CP + EPO (10 mg/kg) | 56 ± 3.8 bc | 41.3 ± 3.9 ab | 3.9 ± 0.39 abc | 0.39 ± 0.04 b |
| Groups | Grades | Mean Scoring Grades for Hepatic Histopathological Changes | |||
| 1 | 2 | 3 | 4 | ||
| Control | 7 | 1 | 0 | 0 | 1.125 ± 0.13 |
| CP-intoxicated | 0 | 1 | 4 | 3 | 3.25 ± 0.25 a |
| CP + EPO (5 mg/kg) | 0 | 6 | 1 | 1 | 2.37 ± 0.26 ab |
| CP + EPO (10 mg/kg) | 6 | 1 | 1 | 0 | 1.63 ± 0.42 b |
| (a) | |||||
| Groups | Grades | Mean Scoring Grades for Pancreatic Histopathological Changes | |||
| 1 | 2 | 3 | 4 | ||
| Control | 6 | 1 | 1 | 0 | 1.37 ± 0.26 |
| CP-intoxicated | 0 | 1 | 4 | 3 | 3.37 ± 0.18 a |
| CP + EPO (5 mg/kg) | 0 | 6 | 1 | 1 | 2.5 ± 0.26 ab |
| CP + EPO (10 mg/kg) | 6 | 1 | 1 | 0 | 1.5 ± 0.26 bc |
| (b) | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodeer, D.M.; Mehanna, E.T.; Abushouk, A.I.; Abdel-Daim, M.M. Protective Effects of Evening Primrose Oil against Cyclophosphamide-Induced Biochemical, Histopathological, and Genotoxic Alterations in Mice. Pathogens 2020, 9, 98. https://doi.org/10.3390/pathogens9020098
Khodeer DM, Mehanna ET, Abushouk AI, Abdel-Daim MM. Protective Effects of Evening Primrose Oil against Cyclophosphamide-Induced Biochemical, Histopathological, and Genotoxic Alterations in Mice. Pathogens. 2020; 9(2):98. https://doi.org/10.3390/pathogens9020098
Chicago/Turabian StyleKhodeer, Dina M., Eman T. Mehanna, Abdelrahman I. Abushouk, and Mohamed M. Abdel-Daim. 2020. "Protective Effects of Evening Primrose Oil against Cyclophosphamide-Induced Biochemical, Histopathological, and Genotoxic Alterations in Mice" Pathogens 9, no. 2: 98. https://doi.org/10.3390/pathogens9020098
APA StyleKhodeer, D. M., Mehanna, E. T., Abushouk, A. I., & Abdel-Daim, M. M. (2020). Protective Effects of Evening Primrose Oil against Cyclophosphamide-Induced Biochemical, Histopathological, and Genotoxic Alterations in Mice. Pathogens, 9(2), 98. https://doi.org/10.3390/pathogens9020098







