1. Introduction
Mycobacterium microti, a member of the
Mycobacterium tuberculosis complex (MTBC), is the main causative agent of tuberculosis (TB) in wild rodents, particularly field voles (
Microtus agrestis), bank voles (
Myodes glareolus), wood mice (
Apodemus sylvaticus), yellow-necked mice (
Apodemus flavicollis), and shrews (
Sorex araneus) [
1,
2,
3,
4,
5]. Cases of TB due to
M. microti have also been described in humans [
6], a wide-range of domestic species, including cattle [
7,
8], goats [
9], pigs [
10], dogs [
11], cats [
12,
13], and wildlife other than micromammals, such as meerkats [
14], squirrel monkeys [
15], badgers [
16], foxes [
17], deer [
18], and wild boar [
19,
20], presenting a distribution across different European countries, including France, UK, Italy, Spain, Germany, Austria, and Switzerland [
3,
9,
18,
19,
20,
21].
Although differentiation of
M. microti from the other members of MTBC is possible mainly by genotyping methods [
21], it is rarely diagnosed because of the apparently lower susceptibility in humans and livestock [
6,
7,
9], but also due to
M. microti’s slow growing rate that that impedes its isolation by classical bacteriological methods [
22]. However, the extended distribution of this mycobacteria, together with the newly sensitive hosts presents a concern for the identification and management of its role in the control strategies of animal TB. Indeed, it has been suggested that it may interfere with current
M. bovis/
M. caprae diagnosis conducted in livestock in the frame of TB eradication campaigns [
8]. This poses a major concern in low bovine TB prevalence settings, such as in the Pyrenees, where
M. microti has recently been detected in different hosts [
20,
23].
Since voles are described as the main maintenance host for this M. microti, an experimental infection was designed to reproduce the natural disease and better understand the pathogenesis and transmission of TB in voles.
2. Materials and Methods
2.1. Experimental Animals and Housing
Bank voles (Myodes glareolus) were bred at the facilities of the Istituto Superiore di Sanità (Rome, Italy). Then, experimental voles, aged between 80 and 167 days, were transported to the BLS-3 facilities of IRTA-CReSA (Bellaterra, Spain) and were kept in a controlled environment at a room temperature of 22 °C, 12 h light–darkness cycle and 60% relative humidity in HEPA filtered cages (both air inflow and extraction) in ventilated racks. The voles were fed ad libitum, observed daily, and their clinical status assessed once a week. Periodically, voles were weighed and oral swabs and individual fecal samples were obtained. Males and females were housed separately and a maximum of 4 animals per cage. All experiments took place at the Biocontainment Level 3 animal facility at IRTA-CReSA.
2.2. Study Design and Experimental Infection
Two separate experiments were conducted sequentially.
2.2.1. Experiment 1
For the first experiment, a
M. microti field strain used as inoculum was initially isolated in a dog of the Ariege region (France). The strain was selected on the basis of its spoligotype profile, SB0423 (Mbovis.org), which has been detected in different mammals in cross-border regions of France [
23] and Spain [
20] (Ariege, Haute Garonne, and Catalonia).
The inoculum was prepared at Anses (Maisons-Alfort, France) as follows: The isolate was subcultured in Middlebrook 7H9 medium with mycobactin and a titer of 3 × 107 CFU/mL was determined by measurement of optic density at the beginning of the stationary phase. Once the master seed of M. microti was titered, it was diluted in sterile PBS at final suspension of 106 CFU/mL.
This experiment involved two groups (n = 8, male and female balanced, thus, a total of 16 animals) each group with a different inoculation route: (1) intragastric (IG), 0.1 mL of 105 CFU/0.1 mL of M. microti using a plastic flexible intragastric mice probe and a 1 mL syringe; and (2) intraperitoneal (IP) via inoculation of 0.1 mL of 106 CFU/mL of M. microti with a 25 G needle and a 1 mL syringe. The inoculation was carried out under general gaseous anesthesia (Isofluorane 5%). Two time points were established at 28- and 114-days post inoculation (DPI) for euthanasia and postmortem examination. Animals that died spontaneously or had to be euthanized for humanitarian reason were also analyzed.
2.2.2. Experiment 2
For the second experiment, the inoculum was prepared as following: A
M. microti SB0423 isolate, obtained from the spleen of a vole of the experiment 1 (IP group, euthanized at 28 dpi), was subcultured in Middlebook 7H9 medium (BD Diagnostics, Sparks, NV, USA) for 28 days at 37 ± 1 °C. This second inoculum was titrated by qPCR as following: an aliquot of 1 mL of the suspension was inactivated at 75 °C for 1 h. In parallel, an aliquot of the
M. microti master seed (3 × 10
7 CFU/mL) used for the challenge inoculum of the experiment 1, was also inactivated and then ten-fold serially diluted to establish titrated standards. DNA samples were extracted and amplified using the ID Gene™ spin universal extraction and ID Gene™
Mycobacterium tuberculosis complex Duplex commercial kits (ID.vet, Grabels, France), respectively, according to the manufacturer procedures. Amplification was performed in a 7500 fast real-time PCR system (Applied Biosystems, Walham, MA, USA).
M. microti CFU genomic equivalents were calculated by the extrapolation of Ct value obtained for each DNA sample according to previously described [
24]. Once titrated, the remaining subcultured suspension was diluted in sterile PBS at a final suspension of 10
7 CFU/mL.
In this experiment, the IP route was chosen and a higher dose was used, it consisted of a group (n = 14, male and female balanced) inoculated IP with 0.1 mL of 107 CFU/mL of M. microti. Four additional unchallenged animals were housed in 4 cages, each of them sharing cage with 3 inoculated animals to assess the occurrence of horizontal transmission. An early time point was established at 28–37 dpi (a first batch of 3 animals were euthanized at 29 dpi and were grouped with other three animals that were found dead at 28 and 37 dpi, due to TB-unrelated causes) and a second late time point at 69 dpi for euthanasia and postmortem examination. Two additional voles remained uninoculated and unexposed to infected animals and were used as negative controls.
2.3. Ethics Statement
All experimental animal procedures were approved by the Animal Research Ethics Commission of the Generalitat de Catalunya (procedure number FUE-2020-01337124 and ID 46KZF9ZM4), according to current European legislation for the protection of experimental animals (86/609/EEC, 91/628/EEC, 92/65/EEC, and 90/425/EEC).
2.4. Postmortem Examination and Pathological Evaluation
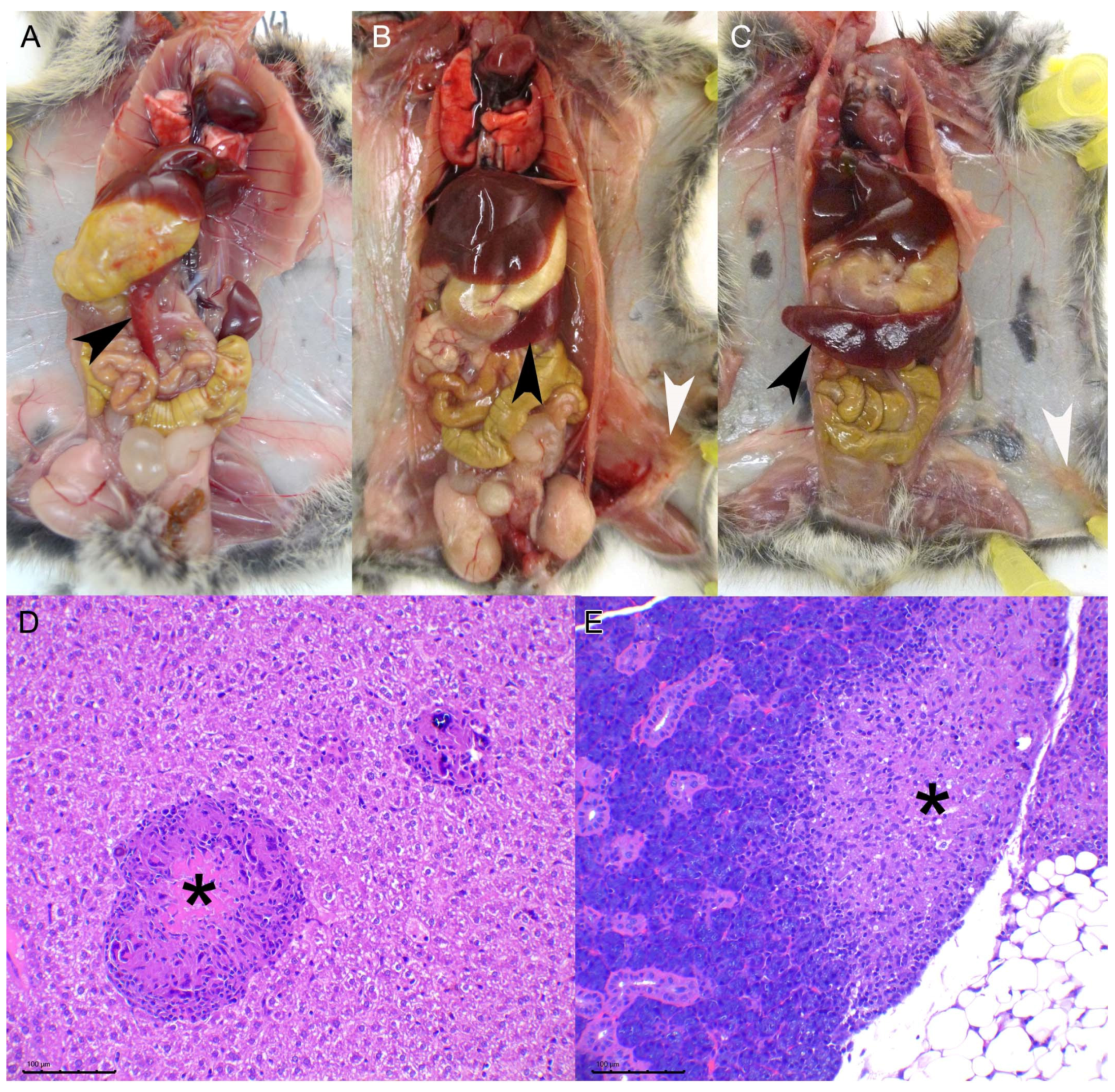
Animals were humanely euthanized upon reaching end point criteria or at established time points by general gaseous anesthesia (Isofluorane 5%) and subsequent decapitation. After visual examination of the viscera, samples of liver, spleen, and lung tissue were obtained and frozen at −75 °C, the remaining tissues were immediately immersed in formalin. Two weeks later the tissues were routinely processed for histopathology, i.e., paraffin embedded, 3–4 µm sections were obtained and further stained with Hematoxylin and Eosin (HE) for morphological assessment. Ziehl Neelsen (ZN) stain was used to detect AFB. The tissues processed for microscopic evaluation included: kidney, liver, lung, thoracic lymph node (LN), spleen, stomach, intestines, mesenteric LN, cervical LN, inguinal LN, and any tissue with visible lesions.
To objectively assess the progression of the TB lesions, a pathology index was established based on a mixed scoring system that integrated different parameters evaluated separately: the absence or presence of lesions (0/1) inguinal LN, cervical LN, and peritoneum; for the spleen a semiquantitative scoring system (0–3) was established (from 0—absence of lesion— to 3—extensive splenomegaly due to diffuse granulomatous inflammatory infiltrate—scores 1, 2, and 3 were assigned to mild, moderate, and evident levels of the same lesional pattern, respectively); thoracic lesions were evaluated as summatory of the absence/presence (0/1) of TB lesions in the lung and thoracic lymph nodes combined. Finally, lesions in the hepatic tissue were quantitatively evaluated using Image J software [
25] including different parameters: number of granulomas found in five 10× randomly selected liver fields, the area of the granulomas was calculated, as well as the absence/presence (0/1) of a central area on necrosis and absence/presence (0/1) of mineralization. For the pathology index, the liver score included three parameters: necrosis (0/1), Mineralization (0/1) and a third one regarding the size of granulomas. For this third parameter a score of 0 was given when the animal’s total liver granuloma area median was below (smaller granulomas) the global liver granuloma area median (5729.985 μm
2) and 1 when it was above (bigger granulomas).
2.5. Mycobacterial Cultures
Liver, spleen, and lung tissues were thawed and sliced with sterile scissors and subsequently mechanically homogenized in 1.5 mL of sterile distilled water, and an aliquot of 1 mL of each homogenate was separated for subsequent bacterial load assessment by qPCR. The remaining 0.5 mL was decontaminated with 0.5 mL of oxalic acid at 5%
w/
v for 30 min and then neutralized with 250 μL of NaOH 1 M. Afterwards, samples were centrifuged at 2451×
g for 30 min. Supernatants were discarded and pellets were suspended in 1 mL of sterile PBS. Suspensions were cultured as following: 100 μL were inoculated in BBL tubes and incubated in BACTEC MGIT 320 system (BD diagnostics, Sparks, MD, USA), 100 μL were cultured in Middlebrook 7H11 plates (BD diagnostics, Sparks, MD, USA), and a swab was immersed in the remaining suspension for culture in Löwenstein–Jensen with pyruvate and Coletsos solid medium tubes (BD Diagnostics, Sparks, MD, USA). Growth in positive cultures were confirmed as MTBC by multiplex PCR [
26]. A culture was considered negative when no growth was observed in Middlebrook 7H11 plates (at 28 days), BACTEC MGIT (at 42 days) or solid medium tubes (at 90 days).
2.6. Mycobacterial Load Assessment by qPCR
Aliquots of 1 mL of liver, spleen, and lung homogenates of each vole were inactivated at 75 °C for 1 h. The M. microti master seed was used to generate the qPCR standard curve. DNA samples from homogenates and standards were extracted and amplified, and M. microti CFU genomic equivalents were calculated as described above.
2.7. Mycobacterial Excretion Assessment by qPCR
Oral swabs and feces were collected from each at animal at days: 7, 14, 21, 28, and 56 p.i. (experiment 1), and 13, 41, and 69 p.i. (experiment 2). Oral swabs were immersed and cut in microtubes containing 1 mL of PBS, while feces was suspended and homogenized in 1 mL of PBS. All samples were inactivated at 100 °C for 10 min at stored at −20 °C.
DNA was extracted from oral swabs using an LSI MagVetTM Universal Isolation Kit (Life Technologies, Villebon sur Yvette, France) with a KingFisherTM Flex automate (ThermoFisher Scientific, Villebon sur Yvette, France), following the manufacturer’s instructions. DNA was extracted from feces with the FastDNA™ Spin Kit for Soil (MP Biomedicals, Eschwege, Germany). Real-time PCR were carried out in a 25 µL reaction mix containing TaqMan™ Fast Advanced Master Mix (ThermoFisher Scientific, Villebon sur Yvette, France), 300 nM forward and reverse primers, 250 nM probes, sterile water, and 5 µL of DNA template. Thermocycling conditions were 50 °C for 2 min (1 cycle), followed by one cycle of 20 s at 95 °C and 40 cycles of 3 s at 95 °C and 30 s at 60 °C. PCR inhibition was tested (Diagenode, Seraing, Belgium). Positive detection of
M. microti was established on the basis of a positive response for IS1081 and IS6110 (
Mycobacterium tuberculosis complex) [
27].
2.8. Serology
Blood was obtained upon sacrifice through decapitation. Sera were separated and a MTBC IgG ELISA was carried out and interpreted as previously described [
28]. Briefly, sera were analyzed for antibodies against the MTBC-specific MPB83 antigen (Lionex, Braunschweig, Germany) by a homemade IgG indirect ELISA. A sample was classified as positive (i.e., seroconversion) when ΔOD (sample wells Optical Density—OD—at 450 nm minus the blank well OD at 450 nm) ≥0.2.
2.9. Data Analysis
A randomized design was used for data analyses. Differences in the spleen pathology score, liver granuloma area, overall pathology index, and bacterial loads in the spleen (log10 CFU equivalents) between the early and late infection groups were compared using the one-tailed Mann–Whitney non-parametric test. Correlation between the spleen pathology score and bacterial loads in the spleen was performed by a non-parametric Spearman rank test. The statistical analysis and visualizations were carried out using the R software version 4.1.1, and the following packages: dplyr (1.0.7), tidyverse (1.3.1), ggplot2 (3.3.5), ggpubr (0.4.0), hrbrthemes (0.8.0), viridis (0.6.1), viridisLite (0.4.0), heatmaply (1.2.1), and plotly (4.9.4.1).
4. Discussion
TB in wild voles was described in 1934 by Wells and Oxon [
5], who initially referred to it as ‘the vole acid-fast bacillus’ [
29]. The aim of the present study was to set up an experimental model of
M. microti TB in one of its natural reservoirs to better characterize the infection and pathogenesis. A specific goal was to precisely determine the phenotype caused in Bank Voles by the
M. microti strain identified circulating in the Pyrenees in other mammal species [
20,
23]. Both inoculation routes tested successfully reproduced a TB infection in bank voles with lesions resembling those previously reported in free-ranging voles [
3]. The most common lesions described in field voles, consisting in granulomatous hepatitis, splenitis, and lymphadenitis, were observed in most of the infected animals in our study. Another type of typically reported lesion are skin granulomas [
2,
3], which we have not observed in this experimental model, this is most likely due to the inoculation route chosen since skin lesions are hypothesized to be the entry portal through bites from other infected animals. We have observed, indeed, the presence of subcutaneous granulomas in the inoculation point of the intraperitoneal group. The miliary lesional pattern observed in the liver parenchyma and the presence of diffuse granulomatous infiltrate in the spleen, in both IG and IP routes, strongly suggest that a hematogenous spread of mycobacteria took place in these animals. Lesions in other viscera such as the lungs, skeletal muscle, or the salivary gland were also observed but with a much lower frequency. No differences in the proportion of animals with these lesions were observed in the high dose vs. the low dose experiment, suggesting that individual host-related variation factors, rather than the dose change, influence the spread of the disease. Given that some animals presented lung and salivary gland lesions suggests that shedding of mycobacteria is likely to occur.
The oral intake of mycobacteria is a likely infectious route in the field, from the environment or even through consumption of infected carcasses. One of the IG-inoculated animals showed, indeed, muscular lesions similar to those described in field voles [
3]. However, the results obtained in the IG route were less consistent than the IP route, which generated a 100% attack rate even with the lower dose at the early time point. The titers obtained by qPCR in the early time point low dose IP inoculated spleens were, in fact, lower than the titer of the inoculum, and thus these results might be interpreted as residual inoculum and not a true infection, but considering the evolution of all the remaining IP animals towards a more severe pathology and titers similar or higher than those inoculated, we interpret these results as early stages of infection. Another potential artifact of the IP route is the presence of granulomatous infiltrate and mycobacteria in the peritoneum of the animals, observed in the pancreatic parenchyma and perirenally, probably resulting from the direct deposition of mycobacteria in the peritoneal cavity. An alternative explanation for these lesions would be the hematogenous spread of mycobacteria from lesions in other viscera to the serous membranes, in a mechanism similar to that causing TB pearly disease in other species [
30]. In any case, the IP route was the chosen inoculation route for the second experiment and the one we recommend for future experimental bioassays.
A log increase in the inoculum titer was tested for the second experiment focused on the IP route. This increase in the dose approximately accelerated the observed phenotype twice. The lesions observed in the early time point (28–37 dpi) in the IP high dose animals were equivalent or even more severe that those observed at 114 dpi in the lower dose group. In any case, longer incubation times would likely lead to the appearance of more lesions in the lungs, salivary glands and probably the kidneys. An increased dose, however, allowed us to optimize the model shortening the incubation period needed to observe widespread lesions.
TB is usually a slowly progressive disease. The rapid progression of the lesional phenotype observed in our experiments can be explained by the relatively high doses inoculated. Indeed, one log increase in the dose resulted in a noticeable acceleration of the phenotype. In the field voles are likely to be exposed to variable doses, probably much lower than the ones used here. Descriptions of field studies report a proportion of animals with positive cultures of
M. microti in the absence of visible TB lesions [
3], this suggests that the severity of lesions observed is heterogeneous and that even animals with latent
M. microti infection might exist. This is in agreement with the variable results observed in the first experiment with intragastric exposure, which likely mimics one of the natural exposure routes. In a laboratory setting, however, the aim of an experimental model is to reproduce a given phenotype in a controlled manner; thus, those conditions giving a fast and reproducible phenotype are the ones to be met. Even if the result is not representative of the full spectrum of the disease phenotype in the field. Eventually, a need to design control tools for wild micromammal populations with high TB prevalence will benefit form a well-characterized model.
In the second experiment, we assessed the pathological and bacteriological findings at two time points after M. microti experimental infection to characterize the progression of the disease in the bank vole model. As expected, the severity of lesions increased in target tissues at 70 dpi compared to 30 dpi (i.e., higher mean pathology score in the spleen and number of large granulomas in the liver). Mycobacterial loads in the spleen also tended to increase at late time points. The presence of lesions or mycobacterial isolation in other localizations also confirmed the progression of the infection. Regarding the liver lesions, not only qualitative changes indicative of pathology progression where observed (presence necrosis and increased granuloma size), but also an increase in the total number of granulomas observed in the late time point animals (although not statistically significant) that could be explained by the formation of new granulomas due to a continued hematogenous spread of mycobacteria. This fact could explain the lack of significant differences when comparing both time points as regards to the size of granulomas.
Moreover, mycobacterial shedding results suggested that infected animals could also be infectious at the late time point. Even though mycobacterial DNA was not detected from oral swabs in any case throughout both experiments, mycobacterial DNA in feces was detected in a few animals, although with a very low bacterial burden. Therefore, a fecal–oral transmission cannot be ruled out. Little evidence could be gathered from the histological study where only four animals with lung lesions and one with salivary gland lesions could be observed that could support mycobacterial excretion through sputum (both from lower respiratory tract exudates and/or from saliva). The characteristic skin lesions described in field TB cases in voles could be explained by transmission trough bites from infected individuals excreting mycobacteria through the saliva [
3]. Still, one of the contact-uninoculated animals seroconverted. Interestingly, this animal shared cage with two inoculated animals in which lesions were not observed neither in the lungs nor in the salivary gland, and additionally qPCR and culture form their lungs, as well as from the feces and oral swabs of these animals that were also negative. Therefore, there is no evidence of
M. microti transmission between these animals. However, certain evidence of infection was found in the seropositive contact animal, consisting of a single multinucleated giant cell and a single AFB compatible structure in the studied sections (
Figure 6), that precluded a solid confirmation of horizontal transmission in our experimental setting. Further studies with more prolonged incubation times are needed to assess this. An alternative explanation for seroconversion could be an oral exposure to mycobacteria from the inoculation point of its cage mates at day 0.
5. Conclusions
M. microti IP and IG challenge successfully reproduced a TB phenotype resembling field infection in bank voles.
The IP route provides more consistent results than IG (100% attack rate and earlier pathology onset). Increasing two logs the infectious titer inoculated accelerates (approximately times two) the onset of TB pathology, thus optimizing the model.
Longer studies are required to confirm likely horizontal transmission between voles.
As disease progresses, splenomegaly increases, the number of liver granulomas remains unchanged but granulomas become bigger and develop a central necrotic core. For surveillance matters in field voles, microscopic examination of the liver is recommended in the absence of macroscopically visible lesions, particularly in voles with a certain degree of splenomegaly. Spread to other organs, such as the lung or salivary glands, leading to mycobacterial shedding, probably depends on host-related individual factors.