APTC-EC-2A: A Lytic Phage Targeting Multidrug Resistant E. coli Planktonic Cells and Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Wastewater Collection and Process
2.3. E. coli Strain Isolation from Wastewater
2.4. Antibiotic Sensitivity Testing
2.5. E. coli Phage Isolation Using a Double Layer Agar Assay
2.6. Phage Isolation Using Enrichment Culture
2.7. Purification of Phage
2.8. Phage Propagation and Phage Titration
2.9. Host Range
2.10. Transmission Electron Microscopy (TEM)
2.11. Phage Stability Assay: pH, Temperature and Long-Term Storage
2.12. Inhibition Assay (Bacteriolytic Characteristic of the Phage)
2.13. Biofilm Quantification by Crystal Violet Staining
2.14. LIVE/DEAD Staining
2.15. Genomic Extraction, Sequencing and Analysis
2.16. Genomic Analysis
2.17. Statistical Analysis
3. Results
3.1. Bacterial Isolation from Wastewater
3.2. Determination of Antimicrobial Susceptibility for E. coli Strains
3.3. Isolation of Phages with E. coli XL10 Gold
3.4. Phages Host Range Analysis
3.5. Characterisation and Stability of Selected Phage
3.5.1. Morphology of Phage
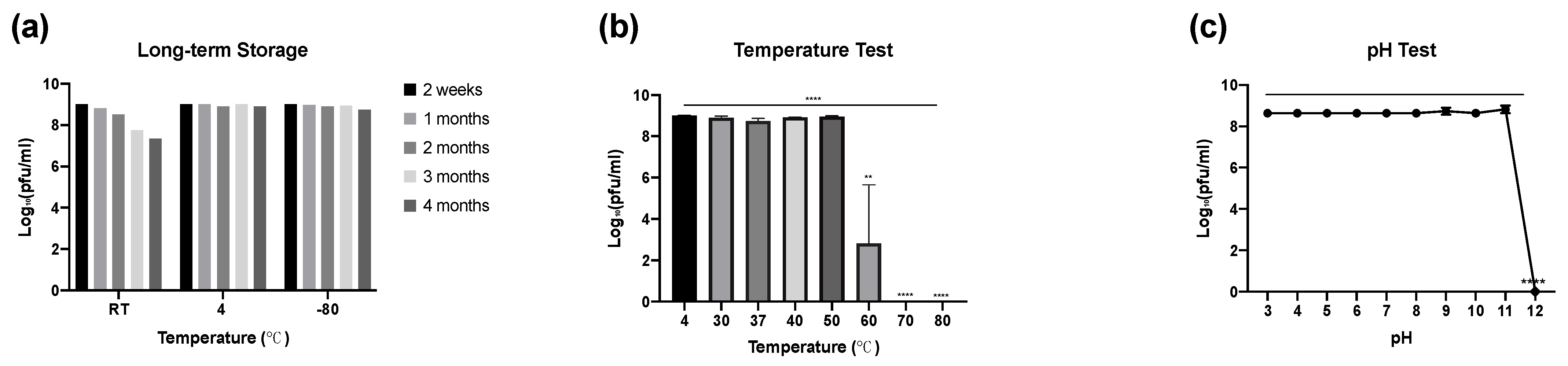
3.5.2. Phage Stability: Long-Term Storage, Temperature and pH
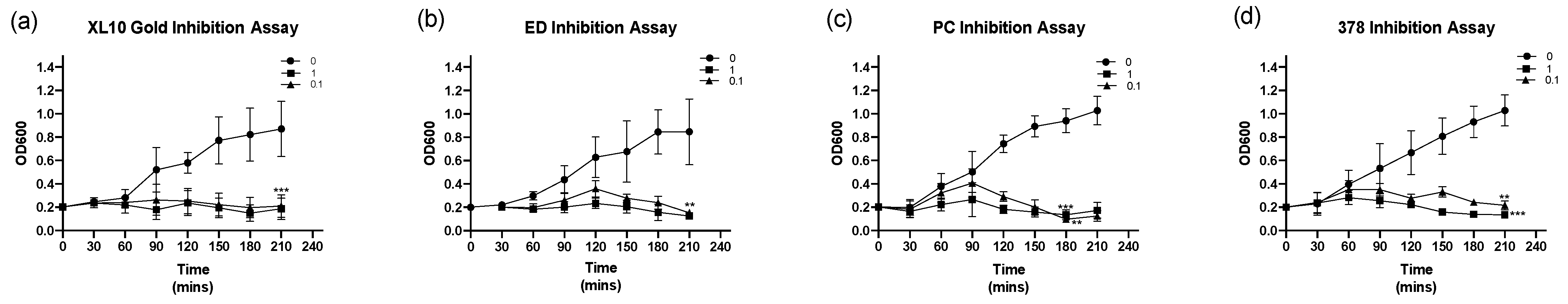
3.6. Inhibition Assay
3.7. Phage Reduces the E. coli Biofilm Biomass and Viability
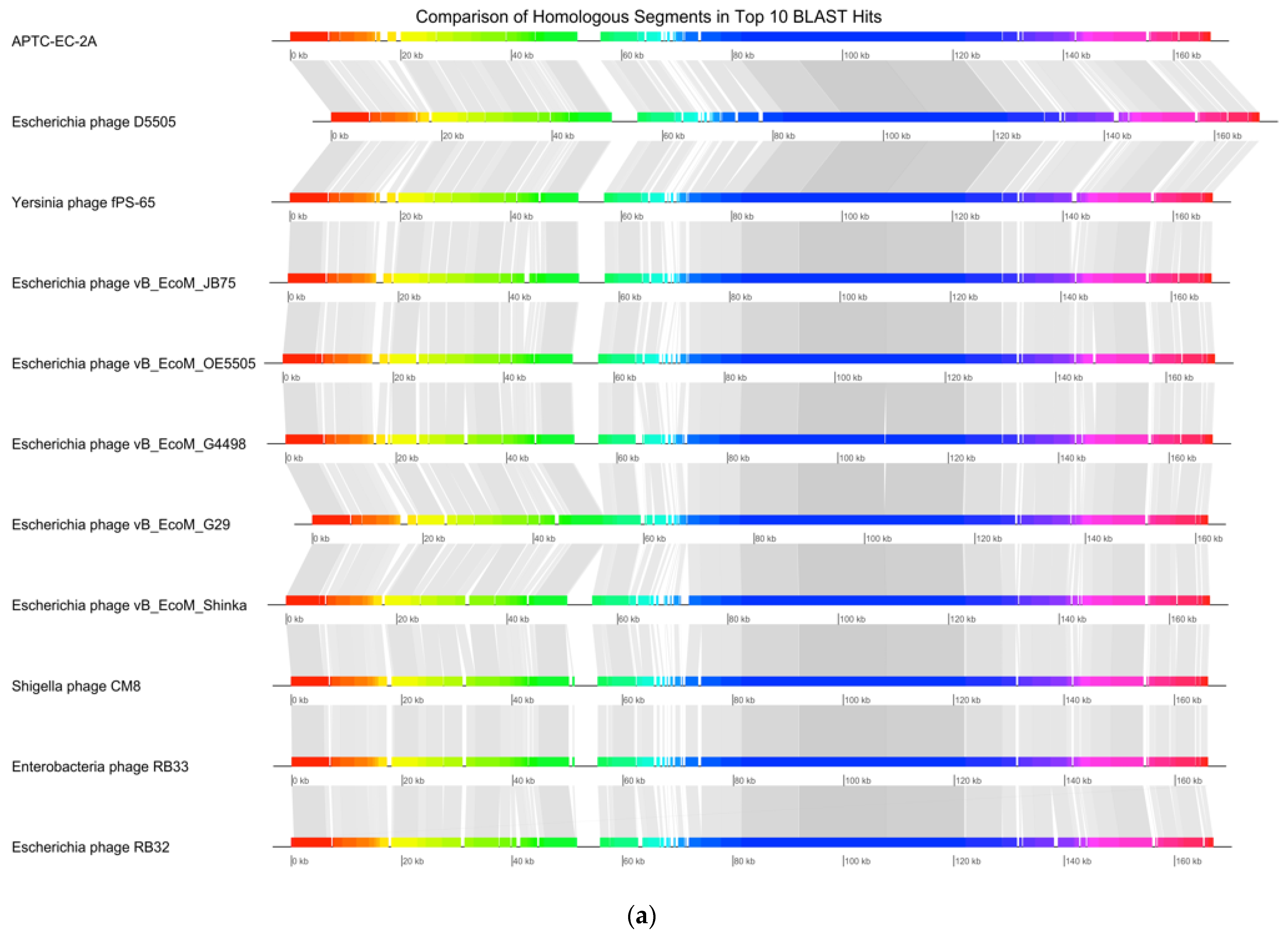
3.8. Genomic Extraction, Sequencing and Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Navab-Daneshmand, T.; Friedrich, M.N.D.; Gächter, M.; Montealegre, M.C.; Mlambo, L.S.; Nhiwatiwa, T.; Mosler, H.J.; Julian, T.R. Escherichia coli Contamination across Multiple Environmental Compartments (Soil, Hands, Drinking Water, and Handwashing Water) in Urban Harare: Correlations and Risk Factors. Am. J. Trop. Med. Hyg. 2018, 98, 803–813. [Google Scholar] [CrossRef]
- Baggi, F.; Demarta, A.; Peduzzi, R. Persistence of viral pathogens and bacteriophages during sewage treatment: Lack of correlation with indicator bacteria. Res. Microbiol. 2001, 152, 743–751. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Olorunmola, F.O.; Kolawole, D.O.; Lamikanra, A. Antibiotic resistance and virulence properties in Escherichia coli strains from cases of urinary tract infections. Afr. J. Infect. Dis. 2013, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.N.; Vannoy, D.; Frederick, A.; Chang, S.; Lawler, E. First-Line Antimicrobial Resistance Patterns of Escherichia coli in Children With Urinary Tract Infection in Emergency Department and Primary Care Clinics. Clin. Pediatr. 2016, 55, 19–28. [Google Scholar] [CrossRef]
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef] [Green Version]
- Temkin, E.; Adler, A.; Lerner, A.; Carmeli, Y. Carbapenem-resistant Enterobacteriaceae: Biology, epidemiology, and management. Ann. N. Y. Acad. Sci. 2014, 1323, 22–42. [Google Scholar] [CrossRef]
- Gill, J.J.; Hyman, P. Phage choice, isolation, and preparation for phage therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Bergh, O.; Børsheim, K.Y.; Bratbak, G.; Heldal, M. High abundance of viruses found in aquatic environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef]
- Williamson, K.E.; Wommack, K.E.; Radosevich, M. Sampling natural viral communities from soil for culture-independent analyses. Appl. Environ. Microbiol. 2003, 69, 6628–6633. [Google Scholar] [CrossRef] [Green Version]
- Durán, A.E.; Muniesa, M.; Méndez, X.; Valero, F.; Lucena, F.; Jofre, J. Removal and inactivation of indicator bacteriophages in fresh waters. J. Appl. Microbiol. 2002, 92, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Aghaee, B.L.; Mirzaei, M.K.; Alikhani, M.Y.; Mojtahedi, A. Sewage and sewage-contaminated environments are the most prominent sources to isolate phages against Pseudomonas aeruginosa. BMC Microbiol. 2021, 21, 132. [Google Scholar] [CrossRef]
- Ribeiro, K.V.G.; Ribeiro, C.; Dias, R.S.; Cardoso, S.A.; de Paula, S.O.; Zanuncio, J.C.; de Oliveira, L.L. Bacteriophage Isolated from Sewage Eliminates and Prevents the Establishment of Escherichia coli Biofilm. Adv. Pharm. Bull. 2018, 8, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Topka, G.; Bloch, S.; Nejman-Faleńczyk, B.; Gąsior, T.; Jurczak-Kurek, A.; Necel, A.; Dydecka, A.; Richert, M.; Węgrzyn, G.; Węgrzyn, A. Characterization of Bacteriophage vB-EcoS-95, Isolated From Urban Sewage and Revealing Extremely Rapid Lytic Development. Front. Microbiol. 2019, 9, 3326. [Google Scholar] [CrossRef]
- Yazdi, M.; Bouzari, M.; Ghaemi, E.A.; Shahin, K. Isolation, Characterization and Genomic Analysis of a Novel Bacteriophage VB_EcoS-Golestan Infecting Multidrug-Resistant Escherichia coli Isolated from Urinary Tract Infection. Sci. Rep. 2020, 10, 7690. [Google Scholar] [CrossRef]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral Phage Therapy of Acute Bacterial Diarrhea with Two Coliphage Preparations: A Randomized Trial in Children from Bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Y.; Paramasivan, S.; Richter, K.; Morales, S.; Wormald, P.-J.; Vreugde, S. Bacteriophage effectively kills multidrug resistant Staphylococcus aureus clinical isolates from chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 2018, 8, 406–414. [Google Scholar] [CrossRef]
- Bozkurt, H.; Bozkurt, E.C.; Ozpinar, H.; Arac, D.; Kaya, I.; Ozer, H.; Egilmez, R. Comparison of the effects of Contractubex gel and benzothiazole after topical application in an experimental model of epidural fibrosis in rats. World Neurosurg. 2018, 117, e403–e410. [Google Scholar] [CrossRef]
- Andrews, S. Babraham Bioinformatics-FastQC a Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 18 August 2021).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Ecale Zhou, C.L.; Kimbrel, J.; Edwards, R.; McNair, K.; Souza, B.A.; Malfatti, S. MultiPhATE2: Code for functional annotation and comparison of phage genomes. G3 Genes Genomes Genet. 2021, 11, jkab074. [Google Scholar] [CrossRef]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef] [Green Version]
- Guy, L.; Kultima, J.R.; Andersson, S.G. genoPlotR: Comparative gene and genome visualization in R. Bioinformatics 2010, 26, 2334–2335. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [Green Version]
- Połaska, M.; Sokołowska, B. Bacteriophages-a new hope or a huge problem in the food industry. AIMS Microbiol. 2019, 5, 324–346. [Google Scholar] [CrossRef]
- Sillankorva, S.M.; Oliveira, H.; Azeredo, J. Bacteriophages and their role in food safety. Int. J. Microbiol. 2012, 2012, 863945. [Google Scholar] [CrossRef] [Green Version]
- González-Gómez, J.P.; González-Torres, B.; Guerrero-Medina, P.J.; López-Cuevas, O.; Chaidez, C.; Avila-Novoa, M.G.; Gutiérrez-Lomelí, M. Efficacy of Novel Bacteriophages against Escherichia coli Biofilms on Stainless Steel. Antibiotics 2021, 10, 1150. [Google Scholar] [CrossRef]
- Li, M.; Shi, D.; Li, Y.; Xiao, Y.; Chen, M.; Chen, L.; Du, H.; Zhang, W. Recombination of T4-like Phages and Its Activity against Pathogenic Escherichia coli in Planktonic and Biofilm Forms. Virol. Sin. 2020, 35, 651–661. [Google Scholar] [CrossRef]
- Dissanayake, U.; Ukhanova, M.; Moye, Z.D.; Sulakvelidze, A.; Mai, V. Bacteriophages Reduce Pathogenic Escherichia coli Counts in Mice Without Distorting Gut Microbiota. Front. Microbiol. 2019, 10, 1984. [Google Scholar] [CrossRef]
- Simpson, D.J.; Sacher, J.C.; Szymanski, C.M. Development of an Assay for the Identification of Receptor Binding Proteins from Bacteriophages. Viruses 2016, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, M.C.; van Alphen, L.B.; Harboe, A.; Li, J.; Christensen, B.B.; Szymanski, C.M.; Brøndsted, L. Bacteriophage F336 recognizes the capsular phosphoramidate modification of Campylobacter jejuni NCTC11168. J. Bacteriol. 2011, 193, 6742–6749. [Google Scholar] [CrossRef] [Green Version]
- Nobrega, F.L.; Vlot, M.; de Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.E.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J.J. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 2018, 16, 760–773. [Google Scholar] [CrossRef]
- Ackermann, H.W. Tailed bacteriophages: The order caudovirales. Adv. Virus Res. 1998, 51, 135–201. [Google Scholar] [CrossRef]
- Litt, P.K.; Jaroni, D. Isolation and Physiomorphological Characterization of Escherichia coli O157:H7-Infecting Bacteriophages Recovered from Beef Cattle Operations. Int. J. Microbiol. 2017, 2017, 7013236. [Google Scholar] [CrossRef] [Green Version]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages—Review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.Y.; Ong, S.L.; Hu, J.Y.; Tan, X.L.; Ng, W.J. Effects of pH and temperature on the survival of coliphages MS2 and Qβ. J. Ind. Microbiol. Biotechnol. 2003, 30, 549–552. [Google Scholar] [CrossRef]
- Guglielmotti, D.M.; Mercanti, D.J.; Reinheimer, J.A.; Quiberoni Adel, L. Review: Efficiency of physical and chemical treatments on the inactivation of dairy bacteriophages. Front. Microbiol. 2011, 2, 282. [Google Scholar] [CrossRef] [Green Version]
- Montso, P.K.; Mlambo, V.; Ateba, C.N. Characterization of Lytic Bacteriophages Infecting Multidrug-Resistant Shiga Toxigenic Atypical Escherichia coli O177 Strains Isolated from Cattle Feces. Front. Public Health 2019, 7, 355. [Google Scholar] [CrossRef]
- Ramirez, K.; Cazarez-Montoya, C.; Lopez-Moreno, H.S.; Castro-del Campo, N. Bacteriophage cocktail for biocontrol of Escherichia coli O157:H7: Stability and potential allergenicity study. PLoS ONE 2018, 13, e0195023. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.D.; Stanford, K.; Kropinski, A.M.; Ackermann, H.-W.; Johnson, R.P.; She, Y.-M.; Ahmed, R.; Villegas, A.; McAllister, T.A. Genomic, Proteomic and Physiological Characterization of a T5-like Bacteriophage for Control of Shiga Toxin-Producing Escherichia coli O157:H7. PLoS ONE 2012, 7, e34585. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, S.; Aikawa, S.; Hinuma, Y. Stepwise degradation of poliovirus capsid by alkaline treatment. J. Gen. Virol. 1971, 13, 101–109. [Google Scholar] [CrossRef]
- Salo, R.J.; Cliver, D.O. Effect of acid pH, salts, and temperature on the infectivity and physical integrity of enteroviruses. Arch. Virol. 1976, 52, 269–282. [Google Scholar] [CrossRef]
- Lukman, C.; Yonathan, C.; Magdalena, S.; Waturangi, D.E. Isolation and characterization of pathogenic Escherichia coli bacteriophages from chicken and beef offal. BMC Res. Notes 2020, 13, 8. [Google Scholar] [CrossRef]
- Kanamaru, S.; Uchida, K.; Nemoto, M.; Fraser, A.; Arisaka, F.; Leiman, P.G. Structure and Function of the T4 Spackle Protein Gp61.3. Viruses 2020, 12, 1070. [Google Scholar] [CrossRef]
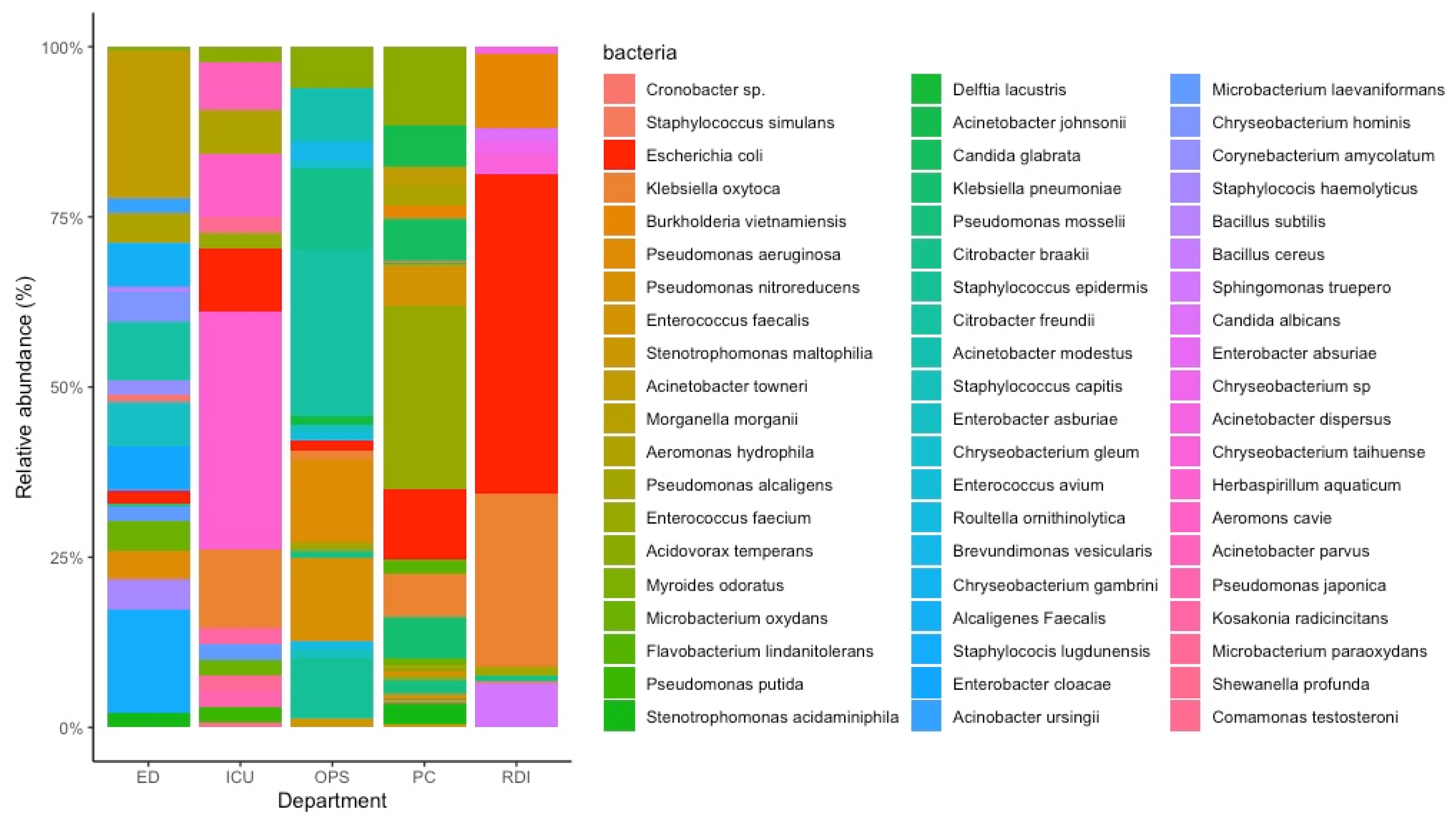



| Antimicrobial Class | Antimicrobial Agent | ECOFF a (µg/mL) | MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| 378 | 452 | ED | PC | RDI | OPS | |||
| Aminoglycosides | Gentamicin | 2 | >128 | 16 | 16 | 64 | 32 | 32 |
| Streptomycin | 16 | >128 | >128 | 64 | 64 | >128 | >128 | |
| ß-lactam/ß-lactam inhibitor | Amoxicillin-clavulanate | - | 128 | 64 | 16 | 16 | 128 | 32 |
| Carbapenem | Ertapenem | - | 1 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 |
| Imipenem | 0.001–4 | 2 | 2 | 4 | 2 | 4 | 4 | |
| Cephems | Cefoxitin | 8 | 16 | 32 | 32 | 32 | 32 | 32 |
| Fluoroquinolones | Ciprofloxacin | 0.06 | 64 | 1 | 0.5 | 0.5 | 0.5 | 16 |
| Folate pathway inhibitors | Trimethoprim | 1 | >128 | >128 | 2 | 4 | >128 | >128 |
| Penicillins | Ampicillin | 8 | >128 | >128 | 8 | 8 | >128 | >128 |
| Phenicol | Chloramphenicol | 16 | 128 | 32 | 16 | 16 | 32 | 16 |
| Tetracyclines | Doxycycline | 8 | 8 | 16 | 8 | 8 | 8 | 64 |
| Department of TQEH | Isolation Method | Presence of E. coli Phages | Phage Isolated |
|---|---|---|---|
| ED | Double-layer agar assay and Enrichment assay | × | |
| PC | Double-layer agar assay | √ | APTC-EC-5, APTC-EC-6 |
| OPS | Double-layer agar assay | √ | APTC-EC-1, APTC-EC-3, APTC-EC-4 |
| ICU | Double-layer agar assay | √ | APTC-EC-2A, APTC-EC-2B |
| RDI | Double-layer agar assay | √ | APTC-EC-7, APTC-EC-8A, APTC-EC-8B |
| Phage | Plaque Morphology | Titre (pfu/mL) |
|---|---|---|
| APTC-EC-1 | Clear, ~3 mm | 6.77 × 1010 |
| APTC-EC-2A | Clear, ~2 mm | 2.4 × 1012 |
| APTC-EC-2B | Clear, ~2.5 mm | 4.11 × 1010 |
| APTC-EC-3 | Clear, ~2 mm | 4.7 × 109 |
| APTC-EC-4 | Clear, ~2 mm | 6.77 × 1010 |
| APTC-EC-5 | Clear, ~2 mm | 2.3 × 109 |
| APTC-EC-6 | Clear, ~2 mm | 8.9 × 1010 |
| APTC-EC-7 | Clear, ~2.5 mm | 3.8 × 108 |
| APTC-EC-8A | Clear, ~3 mm | 4.2 × 1012 |
| APTC-EC-8B | Clear, ~3 mm | 1.07 × 108 |
| APTC-EC-1 | APTC-EC-2A | APTC-EC-2B | APTC-EC-3 | APTC-EC-4 | APTC-EC-5 | APTC-EC-6 | APTC-EC-7 | APTC-EC-8A | APTC-EC-8B | |
|---|---|---|---|---|---|---|---|---|---|---|
| XL10 | + | + | + | + | + | + | + | + | + | + |
| ED 4 | − | + | − | − | +/− | − | − | − | +/− | − |
| PC 22 | − | + | − | − | − | − | − | − | +/− | +/− |
| RDI 14 | − | +/− | − | − | − | − | − | − | − | +/− |
| OPS 13 | − | − | − | − | − | − | − | − | − | − |
| 378 | − | + | − | − | − | − | − | − | +/− | +/− |
| 452 | − | +/− | − | − | − | − | − | − | − | − |
| No. of (semi)sensitive isolates | 1 | 6 | 1 | 1 | 2 | 1 | 1 | 1 | 4 | 4 |
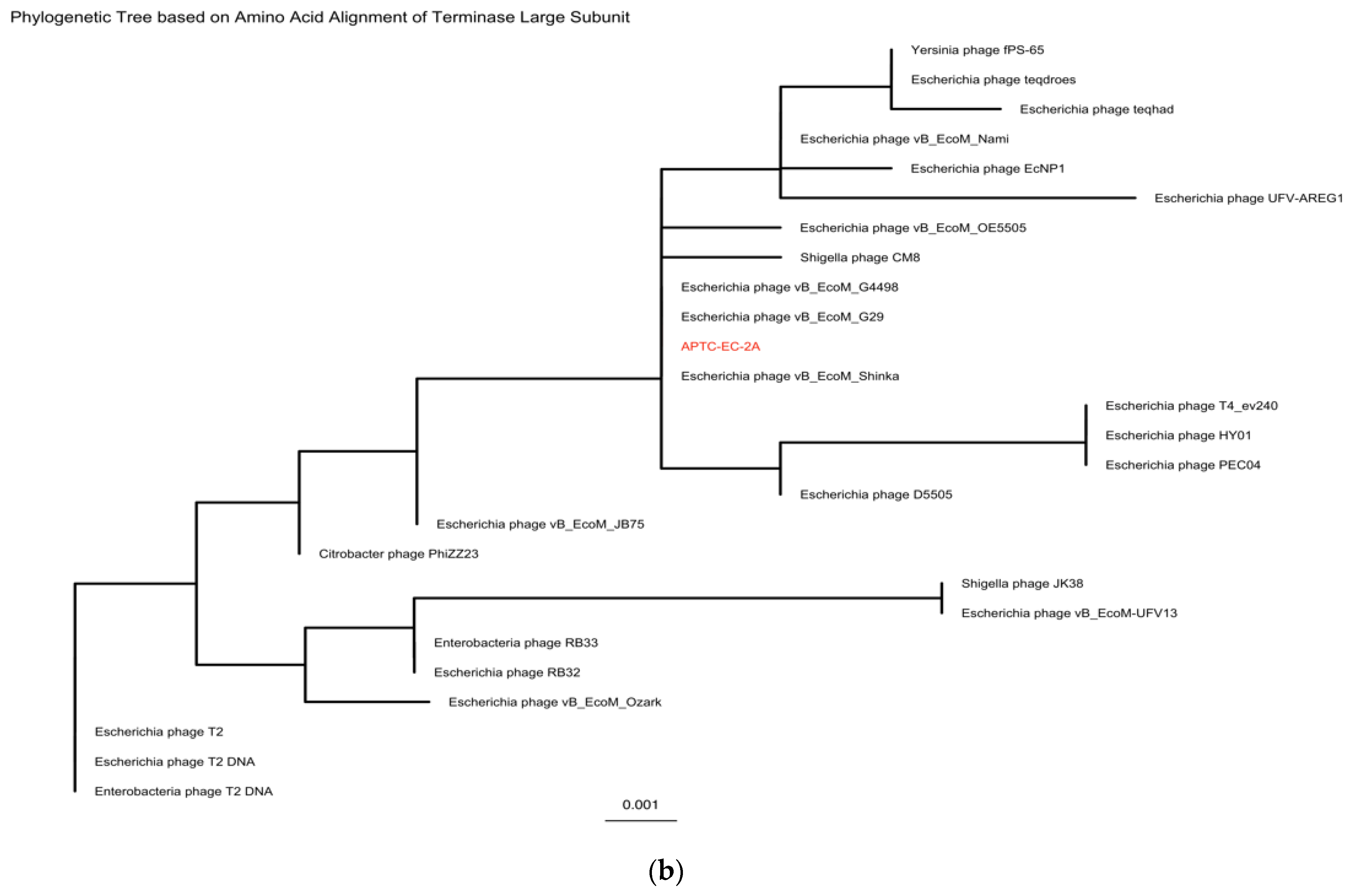
| Accession | Name | Percentage Identity |
|---|---|---|
| NC_054918 | Escherichia phage vB_EcoM_G4498 | 98.97% |
| MK327940 | Escherichia phage vB_EcoM_G29 | 98.41% |
| MK327929 | Escherichia phage D5505 | 97.97% |
| NC_055724 | Yersinia phage fPS-65 | 97.64% |
| NC_054926 | Escherichia phage vB_EcoM_OE5505 | 97.40% |
| MZ502379 | Escherichia phage vB_EcoM_Shinka | 97.39% |
| MH355584 | Escherichia phage vB_EcoM_JB75 | 97.38% |
| NC_054939 | Shigella phage CM8 | 96.88% |
| KM607001 | Enterobacteria phage RB33 | 96.29% |
| DQ904452 | Escherichia phage RB32 | 96.29% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hon, K.; Liu, S.; Camens, S.; Bouras, G.S.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. APTC-EC-2A: A Lytic Phage Targeting Multidrug Resistant E. coli Planktonic Cells and Biofilms. Microorganisms 2022, 10, 102. https://doi.org/10.3390/microorganisms10010102
Hon K, Liu S, Camens S, Bouras GS, Psaltis AJ, Wormald P-J, Vreugde S. APTC-EC-2A: A Lytic Phage Targeting Multidrug Resistant E. coli Planktonic Cells and Biofilms. Microorganisms. 2022; 10(1):102. https://doi.org/10.3390/microorganisms10010102
Chicago/Turabian StyleHon, Karen, Sha Liu, Sophie Camens, George Spyro Bouras, Alkis James Psaltis, Peter-John Wormald, and Sarah Vreugde. 2022. "APTC-EC-2A: A Lytic Phage Targeting Multidrug Resistant E. coli Planktonic Cells and Biofilms" Microorganisms 10, no. 1: 102. https://doi.org/10.3390/microorganisms10010102
APA StyleHon, K., Liu, S., Camens, S., Bouras, G. S., Psaltis, A. J., Wormald, P.-J., & Vreugde, S. (2022). APTC-EC-2A: A Lytic Phage Targeting Multidrug Resistant E. coli Planktonic Cells and Biofilms. Microorganisms, 10(1), 102. https://doi.org/10.3390/microorganisms10010102








