Biochemical Changes in Leaves of Vitis vinifera cv. Sangiovese Infected by Bois Noir Phytoplasma
Abstract
1. Introduction
2. Results
2.1. Plant Symptoms
2.2. Pigments and Soluble Sugars
2.3. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Proanthocyanidins (PAs)
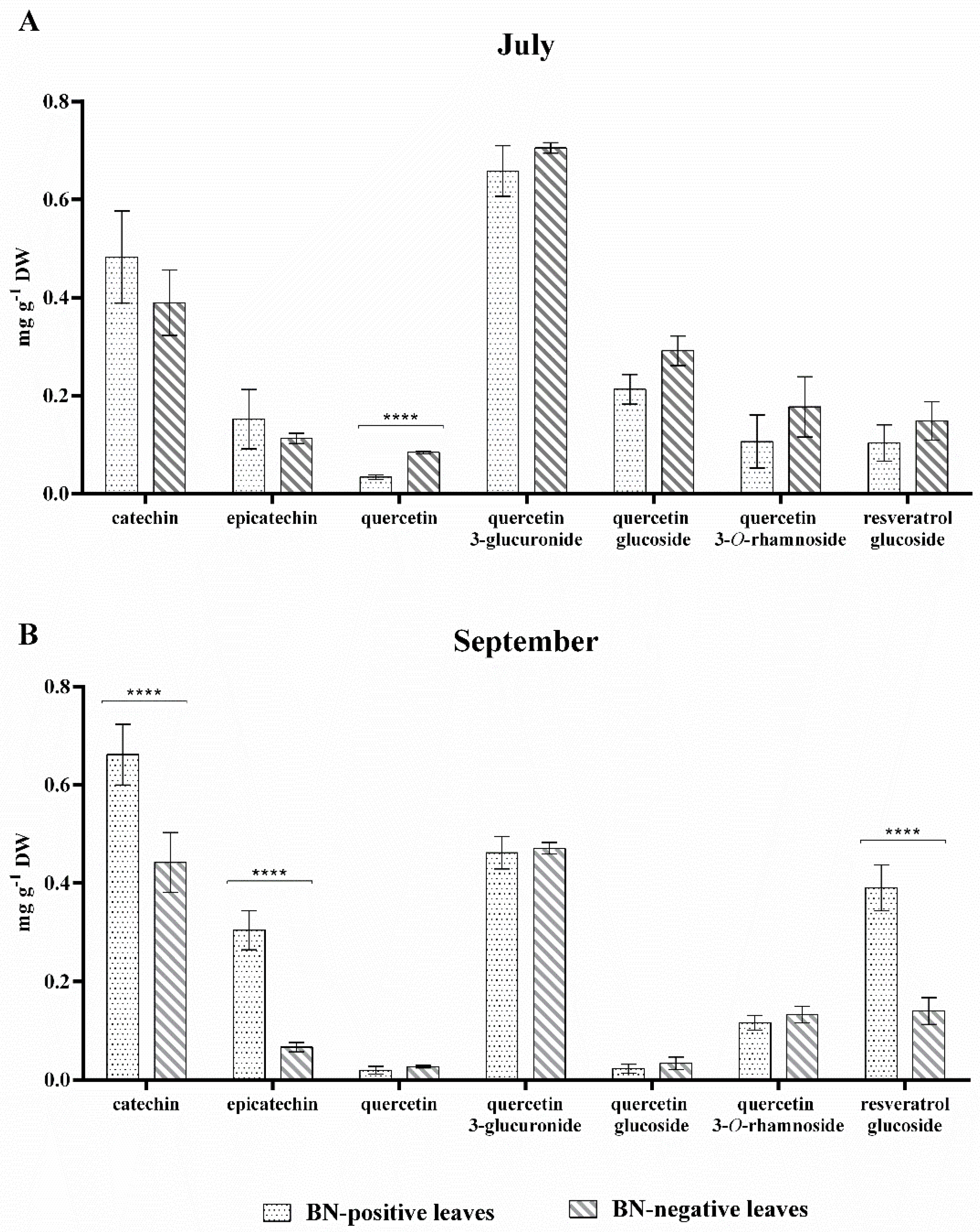
2.4. Quali-Quantitative Analysis of Phenolic Compounds and Anthocyanins in Leaf Extracts
2.5. Lignin Distribution in BN-Positive and BN-Negative Sangiovese Leaves
3. Discussion
4. Materials and Methods
4.1. Plant Samples and Phytoplasma Detection
4.2. Analysis of Pigments and Soluble Sugars
4.3. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Proanthocyanidins (PAs)
4.4. HPLC ESI/MS-TOF Analysis
4.5. Histology: Lignin Distribution in Leaves
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bertaccini, A.; Duduk, B.; Paltrinieri, S.; Contaldo, N. Phytoplasmas and Phytoplasma Diseases: A Severe Threat to Agriculture. Am. J. Plant Sci. 2014, 5, 1763–1788. [Google Scholar] [CrossRef]
- Pierro, R.; Semeraro, T.; Luvisi, A.; Garg, H.; Vergine, M.; De Bellis, L.; Gill, H.K. The distribution of phytoplasmas in South and East Asia: An emerging threat to grapevine cultivation. Front. Plant Sci. 2019, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Belli, G.; Bianco, P.A.; Conti, M. Grapevine yellows: Past, present and future. J. Plant Pathol. 2010, 92, 303–326. [Google Scholar]
- Quaglino, F.; Zhao, Y.; Casati, P.; Bulgari, D.; Bianco, P.A.; Wei, W.; Davis, R.E. ‘Candidatus Phytoplasma solani’, a novel taxon associated with stolbur and Bois Noir related diseases of plants. Int. J. Syst. Evol. Micr. 2013, 63, 2879–2894. [Google Scholar] [CrossRef]
- Lessio, F.; Tedeschi, R.; Alma, A. Population dynamics, host plants and infection rate with Stolbur phytoplasma of Hyalesthes obsoletus Signoret in north-western Italy. J. Plant Pathol. 2007, 89, 97–102. [Google Scholar]
- Osler, R.; Carraro, L.; Loi, N.; Refatti, E. Symptom expression and disease occurrence of a yellows disease of grapevine in north- eastern Italy. Plant Dis. 1993, 77, 496–498. [Google Scholar] [CrossRef]
- Sugio, A.; MacLean, A.M.; Kingdom, H.N.; Grieve, V.M.; Manimekalai, R.; Hogenhout, S.A. Diverse targets of phytoplasma effectors: From plant development to defense against insects. Annu. Rev. Phytopathol. 2011, 49, 175–195. [Google Scholar] [CrossRef]
- Bertamini, M.; Nedunchezhian, N. Effect of phytoplasma, stolbur-subgroup (Bois noir-BN)] of photosynthetic pigments, saccarides, ribulose-1,5-bisphosphate carboxylase, nitrate and nitrite reductases and photosynthetic activities in field-grow grapevine (Vitis vinifera L. cv Chardonnay) leaves. Photosynthetica 2001, 39, 119–122. [Google Scholar] [CrossRef]
- Maust, B.; Espadas, F.; Talavera, C.; Aguilar, M.; Santamaría, J.M.; Oropezaet, C. Changes in carbohydrate metabolism in coconut palms infected with the lethal yellowing phytoplasma. Phytopathology 2003, 93, 976–981. [Google Scholar] [CrossRef]
- Bertamini, M.; Nedunchezhian, N.; Tomasi, F.; Grando, M.S. Phytoplasma [Stolbur-Subgroup (Bois Noir-BN)] Infection Inhibitis Photosynthetic Pigments, Ribulose-1,5-Biphosphate Carboxylase and Photosynthetic Activities in Field Grown Grapevine (Vitis vinifera L. cv. Chardonnay) Leaves. Physiol. Mol. Plant Pathol. 2002, 61, 357–366. [Google Scholar] [CrossRef]
- Hren, M.; Nikolic, P.; Rotter, A.; Blejec, A.; Terrier, N.; Ravnikar, M.; Dermastia, M.; Gruden, K. ‘Bois noir’ phytoplasma induces significant reprogramming of the leaf transcriptome in the field grown grapevine. BMC Genomics 2009, 10, 460. [Google Scholar] [CrossRef]
- Santi, S.; De Marco, F.; Polizzotto, R.; Grisan, S.; Musetti, R. Recovery from stolbur disease in grapevine involves changes in sugar transport and metabolism. Front. Plant Sci. 2013, 4, 171. [Google Scholar] [CrossRef] [PubMed]
- Margaria, P.; Ferrandino, A.; Caciagali, P.; Kedrina, O.; Schubert, A.; Palmano, S. Metabolic and transcript analysis of the flavonoid pathway in diseased and recovered Nebbiolo and Barbera grapevines (Vitis vinifera L.) following infection by Flavescence dorée phytoplasma. Plant Cell Environ. 2014, 37, 2183–2200. [Google Scholar] [CrossRef] [PubMed]
- Prezelj, N.; Covington, E.; Roitsch, T.; Gruden, K.; Fragner, L.; Weckwerth, W.; Chersicola, M.; Vodopivec, M.; Dermastia, M. Metabolic Consequences of Infection of Grapevine (Vitis vinifera L.) cv. “Modra frankinja” with Flavescence Dorée Phytoplasma. Front. Plant Sci. 2016, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Righetti, L.; Tassoni, A. Increasing sucrose concentration promote phenylpropanoid biosynthesis in grapevine cell cultures. J. Plant Physiol. 2011, 168, 189–195. [Google Scholar] [CrossRef]
- Albertazzi, G.; Milc, J.; Caffagni, A.; Francia, E.; Roncaglia, E.; Ferrari, F.; Tagliafico, E.; Stefani, E.; Pecchioni, N. Gene expression in grapevine cultivars in response to Bois noir phytoplasma infection. Plant Sci. 2009, 176, 792–804. [Google Scholar] [CrossRef]
- Rusjan, D.; Veberič, R.; Mikulič-Petkovšek, M. The response of phenolic compounds in grapes of the variety ‘Chardonnay’ (Vitis vinifera L.) to the infection by phytoplasma Bois noir. Eur. J. Plant Pathol. 2012, 133, 965–974. [Google Scholar] [CrossRef]
- Himeno, M.; Kitazawa, Y.; Yoshida, T.; Maejima, K.; Yamaji, Y.; Oshima, K.; Namba, S. Purple top symptoms are associated with reduction of leaf cell death in phytoplasma-infected plants. Sci. Rep. 2014, 4, 4111. [Google Scholar] [CrossRef]
- Rusjan, D.; Mikulic-Petkovšek, M. Phenolic responses in 1-year-old canes of Vitis vinifera cv. Chardonnay induced by grapevine yellows (Bois noir). Aust. J. Grape Wine Res. 2014, 21, 123–134. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide analysis of phenylpropanoid defence pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Pierro, R.; Passera, A.; Panattoni, A.; Rizzo, D.; Stefani, L.; Bartolini, L.; Casati, P.; Luvisi, A.; Quaglino, F.; Materazzi, A. Prevalence of a ‘Candidatus Phytoplasma solani’ strain, so far associated only with other hosts, in Bois noir-affected grapevines within Tuscan vineyards. Ann. Appl. Biol. 2018, 173, 202–212. [Google Scholar] [CrossRef]
- Cruz, A.; Ampatzidis, Y.; Pierro, R.; Materazzi, A.; Panattoni, A.; De Bellis, L.; Luvisi, A. Detection of Grapevine Yellows symptoms in Vitis vinifera L. with Artificial Intelligence. Comput. Electron. Agric. 2019, 157, 63–76. [Google Scholar] [CrossRef]
- Perestrelo, R.; Lu, Y.; Santos, S.A.O.; Silvestre, A.J.D.; Neto, C.P.; Camara, J.S.; Rocha, S.M. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC–DAD–ESI-MSn Novel phenolic compounds in Vitis vinifera L. grape. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef]
- Fang, N.; Yu, S.; Prior, R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002, 50, 3579–3585. [Google Scholar] [CrossRef] [PubMed]
- Kedrina-Okutan, O.; Novello, V.; Hoffmann, T.; Hadersdorfer, J.; Occhipinti, A.; Schwab, W.; Ferrandino, A. Constitutive Polyphenols in Blades and Veins of Grapevine (Vitis vinifera L.) Healthy Leaves. J. Agric. Food Chem. 2018, 66, 10977–10990. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef]
- Vergine, M.; Nicolì, F.; Negro, C.; Luvisi, A.; Nutricati, E.; Accogli, R.A.; Sabella, E.; Miceli, A. Phytochemical profiles and antioxidant activity of Salvia species from Southern Italy. Rec. Nat. Prod. 2019, 13, 205–215. [Google Scholar] [CrossRef]
- El Gengaihi, S.; Faten, M.A.E.; Emad, M.H.; Shalaby, E.; Doha, H. Antioxidant activity of phenolic compounds from different grape wastes. J. Food Process. Technol. 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gu, L. Antioxidant Capacity, Phenolic Content, and Profiling of Phenolic Compounds in the Seeds, Skin, and Pulp of Vitis rotundifolia (Muscadine Grapes) As Determined by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef]
- Arbulu, M.; Sampedro, M.C.; Gómez-Caballero, A.; Goicolea, M.A.; Barrio, R.J. Untargeted metabolomic analysis using liquid chromatography quadrupole time-of-flight mass spectrometry for non-volatile profiling of wines. Anal. Chim. Acta 2015, 858, 32–41. [Google Scholar] [CrossRef]
- Castillo-Munõz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera white grape cultivars. J. Food Compos. Anal. 2010, 23, 699–705. [Google Scholar] [CrossRef]
- Mattivi, F.; Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Stefanini, M.; Moser, C.; Velasco, R.; Guella, G. Profiling of Resveratrol Oligomers, Important Stress Metabolites, Accumulating in the Leaves of Hybrid Vitis vinifera (Merzling Teroldego) Genotypes Infected with Plasmopara viticola. J. Agric. Food Chem. 2011, 59, 5364–5375. [Google Scholar] [CrossRef]
- Russo, D.; Kenny, O.; Smyth, T.J.; Milella, L.; Hossain, M.B.; Diop, M.S.; Rai, D.K.; Brunton, N.P. Profiling of Phytochemicals in Tissues from Sclerocarya birrea by HPLC-MS and Their Link with Antioxidant Activity. ISRN Chromatogr. 2013, 1–11. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Dettmann, J.; Nimalaratne, C.; Schieber, A. Characterization and quantification of polyfenols in Amazon grape (Pourouma cecropiifolia Martius). Molecules 2010, 15, 8543–8552. [Google Scholar] [CrossRef] [PubMed]
- Nicolì, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 9, 1998. [Google Scholar] [CrossRef] [PubMed]
- De Nisco, M.; Manfra, M.; Bolognese, A.; Sofo, A.; Scopa, A.; Tenore, G.C.; Pagano, F.; Milite, C.; Russo, M.T. Nutraceutical properties and polyphenolic profile of berry skin and wine of Vitis vinifera L. (cv. Aglianico). Food Chem. 2013, 140, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of primary metabolism during plant-pathogen interactions and its contribution to plant. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef]
- Endeshaw, S.; Murolo, S.; Romanazzi, G.; Neri, D. Effects of Bois noir on carbon assimilation, transpiration, stomatal conductance of leaves and yield of grapevine (Vitis vinifera) cv. Chardonnay. Physiol. Plant. 2012, 145, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Rusjan, D.; Halbwirth, H.; Stich, K.; Mikulič-Petkovšek, M.; Veberič, R. Biochemical response of grapevine variety ‘Chardonnay’ (Vitis vinifera L.) to infection with grapevine yellows (Bois noir). Eur. J. Plant Pathol. 2012, 134, 231–237. [Google Scholar] [CrossRef]
- Ehness, R.; Ecker, M.; Godt, D.E.; Roitsch, T. Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 1997, 9, 1825–1841. [Google Scholar] [CrossRef] [PubMed]
- Vitali, M.; Chitarra, W.; Galetto, L.; Bosco, D.; Narzachi, C.; Guillino, M.L.; Spanna, F.; Lovisolo, C. Flavescence dorée phytoplasma deregulates stomatal control of photosynthesis in Vitis vinifera. Ann. Appl. Biol. 2013, 162, 335–346. [Google Scholar] [CrossRef]
- Gutha, L.R.; Casassa, L.F.; Harbertson, J.F.; Naidu, R.A. Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera L.) leaves. BMC Plant Biol. 2010, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Agudelo-Romero, P.; Erban, A.; Rego, C.; Carbonell-Bejerano, P.; Nascimento, T.; Sousa, L.; Martínez-Zapater, J.M.; Kopka, J.; Fortes, A.M. Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. J. Exp. Bot. 2015, 66, 1769–1785. [Google Scholar] [CrossRef]
- Latouche, G.; Bellow, S.; Poutaraud, A.; Meyer, S.; Cerovic, Z.G. Influence of constitutive phenolic compounds on the response of grapevine (Vitis vinifera L.) leaves to infection by Plasmopara viticola. Planta 2013, 237, 351–361. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Stampar, F.; Veberic, R.; Koron, D. Changes in phenolic content induced by infection with Didymella applanata and Leptosphaeria coniothyrium, the causal agents of raspberry spur and cane blight. Plant Pathol. 2014, 63, 185–192. [Google Scholar] [CrossRef]
- Pierro, R.; Passera, A.; Panattoni, A.; Casati, P.; Luvisi, A.; Rizzo, D.; Bianco, P.A.; Quaglino, F.; Materazzi, A. Molecular typing of ‘bois noir’ phytoplasma strains in the Chianti Classico area (Tuscany, central Italy) and their association with symptom severity in Vitis vinifera L. cv. Sangiovese. Phytopathology 2018, 108, 362–373. [Google Scholar] [CrossRef]
- Edwards, K.; Johnstone, C.; Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991, 19, 1349. [Google Scholar] [CrossRef]
- Nicolì, F.; Negro, C.; Nutricati, E.; Vergine, M.; Aprile, A.; Sabella, E.; Damiano, G.; De Bellis, L.; Luvisi, A. Accumulation of azelaic acid in Xylella fastidiosa-infected olive trees: A mobile metabolite for health screening. Phytopathology 2019, 109, 318–325. [Google Scholar] [CrossRef]
- Angelini, E.; Bianchi, G.L.; Filippin, L.; Morassutti, C.; Borgo, M. A new TaqMan method for the identification of phytoplasmas associated with grapevine yellows by real-time PCR assay. J. Microbiol. Meth. 2007, 68, 613–622. [Google Scholar] [CrossRef]
- Osman, F.; Leutenegger, C.; Golino, D.; Rowhani, A. Realtime RT-PCR (TaqMan®) assays for the detection of Grapevine leafroll associated viruses 1–5 and 9. J. Virol. Methods 2007, 141, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Osman, F.; Leutenegger, C.; Golino, D.; Rowhani, A. Comparison of low-density arrays, RT-PCR and real-time TaqMan RT-PCR in detection of grapevine viruses. J. Virol. Methods 2008, 149, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Lebas, B.S.M.; Shiller, J.B.; Quinn, B.D.; Clover, G.R.G. Detection of five viruses infecting dormant bulbs by TaqMan-based real-time RT-PCR. Australas. Plant Path. 2012, 41, 93–98. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In Advances in Photosynthesis Research; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume II. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Di Stefano, R.; Cravero, M.C.; Gentilini, N. Metodi per lo studio dei polifenoli dei vini. L’Enotecnico 1989, 25, 83–89. [Google Scholar]
- Aprile, A.; Negro, C.; Sabella, E.; Luvisi, A.; Nicolì, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; De Bellis, L. Antioxidant Activity and Anthocyanin Contents in Olives (cv Cellina di Nardò) during Ripening and after Fermentation. Antioxidants 2019, 8, 138. [Google Scholar] [CrossRef]
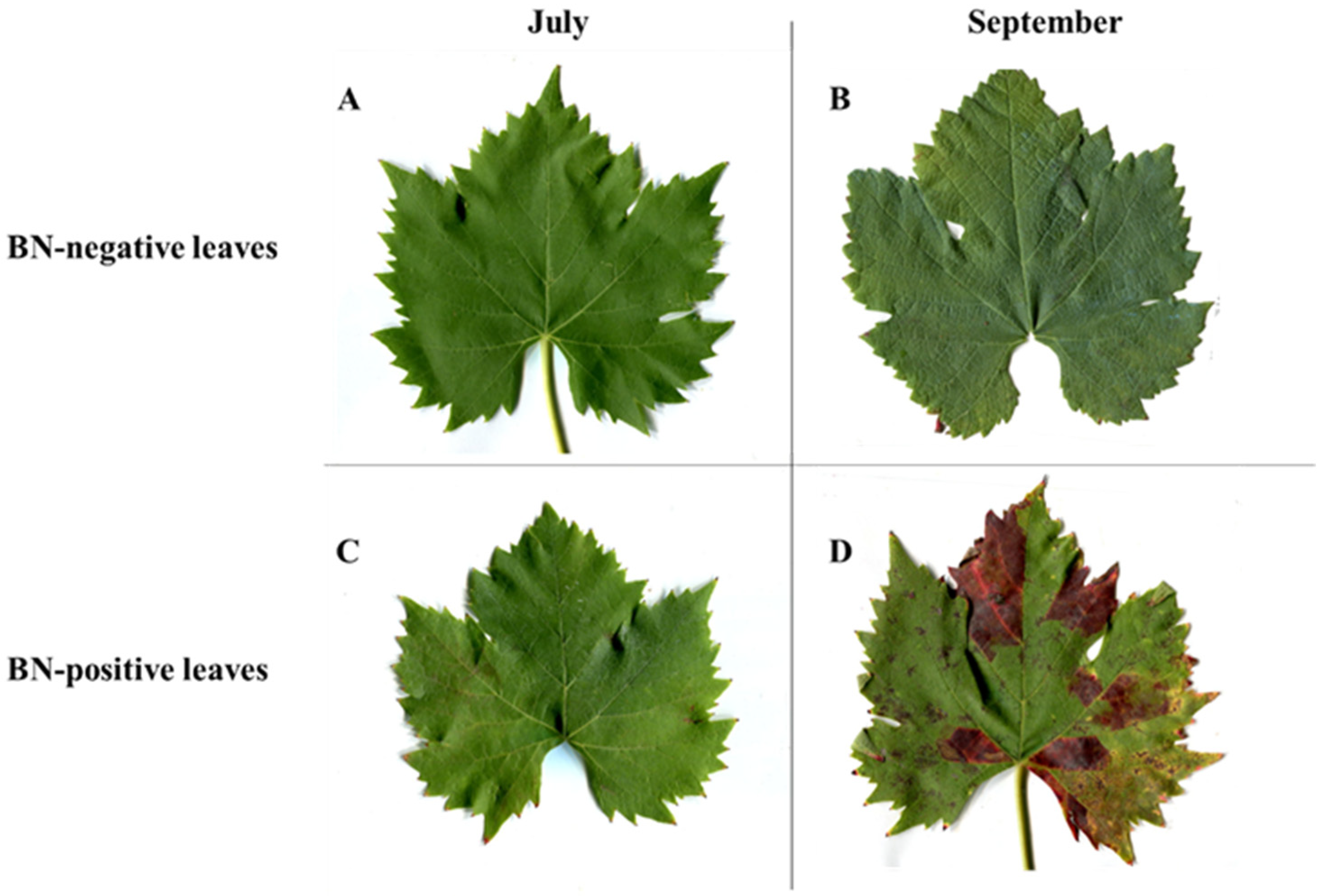




| July | September | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter (mg·g−1 DW) | BN-p | BN-n | BN-p vs. BN-n | %var. | BN-p | BN-n | BN-p vs. BN-n | %var. |
| Chl a | 1.29 ± 0.01 | 1.52 ± 0.04 | **** | −15 | 0.57 ± 0.06 | 1.01 ± 0.03 | **** | −44 |
| Chl b | 0.66 ± 0.04 | 0.67 ± 0.05 | ns | −1 | 0.46 ± 0.06 | 0.66 ± 0.03 | *** | −30 |
| Chls a+b | 1.95 ± 0.17 | 2.19 ± 0.37 | ns | −11 | 1.03 ± 0.21 | 1.67 ± 0.35 | ** | −38 |
| Chls a/b | 1.96 ± 0.01 | 2.29 ± 0.01 | - | −14 | 1.23 ± 0.01 | 1.53 ± 0.01 | - | −19 |
| Cars | 0.25 ± 0.06 | 0.25 ± 0.05 | ns | 0 | 0.12 ± 0.02 | 0.18 ± 0.05 | * | −33 |
| Cars/Chls | 0.13 ± 0.01 | 0.11 ± 0.02 | - | - | 0.12 ± 0.01 | 0.11 ± 0.02 | - | - |
| Soluble sugars | 80.4 ± 6.02 | 47.9 ± 3.21 | **** | +68 | 68.3 ± 5.93 | 52.2 ± 3.39 | *** | +31 |
| N. | Compound (Negative Ion Mode) | RT a (min) | m/z exp. b | m/z calc. c | (M-H)- | Error d | Score e | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | protocatechuic acid 3-glucoside | 2.451 | 315.0737 | 315.0722 | C13H15O9 | −4.98 | 96.36 | [24,25] |
| 2 | caftaric acid isomer 1 | 3.060 | 311.0436 | 311.0409 | C13H11O9 | −8.76 | 94.65 | [24,26,27] |
| 3 | caftaric acid isomer 2 | 3.386 | 311.0404 | 311.0409 | C13H11O9 | 1.49 | 94.65 | [24,26,27] |
| 4 | protocatechuic acid | 3.689 | 153.0571 | 153.0557 | C8H9O3 | −9.28 | 98.83 | [25,27,28] |
| 5 | protocatechuic acid glucoside | 3.704 | 315.1101 | 315.1045 | C14H19O8 | −5.05 | 95.00 | [25,26] |
| 6 | rosmarinic acid | 4.610 | 359.0785 | 359.0772 | C18H15O8 | −3.46 | 94.79 | [29] |
| 7 | caffeic acid glucoside | 4.689 | 341.0893 | 341.0878 | C15H17O9 | −4.45 | 87.82 | [25,30] |
| 8 | tartaric acid | 5.176 | 149.0103 | 149.0092 | C4H5O6 | −7.56 | 99.85 | [31] |
| 9 | coumaric acid | 5.245 | 163.0398 | 163.0401 | C9H7O3 | 1.87 | 98.83 | [24,27] |
| 10 | catechin* | 5.307 | 289.0695 | 289.0718 | C15H13O6 | 7.67 | 98.49 | [24,26,27] |
| 11 | isorhamnetin 3-glucuronide isomer 1 | 5.568 | 491.0841 | 491.0831 | C22H19O1 | −2.09 | 94.41 | [32] |
| 12 | viniferin | 6.555 | 453.1348 | 453.1337 | C28H21O6 | 2.42 | 88.00 | [33] |
| 13 | unknown | 6.907 | 447.1492 | 447.1508 | C19H27O1 | 3.57 | 43.82 | - |
| 14 | unknown | 7.107 | 451.2193 | 451.2185 | C20H35O1 | −1.90 | 90.34 | - |
| 15 | epicatechin* | 7.193 | 289.0735 | 289.0718 | C15H13O6 | −6.02 | 91.43 | [24,27] |
| 16 | myricetin 3-O-glucuronide is.1 | 7.333 | 493.0601 | 493.0624 | C21H17O1 | 4.67 | 88.49 | [26] |
| 17 | hexose derivative isomer1 | 7.361 | 431.1920 | 431.1923 | C20H31O1 | 0.65 | 92.50 | [34] |
| 18 | hexose derivative isomer 2 | 7.704 | 431.1928 | 431.1923 | C20H31O1 | −1.15 | 99.00 | [34] |
| 19 | quercetin-pentoside | 7.872 | 433.2081 | 433.2079 | C20H33O1 | −0.31 | 94.94 | [24,26,35] |
| 20 | hexose derivative isomer 3 | 8.073 | 431.1914 | 431.1923 | C20H31O1 | −1.98 | 95.00 | [34] |
| 21 | unknown | 8.243 | 657.1084 | 657.1097 | C30H25O1 | 1.94 | 94.94 | - |
| 22 | myricetin 3-O-glucuronide isomer 2 | 8.257 | 493.0629 | 493.0624 | C21H17O1 | −1.05 | 86.75 | [26] |
| 23 | myricetin 3-O-glucuronide isomer 3 | 8.505 | 493.0643 | 493.0624 | C21H17O1 | −3.86 | 91.97 | [26] |
| 24 | myricetin-3-glucoside | 8.604 | 479.0849 | 479.0831 | C21H19O1 | −3.73 | 91.89 | [27] |
| 25 | unknow | 8.967 | 387.2021 | 387.2024 | C19H31O8 | 0.86 | 92.39 | - |
| 26 | quercetin-glucoside isomer 1* | 9.488 | 463.0902 | 463.0882 | C21H19O1 | −4.43 | 93.41 | [24,26,30] |
| 27 | quercetin 3-glucuronide | 9.615 | 477.0697 | 477.0675 | C21H17O1 | −4.77 | 95.94 | [26,27] |
| 28 | quercetin-glucoside isomer 2* | 9.750 | 463.0900 | 463.0882 | C21H19O1 | −3.91 | 93.21 | [24,26,30] |
| 29 | kaempferol 3-O-glucoside* | 10.274 | 447.0955 | 447.0933 | C21H19O1 | −5.00 | 91.62 | [24,26] |
| 30 | unknown | 10.435 | 549.2544 | 549.2553 | C25H41O1 | 1.62 | 62.74 | - |
| 31 | kaempferol-rutinoside | 10.519 | 593.1513 | 593.1512 | C27H29O1 | −0.10 | 90.62 | [24,30] |
| 32 | kaempferol 3-O-glucuronide | 10.673 | 461.0740 | 461.0725 | C21H17O1 | −3.23 | 91.22 | [26] |
| 33 | quercetin 3-O-rhamnoside | 10.678 | 447.0960 | 447.0933 | C21H19O1 | −6.05 | 93.66 | [30,34] |
| 34 | unknown | 10.734 | 429.1778 | 429.1776 | C20H29O1 | −2.71 | 88.77 | - |
| 35 | isorhamnetin 3 glucuronide isomer 2 | 11.007 | 491.0841 | 491.0831 | C22H19O1 | −2.01 | 92.57 | [32] |
| 36 | resveratrol glucoside | 11.122 | 389.1228 | 389.1242 | C20H21O8 | 3.49 | 95.85 | [30] |
| 37 | caffeic acid and catechin condensation product | 11.622 | 451.1010 | 451.1028 | C24H19O6 | −3.99 | 87.00 | [33] |
| 38 | quercetin | 13.100 | 301.0939 | 301.0412 | C15H9O7 | 6.44 | 91.77 | [36] |
| 39 | syringetin-3-O-galactoside | 13.990 | 507.2089 | 507.2083 | C23H23O1 | −1.11 | 96.44 | [37] |
| N. | Compound (Positive Ion Mode) | RT a (min) | m/z exp. b | m/z calc. c | (M-H)+ | Error d | Score e | Reference |
| 40 | delphinidin 3-glucoside | 8.330 | 465.1032 | 465.1028 | C21H21O1 | −0.94 | 98.36 | [26,35] |
| 41 | cyanidin 3-glucoside* | 9.615 | 449.1104 | 449.1078 | C21H21O1 | −5.78 | 96.25 | [35] |
| 42 | peonidin 3-glucoside | 12.200 | 463.1232 | 463.1235 | C22H23O1 | 0.30 | 97.02 | [35] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negro, C.; Sabella, E.; Nicolì, F.; Pierro, R.; Materazzi, A.; Panattoni, A.; Aprile, A.; Nutricati, E.; Vergine, M.; Miceli, A.; et al. Biochemical Changes in Leaves of Vitis vinifera cv. Sangiovese Infected by Bois Noir Phytoplasma. Pathogens 2020, 9, 269. https://doi.org/10.3390/pathogens9040269
Negro C, Sabella E, Nicolì F, Pierro R, Materazzi A, Panattoni A, Aprile A, Nutricati E, Vergine M, Miceli A, et al. Biochemical Changes in Leaves of Vitis vinifera cv. Sangiovese Infected by Bois Noir Phytoplasma. Pathogens. 2020; 9(4):269. https://doi.org/10.3390/pathogens9040269
Chicago/Turabian StyleNegro, Carmine, Erika Sabella, Francesca Nicolì, Roberto Pierro, Alberto Materazzi, Alessandra Panattoni, Alessio Aprile, Eliana Nutricati, Marzia Vergine, Antonio Miceli, and et al. 2020. "Biochemical Changes in Leaves of Vitis vinifera cv. Sangiovese Infected by Bois Noir Phytoplasma" Pathogens 9, no. 4: 269. https://doi.org/10.3390/pathogens9040269
APA StyleNegro, C., Sabella, E., Nicolì, F., Pierro, R., Materazzi, A., Panattoni, A., Aprile, A., Nutricati, E., Vergine, M., Miceli, A., De Bellis, L., & Luvisi, A. (2020). Biochemical Changes in Leaves of Vitis vinifera cv. Sangiovese Infected by Bois Noir Phytoplasma. Pathogens, 9(4), 269. https://doi.org/10.3390/pathogens9040269











