Continuous Reassortment of Clade 2.3.4.4 H5N6 Highly Pathogenetic Avian Influenza Viruses Demonstrating High Risk to Public Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical and Biological Safety Statement
2.2. Virus Isolation, RNA Extraction, RT-PCR, and DNA Sequencing
2.3. Phylogenetic Analyses
2.4. Receptor Binding Specificity Assay
2.5. Growth Kinetics of Influenza in Different Cells
2.6. Mice and Chickens Challenge Studies
3. Results
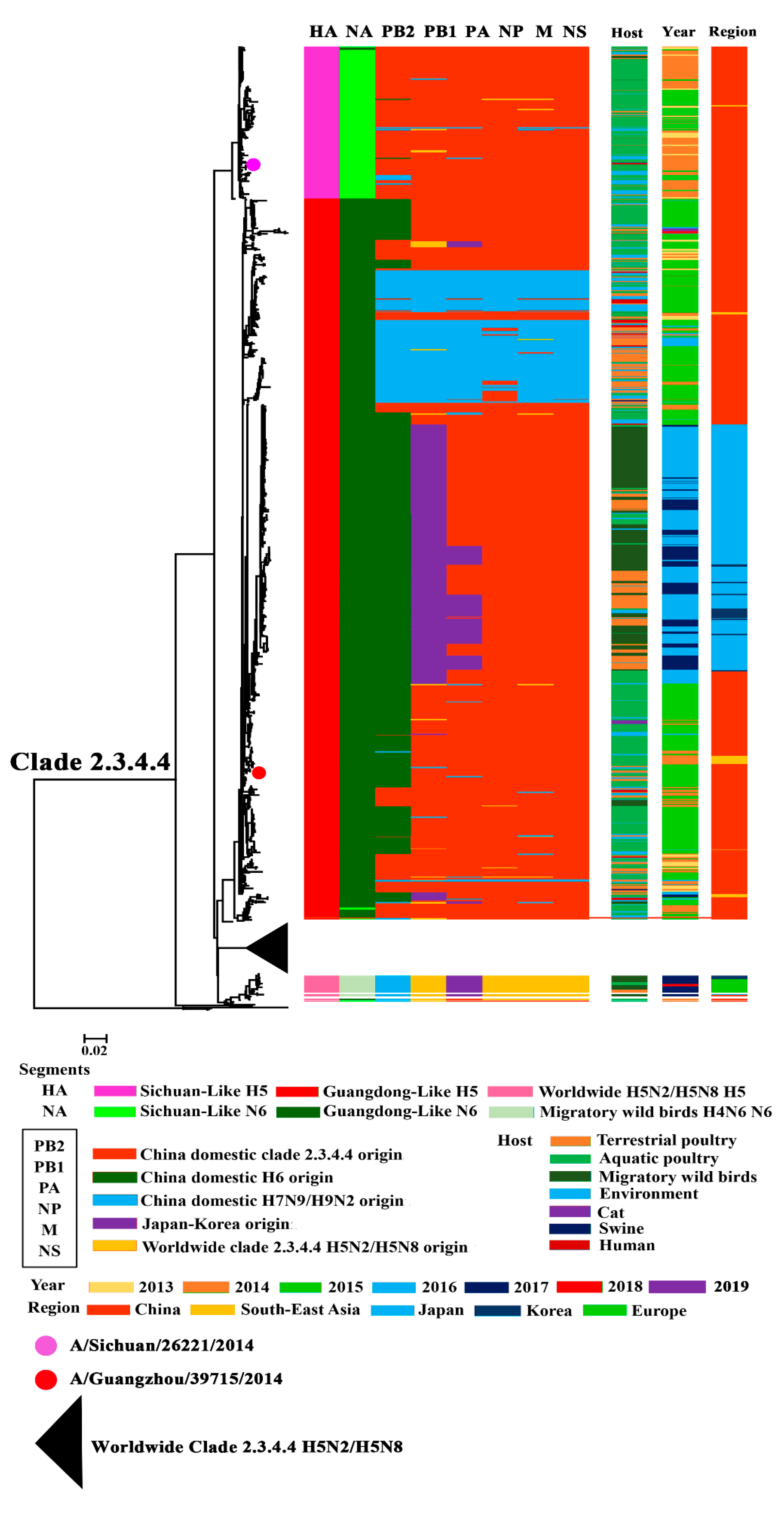
3.1. Phylogenetic Analysis of Surface Genes of H5N6 Viruses
3.2. Phylogenetic Analysis of Internal Genes of Clade 2.3.4.4 H5N6 HPAIVs
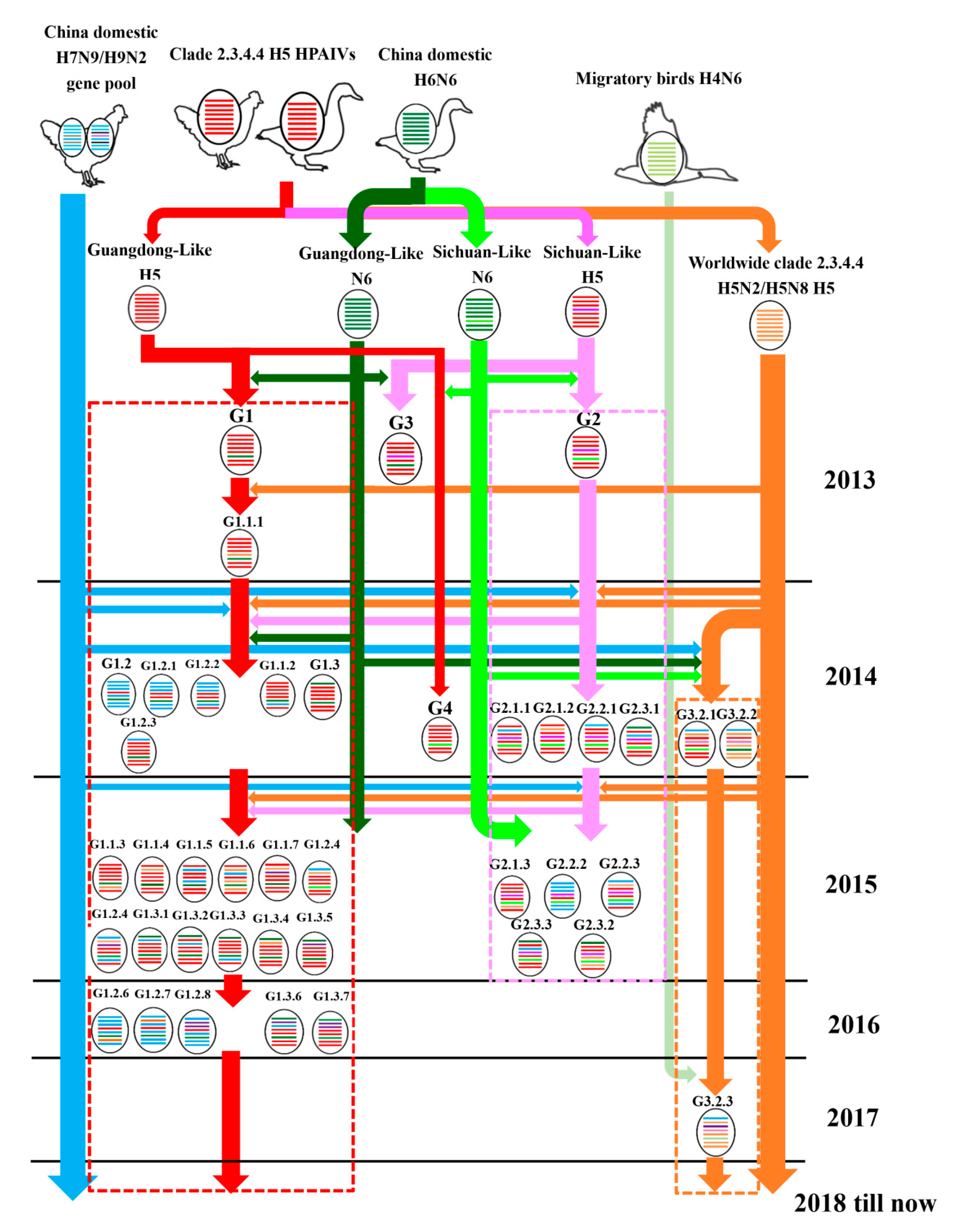
3.3. Continuously Reassortment of Clade 2.3.4.4 H5N6 HPAIVs
3.4. Host, Time, and Region Distribution of Clade 2.3.4.4 H5N6 HPAIVs
3.5. Receptor Binding Ability of Clade 2.3.4.4 H5N6 HPAIVs
3.6. Replication of Clade 2.3.4.4 H5N6 in Different Cells
3.7. Pathogenicity of Clade 2.3.4.4 H5N6 in Mice
3.8. Pathogenicity of Clade 2.3.4.4 H5N6 in Chickens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qi, X.; Cui, L.; Yu, H.; Ge, Y.; Tang, F. Whole-Genome Sequence of a Reassortant H5N6 Avian Influenza Virus Isolated from a Live Poultry Market in China, 2013. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, R.; Peng, X.; Xu, L.; Cheng, L.; Lu, X.; Jin, C.; Xie, T.; Yao, H.; Wu, N. Novel reassortant highly pathogenic H5N6 avian influenza viruses in poultry in China. Infect. Genet. Evol. 2015, 31, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.Y.; Phommachanh, P.; Kalpravidh, W.; Chanthavisouk, C.; Gilbert, J.; Bingham, J.; Davies, K.R.; Cooke, J.; Eagles, D.; Phiphakhavong, S.; et al. Reassortant highly pathogenic influenza A(H5N6) virus in Laos. Emerg. Infect. Dis. 2015, 21, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Jang, Y.; Nguyen, T.D.; Jones, J.; Shepard, S.S.; Yang, H.; Gerloff, N.; Fabrizio, T.; Nguyen, L.V.; Inui, K.; et al. Shifting Clade Distribution, Reassortment, and Emergence of New Subtypes of Highly Pathogenic Avian Influenza A(H5) Viruses Collected from Vietnamese Poultry from 2012 to 2015. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Kwon, J.H.; Lee, D.H.; Swayne, D.E.; Noh, J.Y.; Yuk, S.S.; Erdene-Ochir, T.O.; Hong, W.T.; Jeong, J.H.; Jeong, S.; Gwon, G.B.; et al. Reassortant Clade 2.3.4.4 Avian Influenza A(H5N6) Virus in a Wild Mandarin Duck, South Korea, 2016. Emerg. Infect. Dis. 2017, 23, 822–826. [Google Scholar] [CrossRef]
- Okamatsu, M.; Ozawa, M.; Soda, K.; Takakuwa, H.; Haga, A.; Hiono, T.; Matsuu, A.; Uchida, Y.; Iwata, R.; Matsuno, K.; et al. Characterization of Highly Pathogenic Avian Influenza Virus A(H5N6), Japan, November 2016. Emerg. Infect. Dis. 2017, 23, 691–695. [Google Scholar] [CrossRef]
- Beerens, N.; Koch, G.; Heutink, R.; Harders, F.; Vries, D.P.E.; Ho, C.; Bossers, A.; Elbers, A. Novel Highly Pathogenic Avian Influenza A(H5N6) Virus in the Netherlands, December 2017. Emerg. Infect. Dis. 2018, 24. [Google Scholar] [CrossRef]
- Bi, Y.; Chen, Q.; Wang, Q.; Chen, J.; Jin, T.; Wong, G.; Quan, C.; Liu, J.; Wu, J.; Yin, R.; et al. Genesis, Evolution and Prevalence of H5N6 Avian Influenza Viruses in China. Cell Host Microbe 2016, 20, 810–821. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; Yang, J.; Guo, J.; He, J.; Guo, J.; Weng, S.; Jia, Y.; Liu, B.; Li, X.; et al. Genetic and biological characterization of two novel reassortant H5N6 swine influenza viruses in mice and chickens. Infect. Genet. Evol. 2015, 36, 462–466. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, T.; Ou, X.; Liu, R.; Yang, Y.; Ye, W.; Chen, J.; Yao, D.; Sun, B.; Zhang, X.; et al. Clinical, epidemiological and virological characteristics of the first detected human case of avian influenza A(H5N6) virus. Infect. Genet. Evol. 2016, 40, 236–242. [Google Scholar] [CrossRef]
- Jiang, W.M.; Liu, S.; Chen, J.; Hou, G.Y.; Li, J.P.; Cao, Y.F.; Zhuang, Q.Y.; Li, Y.; Huang, B.X.; Chen, J.M. Molecular epidemiological surveys of H5 subtype highly pathogenic avian influenza viruses in poultry in China during 2007-2009. J. Gen. Virol. 2010, 91, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, J.; Zhong, G.; Deng, G.; Tian, G.; Ge, J.; Zeng, X.; Song, J.; Zhao, D.; Liu, L.; et al. Continued evolution of H5N1 influenza viruses in wild birds, domestic poultry, and humans in China from 2004 to 2009. J. Virol. 2010, 84, 8389–8397. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Chen, H.; Gao, G.F.; Shu, Y.; Kawaoka, Y. H5N1 influenza viruses: Outbreaks and biological properties. Cell Res. 2010, 20, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Bi, Y.; Wong, G.; Gray, G.C.; Gao, G.F.; Li, S. Epidemiology, Evolution, and Recent Outbreaks of Avian Influenza Virus in China. J. Virol. 2015, 89, 8671–8676. [Google Scholar] [CrossRef]
- Cheung, C.L.; Vijaykrishna, D.; Smith, G.J.; Fan, X.H.; Zhang, J.X.; Bahl, J.; Duan, L.; Huang, K.; Tai, H.; Wang, J.; et al. Establishment of influenza A virus (H6N1) in minor poultry species in southern China. J. Virol. 2007, 81, 10402–10412. [Google Scholar] [CrossRef]
- Huang, K.; Zhu, H.; Fan, X.; Wang, J.; Cheung, C.L.; Duan, L.; Hong, W.; Liu, Y.; Li, L.; Smith, D.K.; et al. Establishment and lineage replacement of H6 influenza viruses in domestic ducks in southern China. J. Virol. 2012, 86, 6075–6083. [Google Scholar] [CrossRef]
- Wei, S.H.; Yang, J.R.; Wu, H.S.; Chang, M.C.; Lin, J.S.; Lin, C.Y.; Liu, Y.L.; Lo, Y.C.; Yang, C.H.; Chuang, J.H.; et al. Human infection with avian influenza A H6N1 virus: An epidemiological analysis. Lancet Respir. Med. 2013, 1, 771–778. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Zhang, Y.; Zhao, N.; Yu, Z.; Pan, H.; Chan, T.C.; Zhang, Z.R.; Liu, S.L. Comparative Epidemiology of Human Fatal Infections with Novel, High (H5N6 and H5N1) and Low (H7N9 and H9N2) Pathogenicity Avian Influenza A Viruses. Int. J. Environ. Res. Public Health 2017, 14, 263. [Google Scholar] [CrossRef]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef]
- Qi, W.; Shi, W.; Li, W.; Huang, L.; Li, H.; Wu, Y.; Yan, J.; Jiao, P.; Zhu, B.; Ma, J.; et al. Continuous reassortments with local chicken H9N2 virus underlie the human-infecting influenza A (H7N9) virus in the new influenza season, Guangdong, China. Protein Cell 2014, 5, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhou, X.; Shi, W.; Huang, L.; Xia, W.; Liu, D.; Li, H.; Chen, S.; Lei, F.; Cao, L.; et al. Genesis of the novel human-infecting influenza A(H10N8) virus and potential genetic diversity of the virus in poultry, China. Euro Surveill. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, J.; Guo, J.; Deng, G.; Zhang, Q.; Wang, J.; He, X.; Wang, K.; Chen, J.; Li, Y.; et al. Genetics, receptor binding property, and transmissibility in mammals of naturally isolated H9N2 Avian Influenza viruses. PLoS Pathog. 2014, 10, e1004508. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Lager, K.M.; Lekcharoensuk, P.; Ulery, E.S.; Janke, B.H.; Solorzano, A.; Webby, R.J.; Garcia-Sastre, A.; Richt, J.A. Viral reassortment and transmission after co-infection of pigs with classical H1N1 and triple-reassortant H3N2 swine influenza viruses. J. Gen. Virol. 2010, 91, 2314–2321. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Liu, B.T.; Ma, S.J.; Cui, P.; Liu, W.Q.; Li, Y.B.; Guo, J.; Chen, H.L. High frequency of reassortment after co-infection of chickens with the H4N6 and H9N2 influenza A viruses and the biological characteristics of the reassortants. Vet. Microbiol. 2018, 222, 11–17. [Google Scholar] [CrossRef]
- Urbaniak, K.; Markowska-Daniel, I. In vivo reassortment of influenza viruses. Acta Biochim. Pol. 2014, 61, 427–431. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, W.F.; Li, X.D.; Bo, H.; Zhang, Y.; Zou, S.M.; Gao, R.B.; Dong, J.; Zhao, X.; Chen, W.B.; et al. Genesis and Dissemination of Highly Pathogenic H5N6 Avian Influenza Viruses. J. Virol. 2017, 91, e02199–e02216. [Google Scholar] [CrossRef]
- Napp, S.; Majo, N.; Sanchez-Gonzalez, R.; Vergara-Alert, J. Emergence and spread of highly pathogenic avian influenza A(H5N8) in Europe in 2016-2017. Transbound. Emerg. Dis. 2018, 65, 1217–1226. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, H.M.; Lee, E.K.; Song, B.M.; Jeong, J.; Kwon, Y.K.; Kim, H.R.; Lee, K.J.; Hong, M.S.; Jang, I.; et al. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg. Infect. Dis. 2014, 20, 1087–1089. [Google Scholar] [CrossRef]
- Zhao, K.; Gu, M.; Zhong, L.; Duan, Z.; Zhang, Y.; Zhu, Y.; Zhao, G.; Zhao, M.; Chen, Z.; Hu, S.; et al. Characterization of three H5N5 and one H5N8 highly pathogenic avian influenza viruses in China. Vet. Microbiol. 2013, 163, 351–357. [Google Scholar] [CrossRef]
- Ozawa, M.; Matsuu, A.; Tokorozaki, K.; Horie, M.; Masatani, T.; Nakagawa, H.; Okuya, K.; Kawabata, T.; Toda, S. Genetic diversity of highly pathogenic H5N8 avian influenza viruses at a single overwintering site of migratory birds in Japan, 2014/15. Eurosurveillance 2015, 20, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Dalby, A.R.; Iqbal, M. The European and Japanese outbreaks of H5N8 derive from a single source population providing evidence for the dispersal along the long distance bird migratory flyways. PeerJ 2015, 3, e934. [Google Scholar] [CrossRef] [PubMed]
- Ip, H.S.; Torchetti, M.K.; Crespo, R.; Kohrs, P.; DeBruyn, P.; Mansfield, K.G.; Baszler, T.; Badcoe, L.; Bodenstein, B.; Shearn-Bochsler, V.; et al. Novel Eurasian highly pathogenic avian influenza A H5 viruses in wild birds, Washington, USA, 2014. Emerg. Infect. Dis. 2015, 21, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Pasick, J.; Berhane, Y.; Joseph, T.; Bowes, V.; Hisanaga, T.; Handel, K.; Alexandersen, S. Reassortant highly pathogenic influenza A H5N2 virus containing gene segments related to Eurasian H5N8 in British Columbia, Canada, 2014. Sci. Rep. 2015, 5, 9484. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, E.I.; Xu, D.; Markwick, P.R.L.; Amaro, R.E.; Pao, H.C.; Wu, K.J.; Alam, M.; McCammon, J.A.; Li, W.W. Mechanism of Glycan Receptor Recognition and Specificity Switch for Avian, Swine, and Human Adapted Influenza Virus Hemagglutinins: A Molecular Dynamics Perspective. J. Am. Chem. Soc. 2009, 131, 17430–17442. [Google Scholar] [CrossRef][Green Version]
- Ha, Y.; Stevens, D.J.; Skehel, J.J.; Wiley, D.C. X-ray structures of H5 avian and H9 swine influenza virus hemagglutinins bound to avian and human receptor analogs. Proc. Natl. Acad. Sci. USA 2001, 98, 11181–11186. [Google Scholar] [CrossRef]
- Martin, J.; Wharton, S.A.; Lin, Y.P.; Takemoto, D.K.; Skehel, J.J.; Wiley, D.C.; Steinhauer, D.A. Studies of the binding properties of influenza hemagglutinin receptor-site mutants. Virology 1998, 241, 101–111. [Google Scholar] [CrossRef]
- Vines, A.; Wells, K.; Matrosovich, M.; Castrucci, M.R.; Ito, T.; Kawaoka, Y. The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. J. Virol. 1998, 72, 7626–7631. [Google Scholar] [CrossRef]
- Hatta, M.; Gao, P.; Halfmann, P.; Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 2001, 293, 1840–1842. [Google Scholar] [CrossRef]
- Song, W.; Wang, P.; Mok, B.W.; Lau, S.Y.; Huang, X.; Wu, W.L.; Zheng, M.; Wen, X.; Yang, S.; Chen, Y.; et al. The K526R substitution in viral protein PB2 enhances the effects of E627K on influenza virus replication. Nat. Commun. 2014, 5, 5509. [Google Scholar] [CrossRef]
- Czudai-Matwich, V.; Otte, A.; Matrosovich, M.; Gabriel, G.; Klenk, H.D. PB2 mutations D701N and S714R promote adaptation of an influenza H5N1 virus to a mammalian host. J. Virol. 2014, 88, 8735–8742. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Ma, W.; Sun, N.; Huang, L.; Li, Y.; Zeng, Z.; Wen, Y.; Zhang, Z.; Li, H.; Li, Q.; et al. PB2-588 V promotes the mammalian adaptation of H10N8, H7N9 and H9N2 avian influenza viruses. Sci. Rep. 2016, 6, 19474. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.; Guo, H.; Dai, M.; Rottier, P.J.; van Kuppeveld, F.J.; de Haan, C.A. Rapid Emergence of Highly Pathogenic Avian Influenza Subtypes from a Subtype H5N1 Hemagglutinin Variant. Emerg. Infect. Dis. 2015, 21, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Stech, J.; Guan, Y.; Webster, R.G.; Perez, D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001, 146, 2275–2289. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Lee, C.W.; Jung, K.; Jadhao, S.J.; Suarez, D.L. Evaluation of chicken-origin (DF-1) and quail-origin (QT-6) fibroblast cell lines for replication of avian influenza viruses. J. Virol. Methods 2008, 153, 22–28. [Google Scholar] [CrossRef]
- Vallejo, V.; Reyes-Leyva, J.; Hernandez, J.; Ramirez, H.; Delannoy, P.; Zenteno, E. Differential expression of sialic acid on porcine organs during the maturation process. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 126, 415–424. [Google Scholar] [CrossRef]
- Schmier, S.; Mostafa, A.; Haarmann, T.; Bannert, N.; Ziebuhr, J.; Veljkovic, V.; Dietrich, U.; Pleschka, S. In Silico Prediction and Experimental Confirmation of HA Residues Conferring Enhanced Human Receptor Specificity of H5N1 Influenza A Viruses. Sci. Rep. 2015, 5, 11434. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Li, H.; Wang, X.; Li, B.; Ren, X.; Zeng, Z.; Zhang, X.; Liu, S.; Hu, P.; et al. Biological Characterizations of H5Nx Avian Influenza Viruses Embodying Different Neuraminidases. Front. Microbiol. 2017, 8, 1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, Z.; Zhang, Z.; Zheng, Y.; Li, B.; Su, G.; Li, H.; Huang, L.; Qi, W.; Liao, M. The Appropriate Combination of Hemagglutinin and Neuraminidase Prompts the Predominant H5N6 Highly Pathogenic Avian Influenza Virus in Birds. Front. Microbiol. 2018, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Bahl, J.; Smith, G.J.; Wang, J.; Vijaykrishna, D.; Zhang, L.J.; Zhang, J.X.; Li, K.S.; Fan, X.H.; Cheung, C.L.; et al. The development and genetic diversity of H5N1 influenza virus in China, 1996–2006. Virology 2008, 380, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sun, Y.; Pu, J.; Zhang, Y.; Zhu, Q.; Li, J.; Gu, J.; Chang, K.C.; Liu, J. Comparative virus replication and host innate responses in human cells infected with three prevalent clades (2.3.4, 2.3.2, and 7) of highly pathogenic avian influenza H5N1 viruses. J. Virol. 2014, 88, 725–729. [Google Scholar] [CrossRef]


| Virus | Heart | Liver | Spleen | Kidney | Brain | Lung | Time of Death | |
|---|---|---|---|---|---|---|---|---|
| 673 | inoculated | 3/3(6.70 ± 0.30) | 3/3(6.25 ± 0.25) | 3/3(6.20 ± 0.10) | 3/3(6.70 ± 0.02) | 3/3(5.60 ± 0.02) | 3/3(7.00 ± 0.40) | 2d b |
| contact | 0/2(0) | 0/2(0) | 0/2(0) | 0/2(0) | 0/2(0) | 0/2(0) | 4d c | |
| 674 | inoculated | 3/3(6.70 ± 0.30) | 3/3(6.00 ± 0.20) | 3/3(6.50 ± 0.06) | 3/3(6.40 ± 0.08) | 3/3(5.30 ± 0.50) | 3/3(6.70 ± 0.30) | 2d b |
| contact | 2/2(6.50, 7.25) | 2/2(6.50, 6.25) | 2/2(6.25, 7.25) | 2/2(6.25, 7.25) | 2/2(5.75, 7.25) | 2/2(6.50, 8.50) | 4d b | |
| 39,715 | inoculated | 2/3(6.75, 7.50) | 2/3(5.50, 6.25) | 2/3(4.75, 6.50) | 2/3(6.25, 7.50) | 2/3(6.50, 7.25) | 3/3(7.25 ± 1.19) | 1/2d, 2/3d b |
| contact | 0/3(0) | 0/3(0) | 0/3(0) | 0/3(0) | 0/3(0) | 2/3(1.50, 3.75) | 4d b |
| Virus | Throat Swab b | Cloacal Swab b | Death Amount c | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2d | 3d | 5d | 2d | 3d | 5d | 2d | 3d | 4d | 5d | 7d | 8d | 10d | ||
| 673 | inoculated | 3/3 (2.60 ± 0.3) | ---d | ---d | 1/3 (1.75) | ---d | ---d | 8/9 | 1/9 | ---e | ---e | ---e | ---e | ---e |
| contact | 0/6 (0) | 0/6 (0) | 0/4 (0) | 2/6 (1.75, 2.50) | 0/6 (0) | 0/4 (0) | 0/6 | 0/6 | 2/6 | 1/6 | 0/6 | 0/6 | 0/6 | |
| 674 | inoculated | 1/1 (4.5) | ---d | ---d | 1/1 (3.25) | ---d | ---d | 9/9 | ---e | ---e | ---e | ---e | ---e | ---e |
| contact | 0/6 (0) | 2/6 (2.25, 3.75) | ---d | 0/6 (0) | 1/6 (2.25) | ---d | 0/6 | 0/6 | 3/6 | 3/6 | ---e | ---e | ---e | |
| 39,715 | inoculated | 2/8 (1.75, 2.75) | 4/8 (3.2 ± 0.09) | 0/2 (0) | 2/8 (1.50, 3.50) | 4/8 (2.4 ± 0.8) | 0/2 (0) | 1/9 | 3/9 | 1/9 | 2/9 | 1/9 | 0/9 | 1/9 |
| contact | 2/7 (1.50, 3.50) | 3/7 (1.50 ± 0.06) | 2/4 (1.25, 3.50) | 0/7 (0) | 1/7 (1.25) | 3/4 (2.80 ± 1.3) | 0/9 | 0/9 | 3/9 | 1/9 | 1/9 | 1/9 | 1/9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, Q.; Li, B.; Guo, Y.; Xing, J.; Xu, Q.; Liu, L.; Zhang, J.; Qi, W.; Jia, W.; et al. Continuous Reassortment of Clade 2.3.4.4 H5N6 Highly Pathogenetic Avian Influenza Viruses Demonstrating High Risk to Public Health. Pathogens 2020, 9, 670. https://doi.org/10.3390/pathogens9080670
Li H, Li Q, Li B, Guo Y, Xing J, Xu Q, Liu L, Zhang J, Qi W, Jia W, et al. Continuous Reassortment of Clade 2.3.4.4 H5N6 Highly Pathogenetic Avian Influenza Viruses Demonstrating High Risk to Public Health. Pathogens. 2020; 9(8):670. https://doi.org/10.3390/pathogens9080670
Chicago/Turabian StyleLi, Huanan, Qian Li, Bo Li, Yang Guo, Jinchao Xing, Qiang Xu, Lele Liu, Jiahao Zhang, Wenbao Qi, Weixin Jia, and et al. 2020. "Continuous Reassortment of Clade 2.3.4.4 H5N6 Highly Pathogenetic Avian Influenza Viruses Demonstrating High Risk to Public Health" Pathogens 9, no. 8: 670. https://doi.org/10.3390/pathogens9080670
APA StyleLi, H., Li, Q., Li, B., Guo, Y., Xing, J., Xu, Q., Liu, L., Zhang, J., Qi, W., Jia, W., & Liao, M. (2020). Continuous Reassortment of Clade 2.3.4.4 H5N6 Highly Pathogenetic Avian Influenza Viruses Demonstrating High Risk to Public Health. Pathogens, 9(8), 670. https://doi.org/10.3390/pathogens9080670






