Application of Artificial Neuromolecular System in Robotic Arm Control to Assist Progressive Rehabilitation for Upper Extremity Stroke Patients
Abstract
1. Introduction
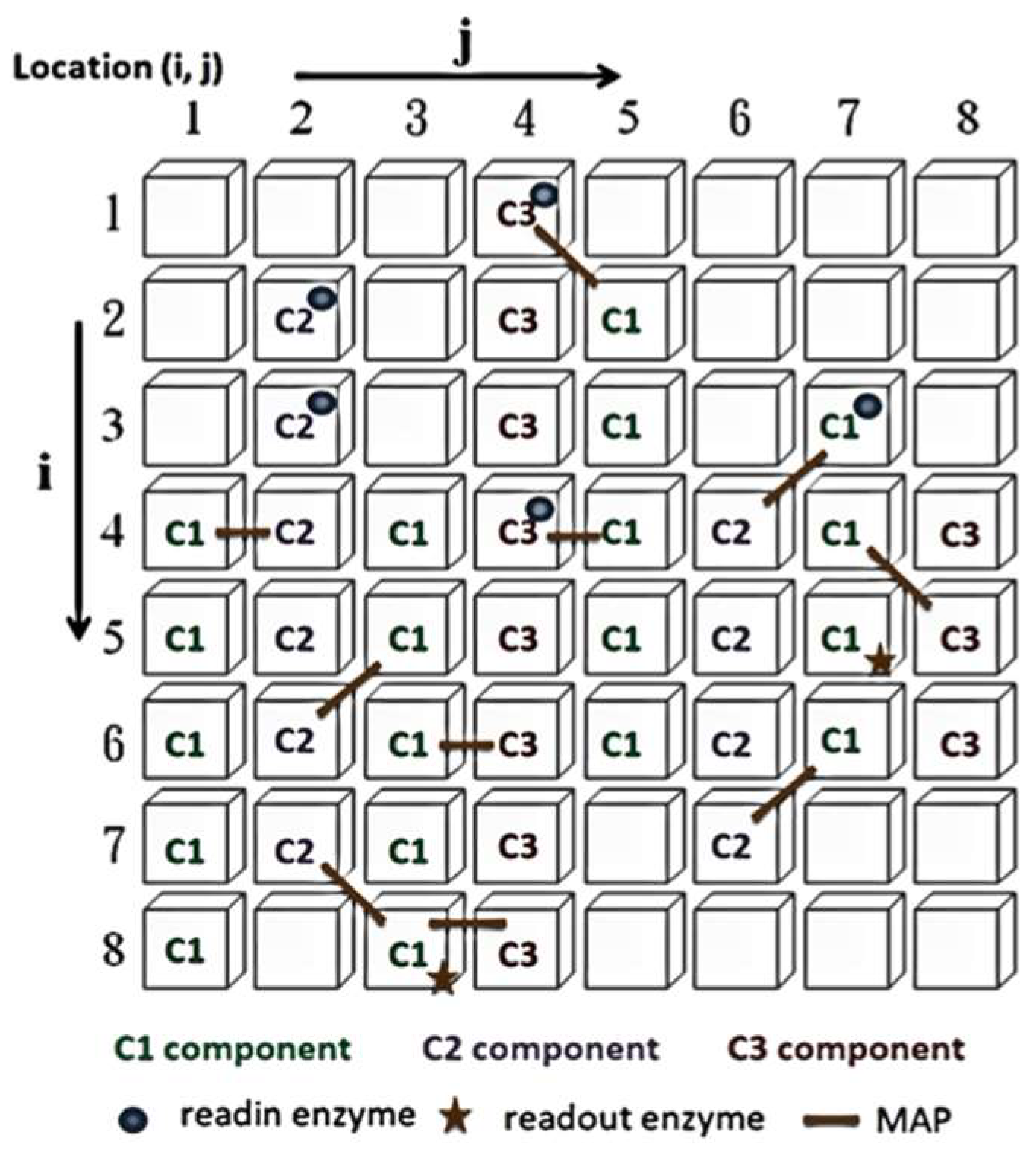
2. The ANM System
- I.
- Evaluation: evaluate the performance of each sub-network according to experimental requirements and find the best sub-network.
- II.
- Copy: copy the variable settings of the best sub-network to other sub-networks.
- III.
- Mutation: randomly change the original settings of each sub-network.
3. Materials and Methods
3.1. Research Model
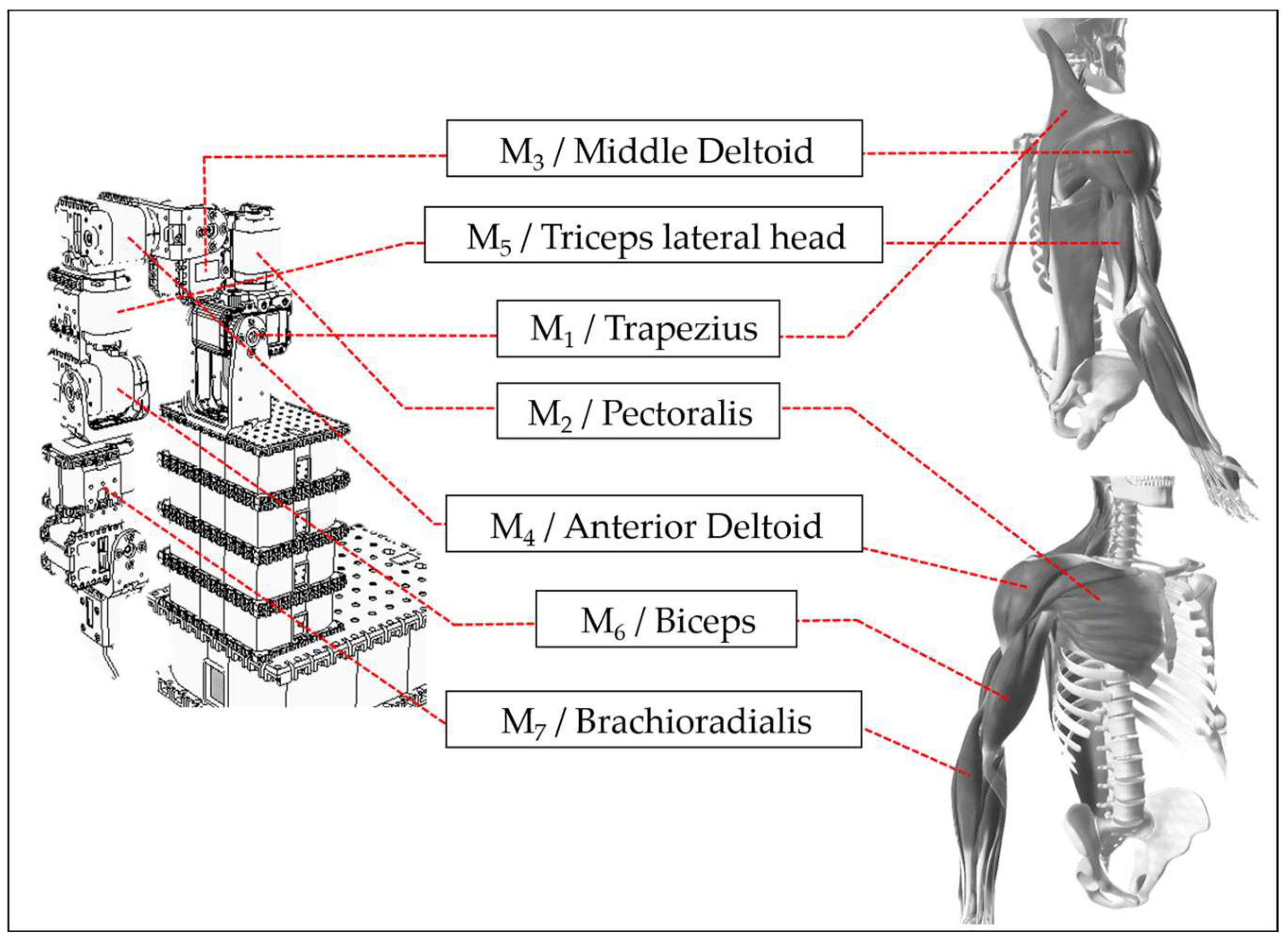
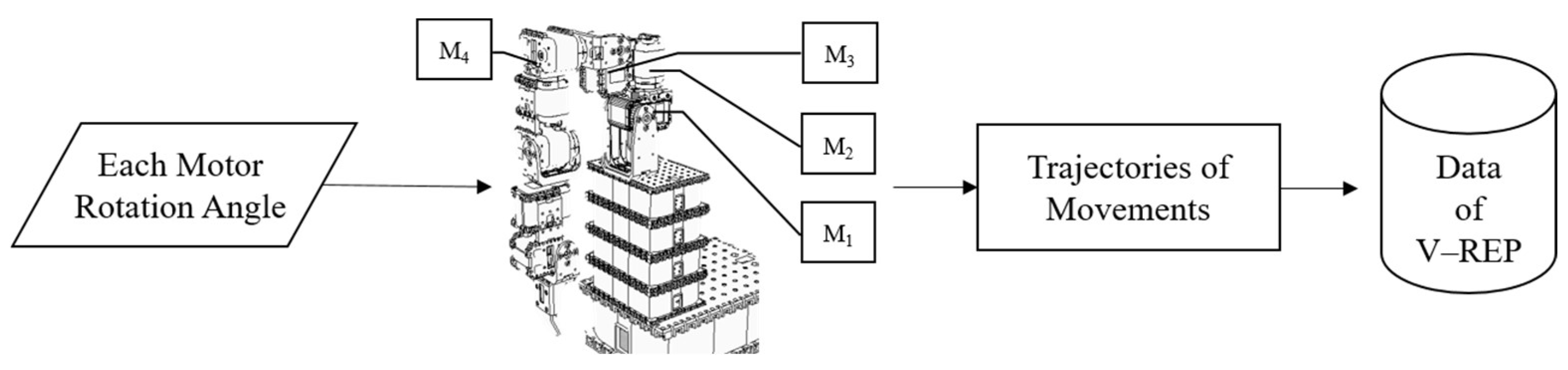
3.2. Equipment and Devices
3.3. Dataset
3.3.1. Artificial World Dataset
3.3.2. Toronto Rehabilitation Dataset
Experimental Tools
Data Collection
- I.
- Using Microsoft Kinect (K4W) with K4W v2 SDK 2.0, 3D positions (x, y, and z coordinates) and orientations of 10 upper body parts and joints relative to the depth sensor were tracked and recorded every 30 milliseconds.
- II.
- Subjects performed a series of short scripted movements, each repeated five times at a comfortable speed and range of motion.
- III.
- Each movement was performed first with the left arm, then the right, for consistency. Participants could rest between repetitions if needed.
- IV.
- Researchers counted each repetition and signaled participants when to start.
- V.
- Stroke patients were asked to perform two types of scripted movements with both upper limbs using a two-degrees-of-freedom haptic robot (handheld end-effector): reach-forward–backward (Fwr_Bck) and reach-side-to-side (Sd2Sd_Bck). Please see Table 1.
- VI.
- To avoid prolonged experimental sessions for stroke patients, healthy participants completed the same scripted movements and then, under expert guidance, performed additional sets simulating everyday post-stroke compensatory movements, including lean-forward (LnFwr), trunk rotation (TrRot), and shoulder elevation (ShElev). Please see Table 1.
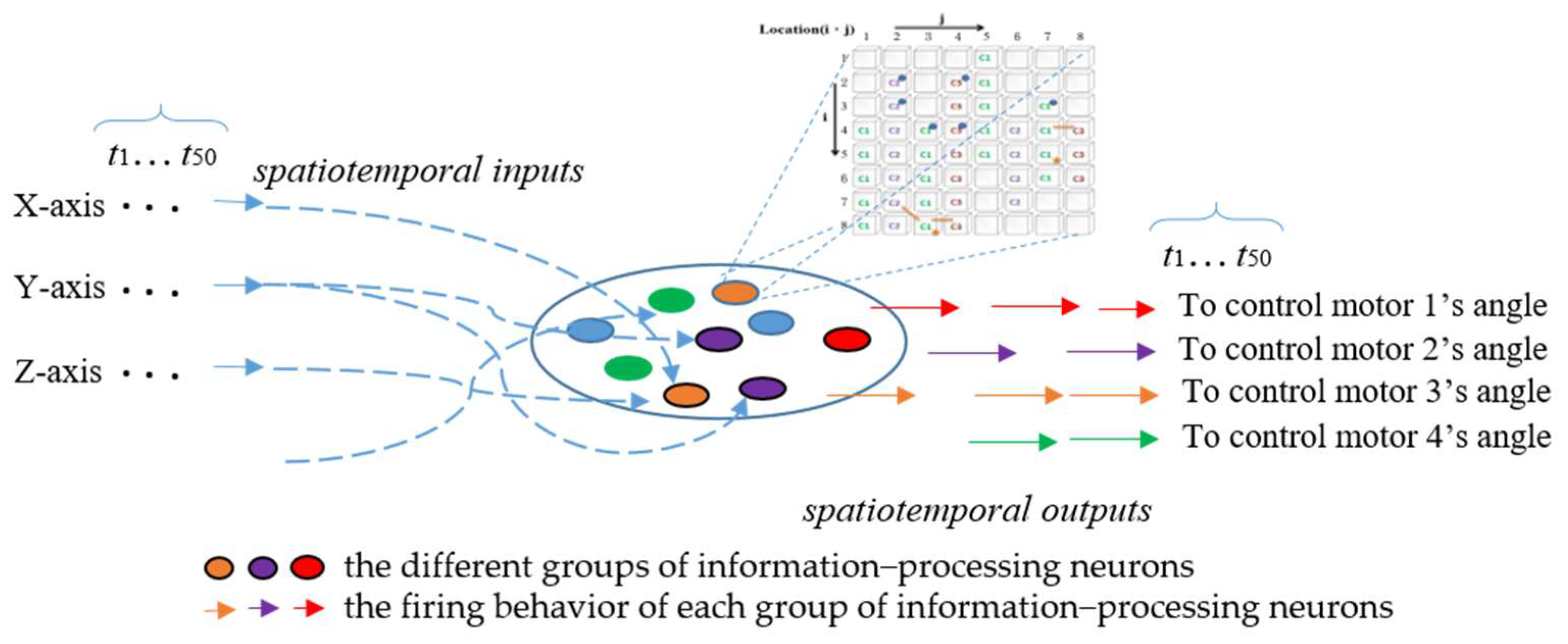
3.4. Input/Output Interface (Linkage to the ANM System)
3.5. Dynamic Time Warping
4. Experiments
4.1. Artificial World Dataset Experiments
- I.
- Focus on the system’s ability to learn autonomously and continuously in an unknown environment.
- II.
- Allow the ANM system to learn arm movements with reduced degrees of freedom, considering how to minimize the number of muscle groups stimulated during movements.
- III.
- Explore how to repeatedly use knowledge from previous tasks to improve learning performance on another related task.
- IV.
- Investigate how to apply the current learning results of the ANM system to different tasks, testing whether the system can adapt to movement transitions.
4.1.1. Perpetual Learning
4.1.2. Adaptive Learning
4.1.3. Transfer Learning
4.1.4. Cross-Task Learning
4.2. Toronto Rehabilitation Dataset Experiments
4.2.1. Adaptive Learning in Rehabilitation
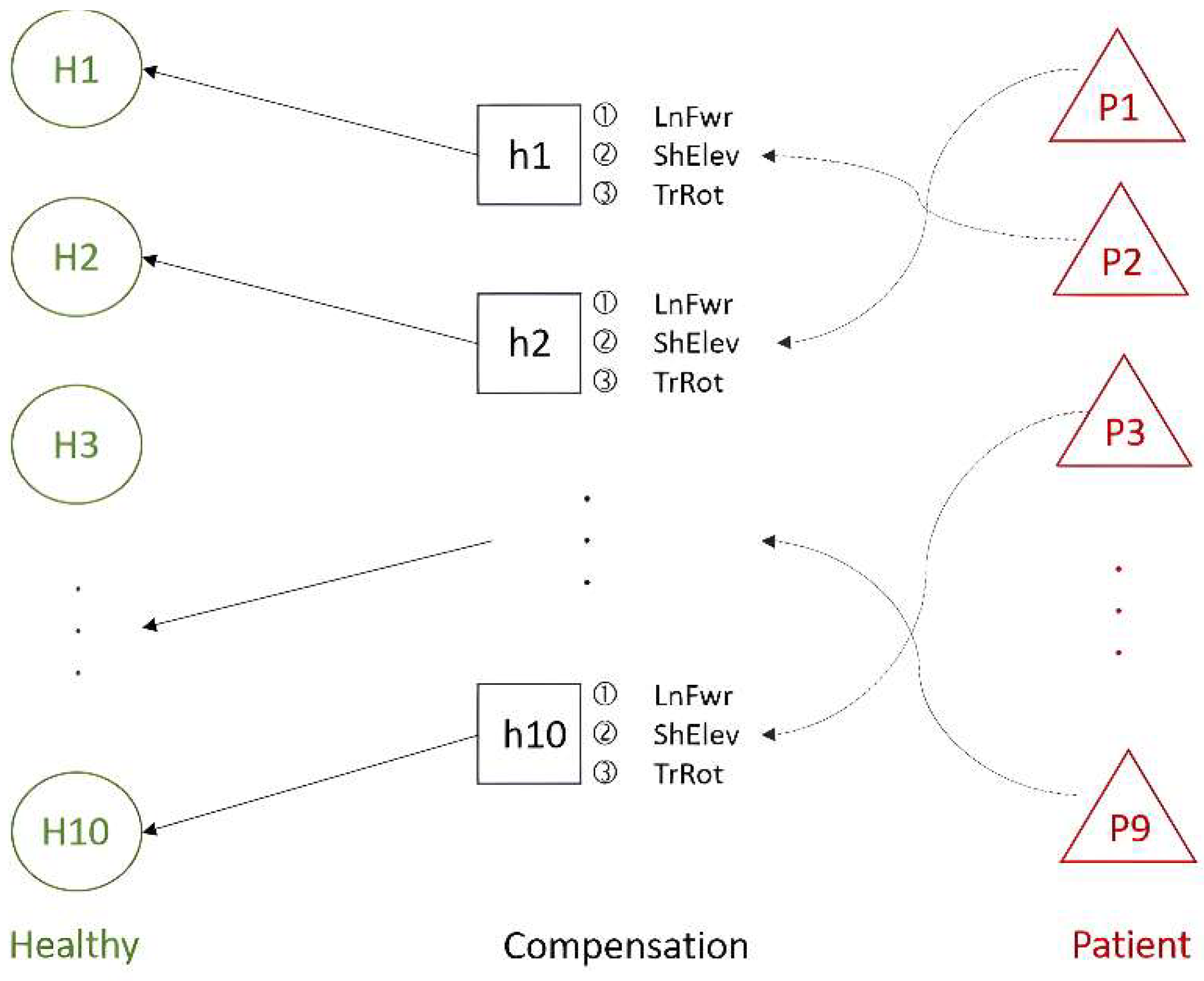
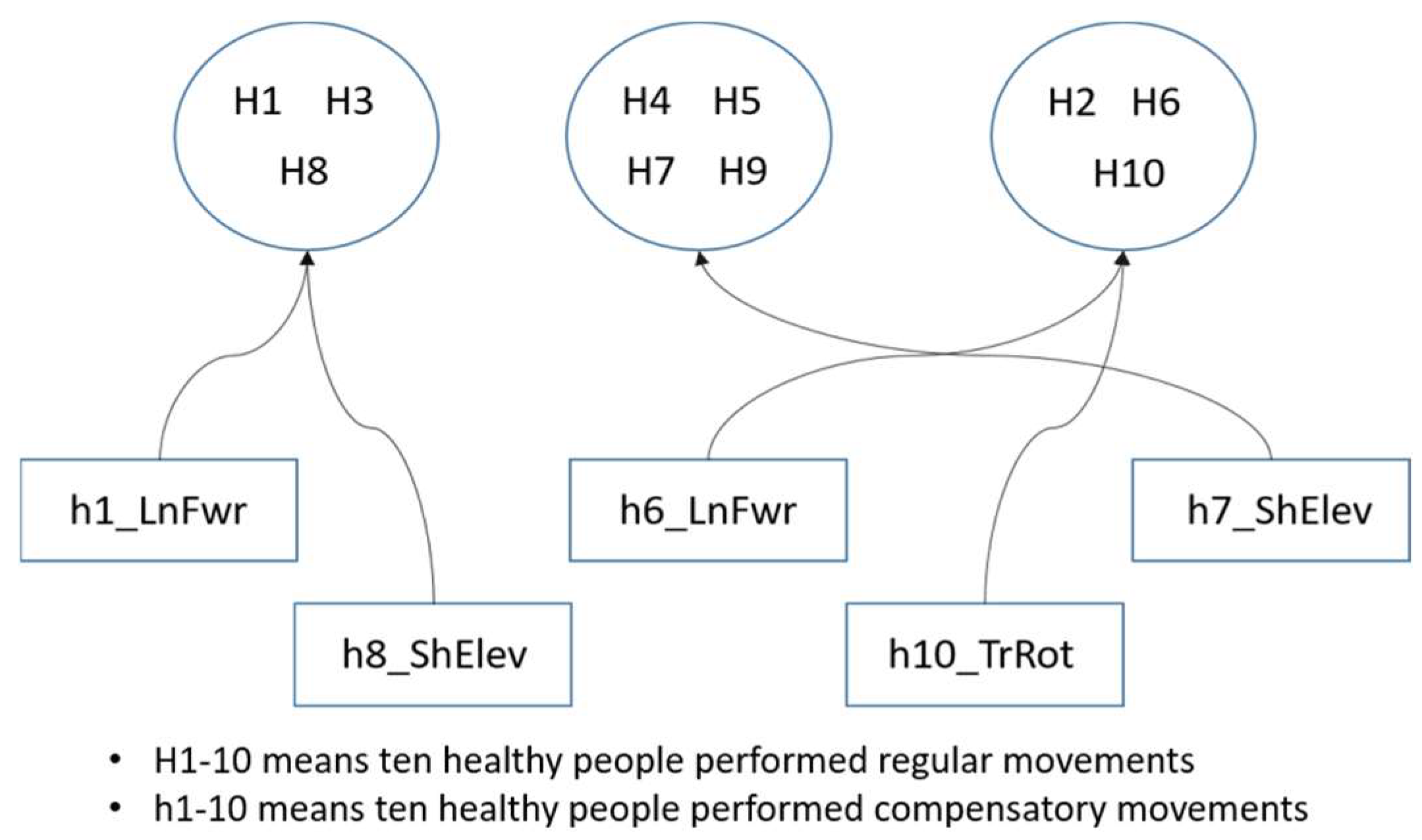
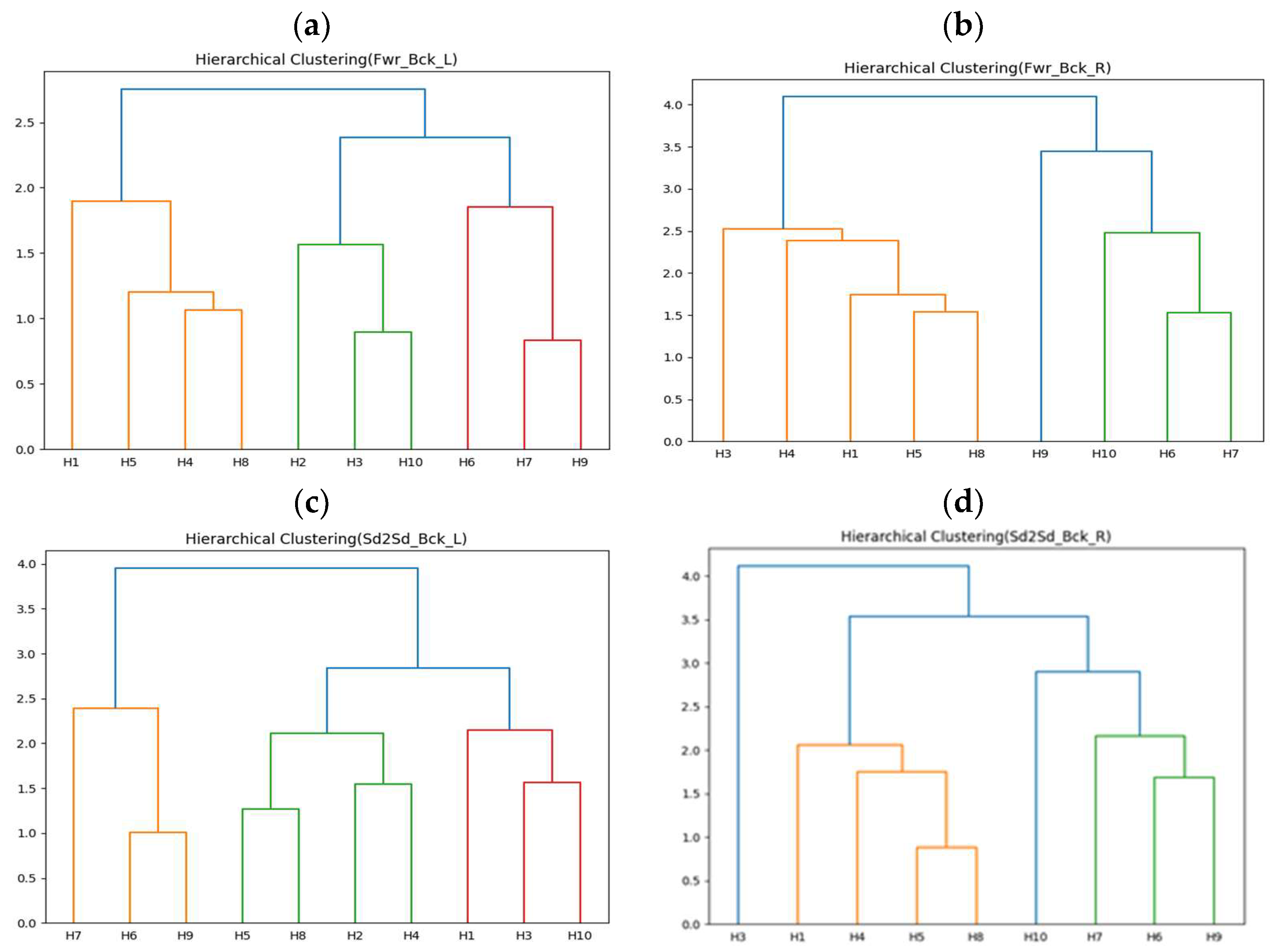
4.2.2. Correlation Analysis between Stroke Patients and Compensatory Movements of Healthy People
4.2.3. Progressive Learning in Rehabilitation
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dewald, J.P.; Beer, R.F. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2001, 24, 273–283. [Google Scholar] [CrossRef]
- Ellis, M.D.; Holubar, B.G.; Acosta, A.M.; Beer, R.F.; Dewald, J.P. Modifiability of abnormal isometric elbow and shoulder joint torque coupling after stroke. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2005, 32, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Lum, P.S.; Reinkensmeyer, D.J.; Lehman, S.L. Robotic assist devices for bimanual physical therapy: Preliminary experiments. IEEE Trans. Rehabil. Eng. 1993, 1, 185–191. [Google Scholar] [CrossRef]
- Barbuto, S.; Stein, J. Rehabilitation Robotics for Stroke. In Stroke Rehabilitation; Elsevier: St. Louis, MI, USA, 2019; pp. 235–247. [Google Scholar]
- Gupta, M.; Bhatia, D.; Kumar, P. Advanced robotic rehabilitation. In Modern Intervention Tools for Rehabilitation; Academic Press: Cambridge, MA, USA, 2023; pp. 69–90. [Google Scholar]
- Stein, J.; Narendran, K.; McBean, J.; Krebs, K.; Hughes, R. Electromyography-controlled exoskeletal upper-limb–powered orthosis for exercise training after Stroke. Am. J. Phys. Med. Rehabil. 2007, 86, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Tong, K.; Hu, X.; Li, L. Assistive control system using continuous myoelectric signal in robot-aided arm training for patients after stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 371–379. [Google Scholar] [CrossRef]
- Su, H.; Hou, X.; Zhang, X.; Qi, W.; Cai, S.; Xiong, X.; Guo, J. Pneumatic Soft Robots: Challenges and Benefits. Actuators 2022, 11, 92. [Google Scholar] [CrossRef]
- Zaghloul, A.; Bone, G.M. Origami-Inspired Soft Pneumatic Actuators: Generalization and Design Optimization. Actuators 2023, 12, 72. [Google Scholar] [CrossRef]
- Pehlivan, A.U.; Celik, O.; O’Malley, M.K. Mechanical design of a distal arm exoskeleton for stroke and spinal cord injury rehabilitation. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011. [Google Scholar]
- Simonetti, D.; Tagliamonte, N.L.; Zollo, L.; Accoto, D.; Guglielmelli, E. Biomechatronic design criteria of systems for robot-mediated rehabilitation therapy. In Rehabilitation Robotics; Academic Press: Cambridge, MA, USA, 2018; pp. 29–46. [Google Scholar]
- Huang, V.S.; Krakauer, J.W. Robotic neurorehabilitation: A computational motor learning perspective. J. Neuroeng. Rehabil. 2009, 6, 5. [Google Scholar] [CrossRef]
- Loureiro, R.; Amirabdollahian, F.; Topping, M.; Driessen, B.; Harwin, W. Upper limb robot mediated stroke therapy—GENTLE/s approach. Auton. Robot. 2003, 15, 35–51. [Google Scholar] [CrossRef]
- Nef, T.; Mihelj, M.; Riener, R. ARMin: A robot for patient-cooperative arm therapy. Med. Biol. Eng. Comput. 2007, 45, 887–900. [Google Scholar] [CrossRef]
- Sanchez, R.J.; Wolbrecht, E.; Smith, R.; Liu, J.; Rao, S.; Cramer, S.; Reinkensmeyer, D.J. A pneumatic robot for re-training arm movement after stroke: Rationale and mechanical design. In Proceedings of the 2005 IEEE 9th International Conference on Rehabilitation Robotics, Chicago, IL, USA, 28 June–1 July 2005. [Google Scholar]
- Sugar, T.G.; He, J.; Koeneman, E.J.; Koeneman, J.B.; Herman, R.; Huang, H.; Ward, J.A. Design and control of RUPERT: A device for robotic upper extremity repetitive therapy. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 336–346. [Google Scholar] [CrossRef]
- Fazekas, G.; Horvath, M.; Troznai, T.; Toth, A. Robot-mediated upper limb physiotherapy for patients with spastic hemiparesis: A preliminary study. J. Rehabil. Med. 2007, 39, 580–582. [Google Scholar] [CrossRef]
- Krebs, H.I.; Hogan, N.; Volpe, B.T.; Aisen, M.L.; Edelstein, L.; Diels, C. Overview of clinical trials with MIT-MANUS: A robot-aided neuro-rehabilitation facility. Technol. Health Care 1999, 7, 419–423. [Google Scholar] [CrossRef]
- Cozens, J.A. Robotic assistance of an active upper limb exercise in neurologically impaired patients. IEEE Trans. Rehabil. Eng. 1999, 7, 254–256. [Google Scholar] [CrossRef]
- Hesse, S.; Schulte-Tigges, G.; Konrad, M.; Bardeleben, A.; Werner, C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Arch. Phys. Med. Rehabil. 2003, 84, 915–920. [Google Scholar] [CrossRef]
- Brokaw, E.B.; Black, I.; Holley, R.J.; Lum, P.S. Hand spring operated movement enhancer (HandSOME): A portable, passive hand exoskeleton for stroke rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 391–399. [Google Scholar] [CrossRef]
- Yurkewich, A.; Kozak, I.J.; Ivanovic, A.; Rossos, D.; Wang, R.H.; Hebert, D.; Mihailidis, A. Myoelectric untethered robotic glove enhances hand function and performance on daily living tasks after stroke. J. Rehabil. Assist. Technol. Eng. 2020, 7, 2055668320964050. [Google Scholar] [CrossRef]
- Park, W.; Jeong, W.; Kwon, G.H.; Kim, Y.H.; Kim, L. A rehabilitation device to improve the hand grasp function of stroke patients using a patient-driven approach. In Proceedings of the IEEE 13th International Conference on Rehabilitation Robotics, Seattle, WA, USA, 24–26 June 2013. [Google Scholar]
- Sivan, M.; Gallagher, J.; Makower, S.; Keeling, D.; Bhakta, B.; O’Connor, R.J.; Levesley, M. Home-based computer assisted arm rehabilitation (hCAAR) robotic device for upper limb exercise after stroke: Results of a feasibility study in home setting. J. Neuroeng. Rehabil. 2014, 11, 163. [Google Scholar] [CrossRef]
- Idà, E.; Mattioni, V. Cable-driven parallel robot actuators: State of the art and novel servo-winch concept. Actuators 2022, 11, 290. [Google Scholar] [CrossRef]
- Rad, C.; Hancu, O.; Lapusan, C. Data-driven kinematic model of PneuNets bending actuators for soft grasping tasks. Actuators 2022, 11, 58. [Google Scholar] [CrossRef]
- Pagliarani, N.; Arleo, L.; Albini, S.; Cianchetti, M. Variable stiffness technologies for soft robotics: A comparative approach for the STIFF-FLOP manipulator. Actuators 2023, 12, 96. [Google Scholar] [CrossRef]
- Banyai, A.D.; Brișan, C. Robotics in physical rehabilitation: Systematic Review. Healthcare 2024, 12, 1720. [Google Scholar] [CrossRef]
- Akbari, A.; Haghverd, F.; Behbahani, S. Robotic Home-Based Rehabilitation Systems Design: From a Literature Review to a Conceptual Framework for Community-Based Remote Therapy During COVID-19 Pandemic. Front. Robot. AI 2021, 8, 612331. [Google Scholar] [CrossRef]
- Maciejasz, P.; Eschweiler, J.; Gerlach-Hahn, K.; Jansen-Troy, A.; Leonhardt, S. A survey on robotic devices for upper limb rehabilitation. J. NeuroEngineering Rehabil. 2014, 11, 3. [Google Scholar] [CrossRef]
- Kwakkel, G.; Kollen, B.J.; Krebs, H.I. Effects of Robot-Assisted Therapy on Upper Limb Recovery After Stroke: A Systematic Review. Neurorehabilit. Neural Repair 2008, 22, 111–121. [Google Scholar] [CrossRef]
- Marchal-Crespo, L.; Reinkensmeyer, D.J. Review of control strategies for robotic movement training after neurologic injury. J. Neuroeng. Rehabil. 2009, 6, 20. [Google Scholar] [CrossRef]
- Fu, J.; Rota, A.; Li, S.; Zhao, J.; Liu, Q.; Iovene, E.; Ferrigno, G.; De Momi, E. Recent Advancements in Augmented Reality for Robotic Applications: A Survey. Actuators 2023, 12, 323. [Google Scholar] [CrossRef]
- Chen, J.-C. Using Artificial Neuro-Molecular System in Robotic Arm Motion Control—Taking Simulation of Rehabilitation as an Example. Sensors 2022, 22, 2584. [Google Scholar] [CrossRef]
- Chen, J.-C.; Cheng, H.-M. Applying an Artificial Neuromolecular System to the Application of Robotic Arm Motion Control in Assisting the Rehabilitation of Stroke Patients—An Artificial World Approach. Biomimetics 2023, 8, 385. [Google Scholar] [CrossRef]
- Toronto Rehab Stroke Pose Dataset. Available online: https://www.kaggle.com/datasets/derekdb/toronto-robot-stroke-posture-dataset (accessed on 11 September 2017).
- BioDigital Human Explore the Body in 3D. Available online: https://www.biodigitalhuman.com/ (accessed on 5 November 2013).
- Dolatabadi, E.; Zhi, Y.X.; Ye, B.; Coahran, M.; Lupinacci, G.; Mihailidis, A.; Wang, R.; Taati, B. The Toronto rehab stroke poses dataset to detect compensation during stroke rehabilitation therapy. In Proceedings of the 11th EAI international Conference on Pervasive Computing Technologies for Healthcare, Barcelona, Spain, 23–26 May 2017. [Google Scholar]
- Zhi, Y.X.; Lukasik, M.; Li, M.H.; Dolatabadi, E.; Wang, R.H.; Taati, B. Automatic detection of compensation during robotic stroke rehabilitation therapy. IEEE J. Transl. Eng. Health Med. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Salvador, S.; Chan, P. Toward accurate dynamic time warping in linear time and space. Intell. Data Anal. 2007, 11, 561–580. [Google Scholar] [CrossRef]




| Movement | Type | Description |
|---|---|---|
| Reach-forward-backward (Fwr_Bck) Reach-side-to-side (Sd2Sd_Bck) | Basic | Move the end-effector back and forth in a straight trajectory (if possible) on the sagittal and transverse planes Move the end-effector from side to side in a straight trajectory (if possible) in the coronal and transverse planes |
| Lean-forward (LnFwr) Trunk-rotation (TrRot) Shoulder-elevation (ShElev) | Compensatory | Hip flexion angle less than 90 degrees in the sagittal plane Turning of the torso in the transverse plane Unilateral shoulder raise in the coronal plane |
| Perpetual Learning | Adaptive Learning | Transfer Learning | Cross-Task Learning | |
|---|---|---|---|---|
| Mechanism | learn from scratch | learn from scratch | use trained weights | use trained weights |
| Input | randomly changing movement trajectories | high degree of freedom of movement | randomly changing movement trajectories | movement with a certain degree of freedom |
| Output | movements combining different degrees of freedom | movements with lower degrees of freedom | movements combining different degrees of freedom | movements with the same degree of freedom |
| Rehabilitation Action | At Cycle 600 | Improvement Rate | R2 Score |
|---|---|---|---|
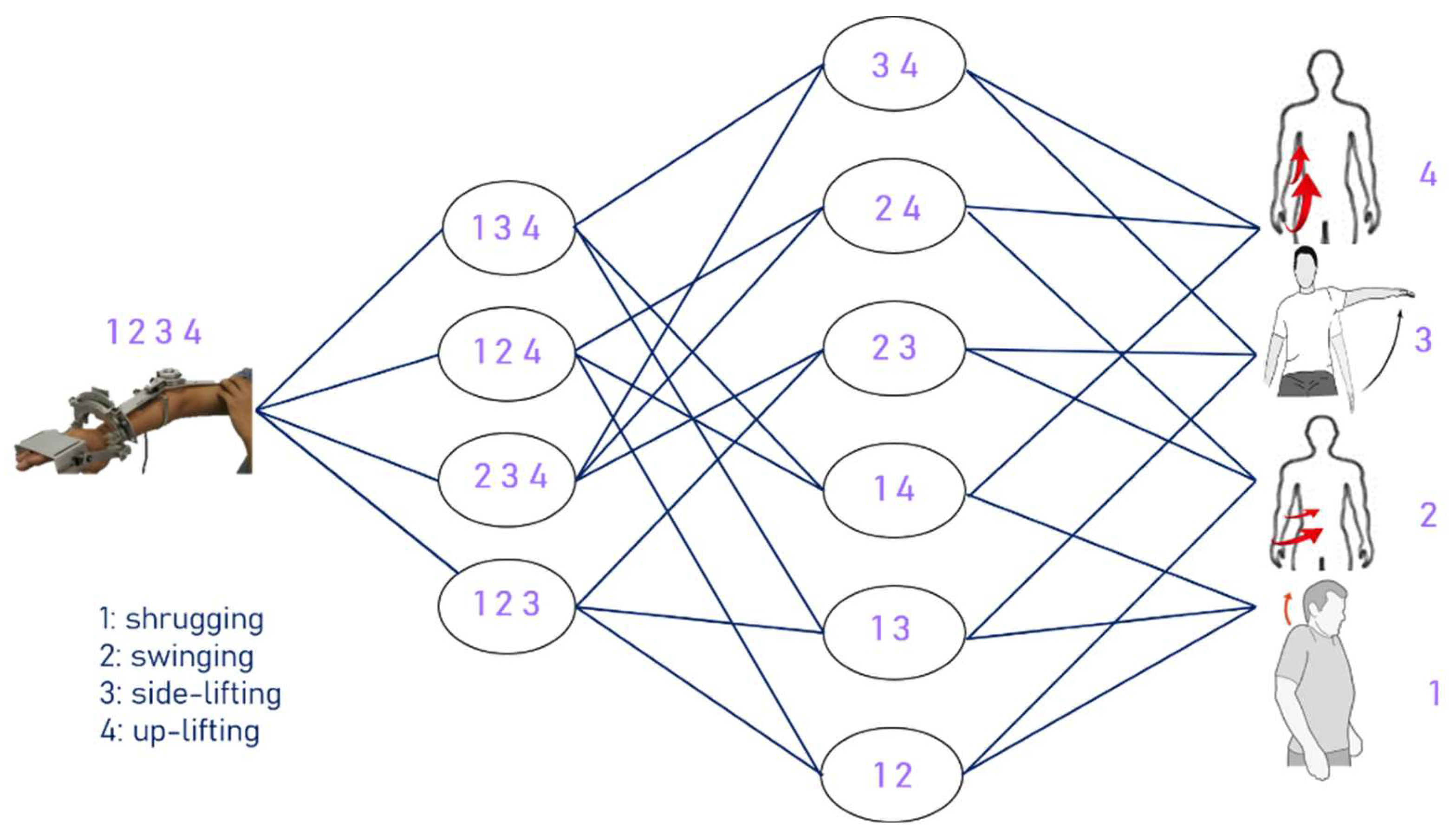
| Shrug, Swing, Side-lift, Up-lift | 1.8 | 86.3% | 0.94 |
| Shrug, Swing, Side-lift | 2.1 | 71.6% | 0.30 |
| Shrug, Swing, Up-lift | 1.5 | 87.1% | 0.96 |
| Shrug, Side-lift, Up-lift | 2.4 | 80.3% | 0.91 |
| Swing, Side-lift, Up-lift | 3.1 | 75.6% | 0.84 |
| Shrug, Swing | 2.5 | 63.8% | −3.06 |
| Shrug, Side-lift | 2.1 | 72.0% | −17.08 |
| Shrug, Up-lift | 2.8 | 73.3% | 0.84 |
| Swing, Side-lift | 3.3 | 54.2% | −0.01 |
| Swing, Up-lift | 4.1 | 65.0% | 0.60 |
| Side-lift, Up-lift | 2.3 | 80.2% | 0.91 |
| Shrug | 1.4 | 80.8% | −4.98 |
| Swing | 2.0 | 73.7% | −3.69 |
| Side-lift | 2.0 | 72.6% | −16.97 |
| Up-lift | 2.9 | 73.2% | 0.80 |
| To | To Three Movements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shrug, Swing, Side-Lift | Shrug, Swing, Up-Lift | Shrug, Side-Lift, Up-Lift | Swing, Side-Lift, Up-Lift | |||||||||
| From | First | After | Rate | First | After | Rate | First | After | Rate | First | After | Rate |
| Shrug, Swing, Side-lift, Up-lift | 21.4 | 2.3 | 89.3% | 23.1 | 5.4 | 76.6% | 28.6 | 2.1 | 92.7% | 21.1 | 3.1 | 85.3% |
| To | To Three Movements | To Two Movements | To Single Movement | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| From | ||||||||||||||
| From Four Movements | Shrug, Swing, Side-Lift | Shrug, Swing, Up-Lift | Shrug, Side-Lift, Up-Lift | Swing, Side-Lift, Up-Lift | Shrug, Swing | Shrug, Side-Lift | Shrug, Up-Lift | Swing, Side-Lift | Swing, Up-Lift | Side-Lift, Up-Lift | Shrug | Swing | Side-Lift | Up-Lift |
| Shrug, Swing, Side-lift, Up-lift | 2.3 | 5.4 | 2.1 | 3.1 | 2.1 | 3.4 | 1.9 | 5.4 | 2.4 | 3.5 | 4.8 | 4.4 | 3.1 | 2.2 |
| To | To Two Movements | To Single Movement | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| From | ||||||||||
| From Three Movements | Shrug, Swing | Shrug, Side-Lift | Shrug, Up-Lift | Swing, Side-Lift | Swing, Up-Lift | Side-Lift, Up-Lift | Shrug | Swing | Side-Lift | Up-Lift |
| Shrug, Swing, Side-lift | 2.1 | 1.5 | 4.6 | 1.4 | 2.3 | 3.1 | 1.4 | 1.7 | 5.8 | 2.7 |
| Shrug, Swing, Up-lift | 2.0 | 3.3 | 1.8 | 1.4 | 1.3 | 4.9 | 1.7 | 1.9 | 2.5 | 3.3 |
| Shrug, Side-lift, Up-lift | 1.8 | 1.8 | 2.6 | 2.6 | 5.2 | 2.3 | 1.7 | 2.6 | 2.3 | 12.4 |
| Swing, Side-lift, Up-lift | 2.5 | 5.3 | 1.7 | 1.5 | 2.6 | 2.2 | 1.7 | 1.6 | 3.3 | 3.7 |
| To | To Single Movement | |||
|---|---|---|---|---|
| From | ||||
| From Two Movements | Shrug | Swing | Side-Lift | Up-Lift |
| Shrug, Swing | 1.0 | 1.7 | 3.9 | 1.9 |
| Shrug, Side-lift | 1.3 | 1.7 | 1.4 | 1.0 |
| Shrug, Up-lift | 1.1 | 1.5 | 2.7 | 1.3 |
| Swing, Side-lift | 2.3 | 3.0 | 1.4 | 2.7 |
| Swing, Up-lift | 0.9 | 1.6 | 1.4 | 1.8 |
| Side-lift, Up-lift | 10.7 | 1.4 | 2.7 | 2.7 |
| At Cycle 1 | At Cycle 800 | |||||
|---|---|---|---|---|---|---|
| Rehabilitation Action | Without TL (DTW) | With TL (DTW) | Improvement Rate | Without TL (DTW) | With TL (DTW) | Improvement Rate |
| Shrug, Swing, Side-lift | 7.4 | 10.1 | −36.5% | 2.1 | 2.1 | 0.0% |
| Shrug, Swing, Up-lift | 11.6 | 4.3 | 62.9% | 1.5 | 1.8 | −20.0% |
| Shrug, Side-lift, Up-lift | 12.2 | 3.1 | 74.6% | 2.4 | 1.6 | 33.3% |
| Swing, Side-lift, Up-lift | 12.7 | 2.2 | 82.7% | 3.1 | 1.7 | 45.2% |
| Shrug, Swing | 6.9 | 11.0 | −59.4% | 2.5 | 2.1 | 16.0% |
| Shrug, Side-lift | 7.5 | 11.5 | −53.3% | 2.1 | 2.6 | −23.8% |
| Shrug, Up-lift | 10.5 | 4.8 | 54.3% | 2.8 | 3.2 | −14.3% |
| Swing, Side-lift | 7.2 | 10.7 | −48.6% | 3.3 | 1.7 | 48.5% |
| Swing, Up-lift | 11.7 | 4.6 | 60.7% | 4.1 | 2.3 | 43.9% |
| Side-lift, Up-lift | 11.6 | 3.2 | 72.4% | 2.3 | 1.9 | 17.4% |
| Shrug | 7.3 | 11.0 | −50.7% | 1.4 | 2.2 | −57.1% |
| Swing | 7.6 | 12.3 | −61.8% | 2.0 | 2.0 | 0.0% |
| Side-lift | 7.3 | 12.4 | −69.9% | 2.0 | 2.4 | −20.0% |
| Up-lift | 10.8 | 4.9 | 54.6% | 2.9 | 2.7 | 6.9% |
| To | Shrug | Swing | Side-Lift | Up-Lift |
|---|---|---|---|---|
| From | ||||
| Shrug | – | 5.0 | 8.9 | 11.6 |
| Swing | 11.6 | – | 10.2 | 12.9 |
| Side-lift | 8.7 | 9.9 | – | 11.7 |
| Up-lift | 10.9 | 11.6 | 10.5 | – |
| To | Shrug, Swing | Shrug, Side-Lift | Shrug, Up-Lift | Swing, Side-Lift | Swing, Up-Lift | Side-Lift, Up-Lift |
|---|---|---|---|---|---|---|
| From | ||||||
| Shrug, Swing | – | 11.9 | 15.7 | 12.0 | 16.1 | 15.3 |
| Shrug, Side-lift | 13.5 | – | 10.2 | 8.9 | 11.9 | 9.8 |
| Shrug, Up-lift | 9.8 | 11.1 | – | 10.9 | 14.1 | 11.3 |
| Swing, Side-lift | 18.7 | 13.0 | 18.1 | – | 19.0 | 15.5 |
| Swing, Up-lift | 13.2 | 10.1 | 17.3 | 10.9 | – | 16.4 |
| Side-lift, Up-lift | 11.6 | 10.6 | 7.9 | 9.6 | 8.9 | – |
| To | Shrug, Swing, Side-Lift | Shrug, Swing, Up-Lift | Shrug, Side-Lift, Up-Lift | Swing, Side-Lift, Up-Lift |
|---|---|---|---|---|
| From | ||||
| Shrug, Swing, Side-lift | – | 16.4 | 18.5 | 18.6 |
| Shrug, Swing, Up-lift | 10.1 | – | 9.8 | 11.6 |
| Shrug, Side-lift, Up-lift | 12.7 | 10.2 | – | 9.1 |
| Swing, Side-lift, Up-lift | 12.3 | 5.5 | 7.5 | – |
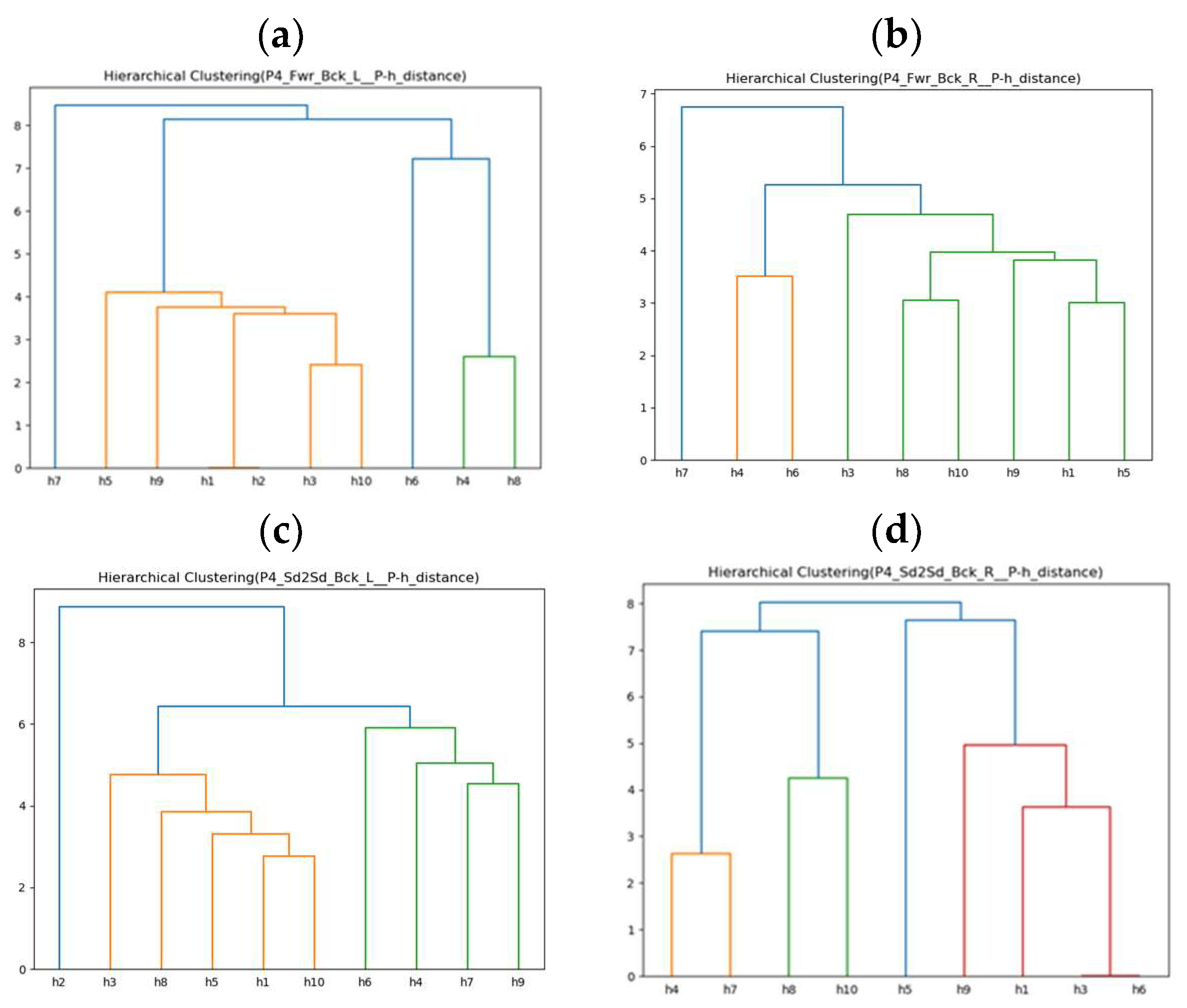
| Rehabilitation Action | Forward–Backward with Left Arm (Fwr_Bck_L) | |||
|---|---|---|---|---|
| Healthy | First | After | Improvement Rate | |
| Cluster 1 | H5 | 73.3 | 7.1 | 90.3% |
| Cluster 2 | H6 | 70.1 | 8.1 | 88.4% |
| Cluster 3 | H10 | 71.7 | 12.6 | 82.4% |
| Rehabilitation Action | Forward–Backward with Left Arm (Fwr_Bck_L) | |||
|---|---|---|---|---|
| Healthy | Without TL | With TL | Improvement Rate | |
| Cluster 1 (Use learning weight of H5) | H1 | 91.0 | 20.2 | 77.8% |
| H4 | 74.4 | 14.8 | 80.1% | |
| H8 | 80.1 | 17.2 | 78.5% | |
| Cluster 2 (Use learning weight of H6) | H7 | 81.3 | 28.1 | 65.4% |
| H9 | 77.9 | 37.2 | 52.2% | |
| Cluster 3 (Use learning weight of H10) | H2 | 67.1 | 27.1 | 59.6% |
| H3 | 78.8 | 30.5 | 61.3% | |
| Rehabilitation Action | Forward–Backward with Right Arm (Fwr_Bck_R) | |||
|---|---|---|---|---|
| Healthy | First | After | Improvement Rate | |
| Cluster 1 | H5 | 85.7 | 9.4 | 89.0% |
| Cluster 2 | H6 | 64.9 | 7.5 | 88.4% |
| Cluster 3 | H9 | 92.7 | 15.9 | 82.8% |
| Rehabilitation Action | Forward–Backward with Right Arm (Fwr_Bck_R) | |||
|---|---|---|---|---|
| Healthy | Without TL | With TL | Improvement Rate | |
| Cluster 1 (Use learning weight of H5) | H1 | 71.8 | 14.9 | 79.2% |
| H3 | 85.5 | 17.8 | 79.2% | |
| H4 | 72.4 | 25.0 | 65.5% | |
| H8 | 83.8 | 13.9 | 83.4% | |
| Cluster 2 (Use learning weight of H6) | H7 | 70.1 | 16.4 | 76.6% |
| H10 | 73.9 | 23.1 | 68.7% | |
| Rehabilitation Action | Side-to-Side with Left Arm (Sd2Sd_Bck_L) | |||
|---|---|---|---|---|
| Healthy | First | After | Improvement Rate | |
| Cluster 1 | H3 | 73.4 | 9.9 | 86.5% |
| Cluster 2 | H4 | 72.6 | 12.5 | 82.8% |
| Cluster 3 | H9 | 79.6 | 10.5 | 86.8% |
| Rehabilitation Action | Side-to-Side with Left Arm (Sd2Sd_Bck_L) | |||
|---|---|---|---|---|
| Healthy | Without TL | With TL | Improvement Rate | |
| Cluster 1 (Use learning weight of H3) | H1 | 81.8 | 34.6 | 57.7% |
| H10 | 64.4 | 22.3 | 65.4% | |
| Cluster 2 (Use learning weight of H4) | H2 | 80.0 | 25.9 | 67.6% |
| H5 | 74.4 | 24.8 | 66.7% | |
| H8 | 79.5 | 29.8 | 62.5% | |
| Cluster 3 (Use learning weight of H9) | H6 | 71.7 | 35.6 | 50.3% |
| H7 | 84.4 | 45.6 | 46.0% | |
| Rehabilitation Action | Side-to-Side with Right Arm (Sd2Sd_Bck_R) | |||
|---|---|---|---|---|
| Healthy | First | After | Improvement Rate | |
| Cluster 1 | H3 | 83.9 | 12.9 | 84.6% |
| Cluster 2 | H8 | 90.0 | 9.2 | 89.8% |
| Cluster 3 | H9 | 72.6 | 9.4 | 87.1% |
| Cluster 4 | H10 | 60.5 | 8.7 | 85.6% |
| Rehabilitation Action | Side-to-Side with Right Arm (Sd2Sd_Bck_R) | |||
|---|---|---|---|---|
| Healthy | Without TL | With TL | Improvement Rate | |
| Cluster 2 (Use learning weight of H8) | H1 | 75.2 | 21.7 | 71.1% |
| H4 | 67.2 | 22.5 | 66.5% | |
| H5 | 80.7 | 14.0 | 82.7% | |
| Cluster 3 (Use learning weight of H9) | H6 | 66.3 | 24.8 | 62.6% |
| H7 | 77.9 | 26.4 | 66.1% | |
| Healthy | H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | H9 | H10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Action | ||||||||||
| Forward–Backward with Left Arm | 4.4 | 4.4 | 7.3 | 17.7 | 2.5 | 12.0 | 2.3 | 12.3 | 2.2 | 0.8 |
| Forward–Backward with Right Arm | 3.7 | — | 9.3 | 32.2 | 5.1 | 13.0 | 1.3 | 8.1 | 5.8 | 11.2 |
| Side-to-Side with Left Arm | 9.1 | 1.7 | 9.2 | 0.3 | 6.8 | 16.7 | 3.6 | 8.5 | 5.6 | 5.0 |
| Side-to-Side with Right Arm | 10.8 | — | 4.2 | 5.7 | 12.6 | 4.2 | 4.6 | 10.7 | 13.9 | 14.4 |
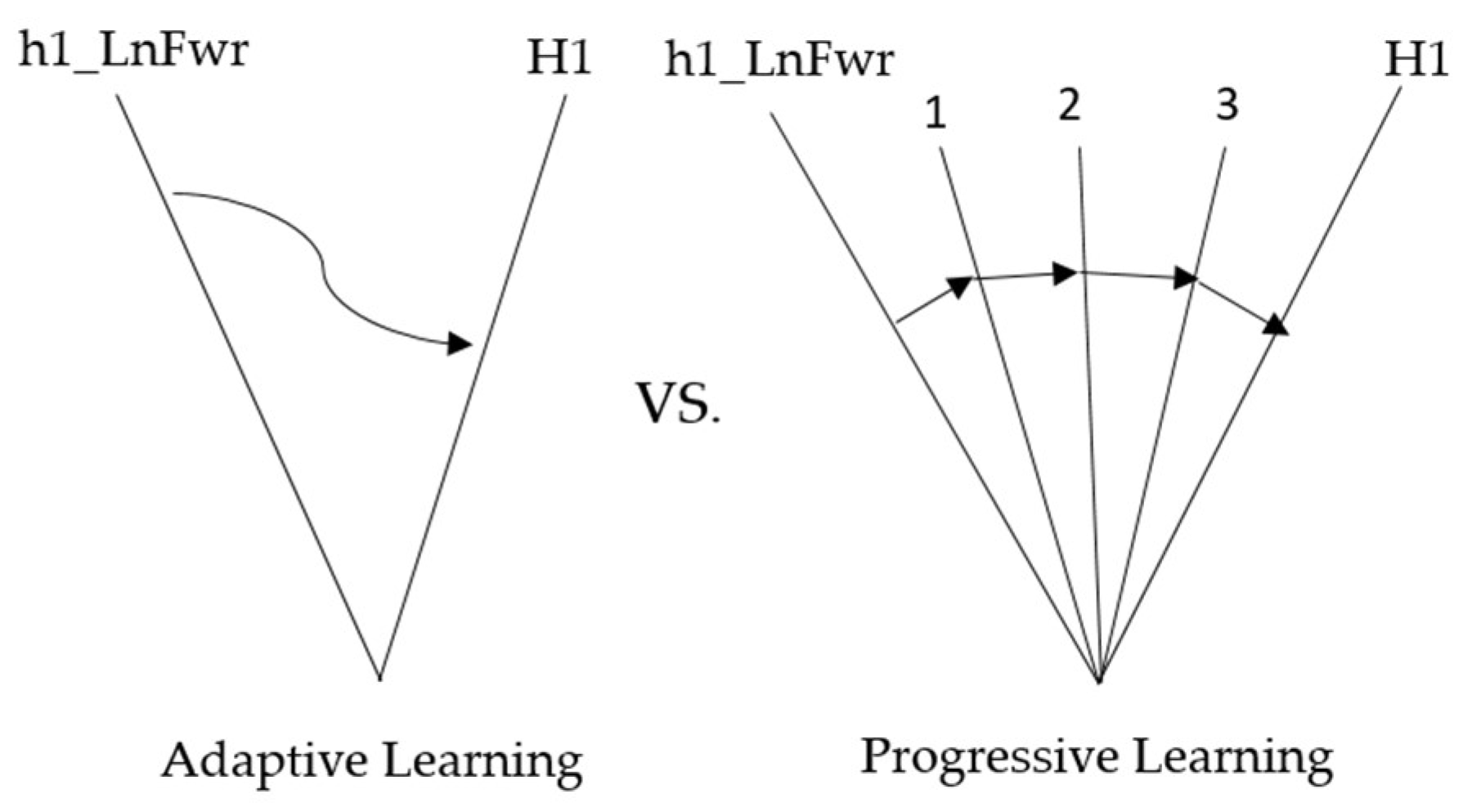
| Adaptive Learning | Progressive Learning | |
|---|---|---|
| Concept | There will only be action trajectories with compensatory effects and average action trajectories. | Find multiple action trajectories between the compensation and the typical action trajectories. |
| Learning Mechanism | Learn from average action trajectories with compensatory effects, as shown in Figure 14. | After finding multiple trajectories, learn from compensatory to everyday actions in sequence, as shown in Figure 14. |
| Learning Stage | A single stage | Multiple Stage |
| Rehabilitation Action | Forward–Backward with Left Arm (Fwr_Bck_L) | |||
|---|---|---|---|---|
| Healthy | First | After | Improvement Rate | |
| H5 | Adaptive Learning | 73.3 | 18.3 | 75.0% |
| Progressive Learning | 76.5 | 20.5 | 73.2% | |
| H6 | Adaptive Learning | 71.7 | 10.0 | 86.1% |
| Progressive Learning | 74.0 | 9.5 | 87.2% | |
| H10 | Adaptive Learning | 70.1 | 15.0 | 78.6% |
| Progressive Learning | 80.1 | 29.3 | 63.4% | |
| Rehabilitation Action | Side-to-Side with Left Arm (Sd2Sd_Bck_L) | |||
|---|---|---|---|---|
| Healthy | First | After | Improvement Rate | |
| H3 | Adaptive Learning | 73.4 | 17.2 | 76.6% |
| Progressive Learning | 77.5 | 12.9 | 83.4% | |
| H4 | Adaptive Learning | 72.6 | 13.8 | 81.0% |
| Progressive Learning | 81.3 | 15.9 | 80.4% | |
| H9 | Adaptive Learning | 79.6 | 15.2 | 80.9% |
| Progressive Learning | 79.4 | 27.2 | 65.7% | |
| Rehabilitation Action | Forward–Backward with Left Arm (Fwr_Bck_L) | |||
|---|---|---|---|---|
| Healthy | First | After | Improvement Rate | |
| H5 | Adaptive Learning | 73.3 | 7.1 | 90.3% |
| Progressive Learning | 76.5 | 14.2 | 81.4% | |
| H6 | Adaptive Learning | 71.7 | 12.6 | 82.4% |
| Progressive Learning | 74.0 | 8.3 | 88.8% | |
| H10 | Adaptive Learning | 70.1 | 8.1 | 88.4% |
| Progressive Learning | 80.1 | 16.7 | 79.2% | |
| Rehabilitation Action | Side-to-Side with Left Arm (Sd2Sd_Bck_L) | |||
|---|---|---|---|---|
| Healthy | First | After | Improvement Rate | |
| H3 | Adaptive Learning | 73.4 | 9.9 | 86.5% |
| Progressive Learning | 77.5 | 9.5 | 87.7% | |
| H4 | Adaptive Learning | 72.6 | 12.5 | 82.8% |
| Progressive Learning | 81.3 | 14.9 | 81.7% | |
| H9 | Adaptive Learning | 79.6 | 10.5 | 86.8% |
| Progressive Learning | 79.4 | 8.5 | 89.3% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-C.; Cheng, H.-M. Application of Artificial Neuromolecular System in Robotic Arm Control to Assist Progressive Rehabilitation for Upper Extremity Stroke Patients. Actuators 2024, 13, 362. https://doi.org/10.3390/act13090362
Chen J-C, Cheng H-M. Application of Artificial Neuromolecular System in Robotic Arm Control to Assist Progressive Rehabilitation for Upper Extremity Stroke Patients. Actuators. 2024; 13(9):362. https://doi.org/10.3390/act13090362
Chicago/Turabian StyleChen, Jong-Chen, and Hao-Ming Cheng. 2024. "Application of Artificial Neuromolecular System in Robotic Arm Control to Assist Progressive Rehabilitation for Upper Extremity Stroke Patients" Actuators 13, no. 9: 362. https://doi.org/10.3390/act13090362
APA StyleChen, J.-C., & Cheng, H.-M. (2024). Application of Artificial Neuromolecular System in Robotic Arm Control to Assist Progressive Rehabilitation for Upper Extremity Stroke Patients. Actuators, 13(9), 362. https://doi.org/10.3390/act13090362








