Abstract
The uncontrolled invasion of moso bamboo (Phyllostachys pubescens) dramatically alters soil nitrogen cycling and destroys the natural habitat of Alsophila spinulosa. Nevertheless, no clear evidence points out the role of denitrifying bacteria in the invasion of bamboo into the habitat of A. spinulosa. In the present study, we found that low (importance value 0.0008), moderate (0.6551), and high (0.9326) bamboo invasions dramatically altered the underground root biomass of both P. pubescens and A. spinulosa. The root biomass of A. spinulosa was maximal at moderate invasion, indicating that intermediate disturbance might contribute to the growth and survival of the colonized plant. Successful bamboo invasion significantly increased rhizospheric soil available nitrogen content of A. spinulosa, coupled with elevated denitrifying bacterial abundance and diversity. Shewanella, Chitinophaga, and Achromobacter were the primary genera in the three invasions, whereas high bamboo invasion harbored more denitrifying bacteria and higher abundance than moderate and low invasions. Further correlation analysis found that most soil denitrifying bacteria were positively correlated with soil organic matter and available nitrogen but negatively correlated with pH and water content. In addition, our findings illustrated that two denitrifying bacteria, Chitinophaga and Sorangium, might be essential indicators for evaluating the effects of bamboo invasion on the growth of A. spinulosa. Collectively, this study found that moso bamboo invasion could change the nitrogen cycling of colonized habitats through alterations of denitrifying bacteria and provided valuable perspectives for profound recognizing the invasive impacts and mechanisms of bamboo expansion.
1. Introduction
Moso bamboo (Phyllostachys pubescens Mazel ex J. Houz.; synonym P. edulis (Carrière) J. Houz) is a member of the Poaceae and tree-like woody bamboo, harboring crucial ecological, practical, and cultural values [1,2]. P. pubescens is able to grow in mountainous areas, possesses a rapid regrowth rate after harvesting, and its culms can reach over 25 m in height with a 20-cm basal diameter [3]. Nevertheless, P. pubescens is also notorious for its invasive characteristics that can, unbridled, invade natural forests throughout subtropical China [2]. The uncontrolled invasion of bamboo has exerted tremendous influences on colonized forests, such as changed forest structure and plant composition [4], decreased species biodiversity and ecological stability [5], altered soil nitrogen (N) pools and cycling rates [6,7], and reshaped microbial diversity and composition [8]. For instance, the bamboo invasion has been documented to promote the input of labile soil organic matter fraction and increase soil bacterial diversity after invading a Japanese cedar (Cryptomeria japonica) plantation [9,10].
Tree fern Alsophila spinulosa (Hook) Tryon, belonging to the family Cyatheaceae, is an endangered relic plant with a tree-like trunk [11,12]. A. spinulosa had been flourishing during the Mesozoic era; nonetheless, after being affected by glaciation, its distribution area declined dramatically. The species mainly grows in mountainous areas, occupying shady niches with acidic soil (pH 4.5~5.5) at 400~900 m [13]. Currently, due to some deficiencies (e.g., short spore life-span, relatively high temperature, humidity and illumination requirements, and primitive root system and transfusion tissue), its modern distribution is restricted to southern China (18.5° N~30.5° N), Japan, and southeastern Asia [14]. In addition, A. spinulosa has been growingly threatened by exotic plant invasion, especially in nature reserves with minor artificial disturbance. For example, the severe invasion of moso bamboo significantly decreased root biomass and altered the root morphological plasticity of A. spinulosa in the Chishui Alsophila National Nature Reserve [15]. Hence, evaluating the impact of invasion on local plants and investigating the invasive mechanisms are essential to protect the endangered plant.
N is one of the most critical components in the ecosystem and an essential nutrient for plant growth and survival. Several processes regulate the N cycle, one of which is denitrification, with the help of denitrifying bacteria (e.g., Shewanella, Achromobacter, and Pseudoxanthomonas) [16,17,18,19]. Unconstrained bamboo expansion is well-reported to alter soil N cycle in the colonized habitat. A previous study found that the invasion of P. pubescens elevated the soil labile N pool, as well as microbial biomass carbon [10]. Similarly, Liu et al. [20] and Fukushima et al. [21] observed that the content of soil total N content in the adjacent evergreen broadleaved forest was lower than that of the bamboo forest, and the invasion of P. pubescens altered the distribution pattern of N stored in plants and soil. Yet, little is known about the impacts of P. pubescens invasion on A. spinulosa, especially from the viewpoint of denitrification, which would be critical for future understanding of the invasive mechanisms of bamboo expansion.
Thus, our objective in this study was to explore the influences and mechanisms of P. pubescens invasion on the highly endangered plant A. spinulosa. Specifically, the primary focuses of this study aim to: (1) reveal how properties of soil colonized with A. spinulosa respond to bamboo expansion; (2) identify the dominant rhizosphere denitrifying bacteria community and explore how invasion reshapes the denitrifying bacterial community; (3) evaluate the relationships between the denitrifying bacterial community and soil properties and growth of A. spinulosa. To address these questions, forest composition, root biomass, and denitrifying bacterial composition were examined meticulously in low, moderate, and high P. pubescens-invasive forests in a natural ecosystem located in Chishui Alsophila National Nature Reserve, Guizhou Province, China.
2. Materials and Methods
2.1. Community Survey and Soil Samples Collection
Chishui Alsophila National Nature Reserve is located in Guizhou Province, China (105°57′54″~106°7′7″ E 28°20′19″~28°28′48″ N), characterized by steep slope, deep valley, and closed terrain. The protected area belongs to a mid-subtropical humid monsoon climate, with 17.7 °C annual average temperature, 1200~1300 mm annual average precipitation, and 90% relative humidity. The soil type where A. spinulosa is grown is purple soil with pH 4.5~5.5.
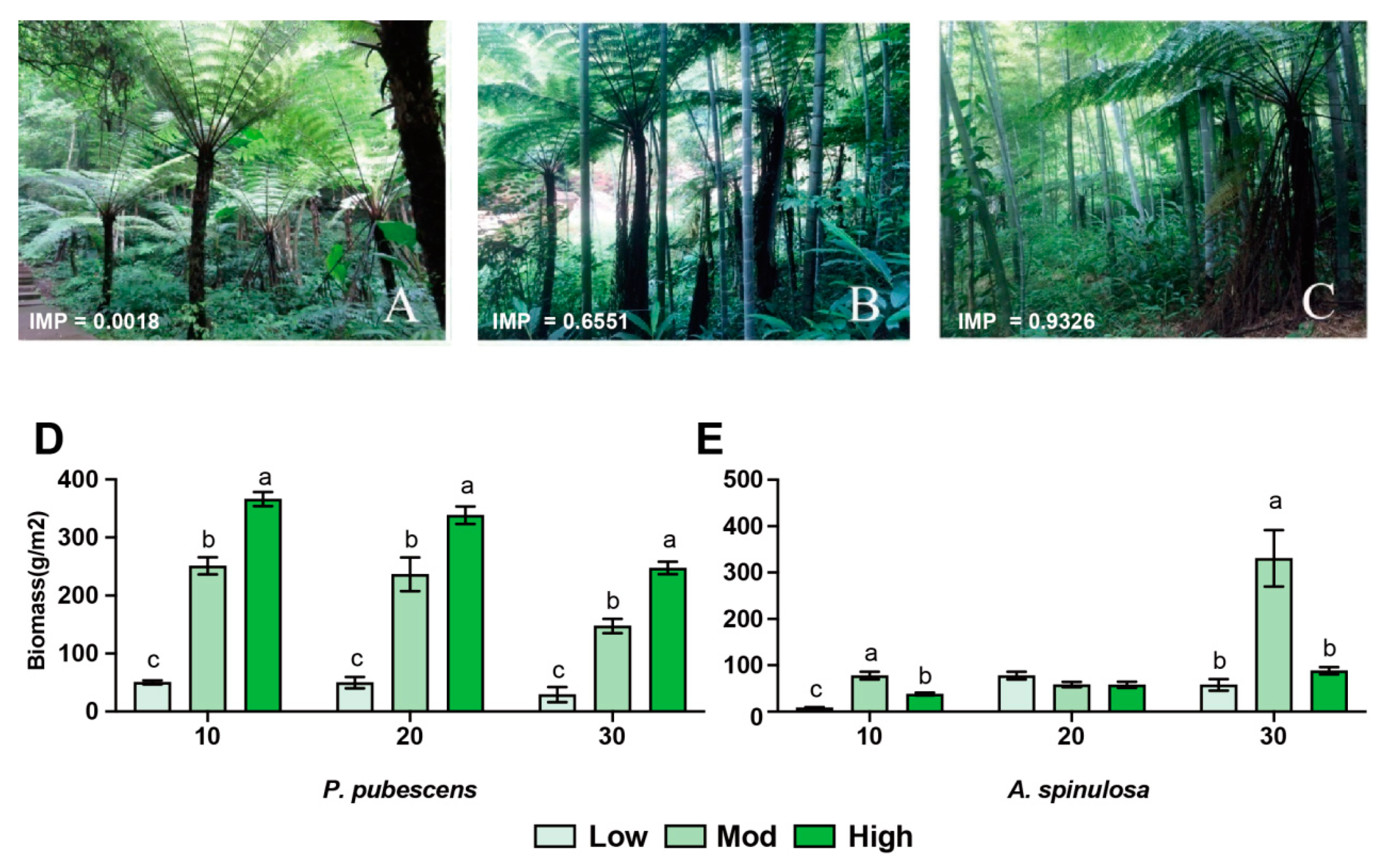
The importance value (IMP) was calculated by dividing the frequency of P. pubescens by the frequency of all species. In this study, three sampling groups were chosen based on the IMP (range from 0~1) of P. pubescens, and three replicates for each group were collected at low (IMP < 0.3), moderate (0.3 < IMP < 0.7), and high (IMP > 0.7) P. pubescens-invasive sites (Figure 1A–C). The detailed information related to sampling site and forest structure are listed in Table 1. The characteristics (e.g., height and diameter at breast height) of A. spinulosa were recorded as well. In addition, to evaluate the effects of invasion on the root system, root samples at depths of 10 cm, 20 cm, and 30 cm (three replicates) were collected from both P. pubescens and A. spinulosa, respectively. The biomass value of root samples was calculated based on the equation B = W/[π × (0.1/2)2]. B~biomass (g/m2), W~root dry weight (g), and 0.1~diameter of the drill (m).
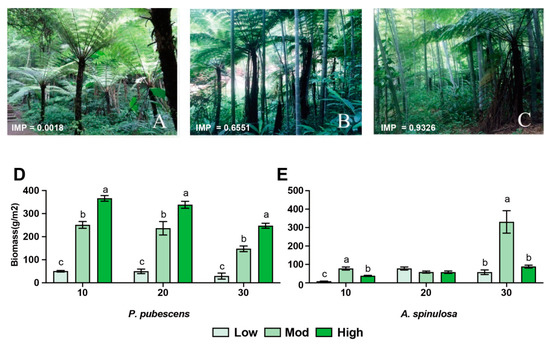
Figure 1.
Effects of moso bamboo invasion on forest structure and tree characteristics. (A–C) Field pictures of A. spinulosa community disturbed by low (A), moderate (B), and high (C) moso bamboo invasions. IMP indicates the importance value of moso bamboo. (D,E) Root biomass of moso bamboo (D) and A. spinulosa (E) at different vertical depths, respectively. abc Different superscripts indicate the significant difference (p < 0.05).

Table 1.
The basic information about three sampling sites.
In order to investigate the role of denitrifying bacteria on the invasion of P. pubescens into A. spinulosa, rhizospheric soil samples (three replicates per group, with depth of 0~20 cm) of A. spinulosa from three invasive sites were excavated and shaken carefully to separate the soil from the roots, as described previously [22]. Finally, a total of nine soil samples were collected from the three invasive groups. A portion of the soil samples was immediately stored at −80 °C for subsequent molecular analysis, whereas the remaining soil was used for soil physical and chemical characterization.
2.2. Soil Chemical and Physical Properties
Soil physical and chemical analyses for pH, OM, WC, and available nitrogen (AN) were performed as previously reported [23]. Specifically, after conventional treatment, soil WC was measured by drying method; pH was measured by potentiometric method; OM was determined by potassium dichromate and sulfuric acid digestion method; and AN was measured by the alkali hydrolysis diffusion method.
2.3. DNA Extraction, QnorB Gene Amplification, and MiSeq Sequencing
Soil samples (0.5 g) were used to extract microbial DNA according to the QIAmp DNA Stool Mini Kit. To explore the soil denitrifying bacteria communities at three invasive sites, the target fragment qnorB was amplified with barcoded 2F 5′-GGNCAYCARGGNTAYGA-3′ and 5R 5′-ACCCANAGRTGNACNACCCACCA-3′. PCR amplification was conducted in an eight-cycle and the PCR products were purified with a GeneJET Gel Extraction Kit (Thermo Scientific, Waltham, MA, USA). Subsequently, the PCR product from each sample was sequenced on the Illumina MiSeq (300-bp paired-end reads) platform (Illumina Inc., San Diego, CA, USA) at the TinyGene Bio-Tech Co., Ltd. (Shanghai, China). The reads were distinguished from each sample and any sequences of low quality were deleted with the ultra-fast sequence analysis (USEARCH). The splicing sequence was qualified and filtered to yield the optimized sequences. Furthermore, operational taxonomic units (OTU) were obtained at a 97% similarity level using the UPARSE pipeline [24].
2.4. Data Analysis
Community diversity indexes, including Ace, Chao1, Shannon, and Simpson, were used to estimate bacterial abundance and diversity. The Chao1 index and Ace index indicated the richness of the bacterial community, and the Shannon index and Simpson index showed the diversity of the bacterial communities. An analysis of variance (ANOVA) test was conducted to assess the significance of tree characteristics and soil properties by IBM SPSS Statistics 26. The relationships of denitrifying bacteria with A. spinulosa’s growth and soil properties were determined using Spearman rank correlation analysis. Graphics were processed by Adobe Illustrator CC, version 2021, and Adobe Photoshop CC, version 2021.
3. Results
3.1. Effects of Invasion on Forest Structure and Soil Properties
In this study, we identified three sampling sites with different invasion degrees of P. pubescens, whose importance values were 0.0008 (low), 0.6551 (mod), and 0.9326 (high), respectively (Figure 1A–C). The biomass of P. pubescens roots under high invasion was significantly higher than that under moderate and low invasions (p < 0.01) and the results were consistent in the soil layers of 10 cm, 20 cm, and 30 cm (Figure 1D). At the same time, the biomass of A. spinulosa under moderate invasion was significantly higher than that at low and high invasions in the soil layers of 10 cm and 30 cm (Figure 1E, p < 0.01), suggesting that intermediate disturbance might contribute to the survival of A. spinulosa.
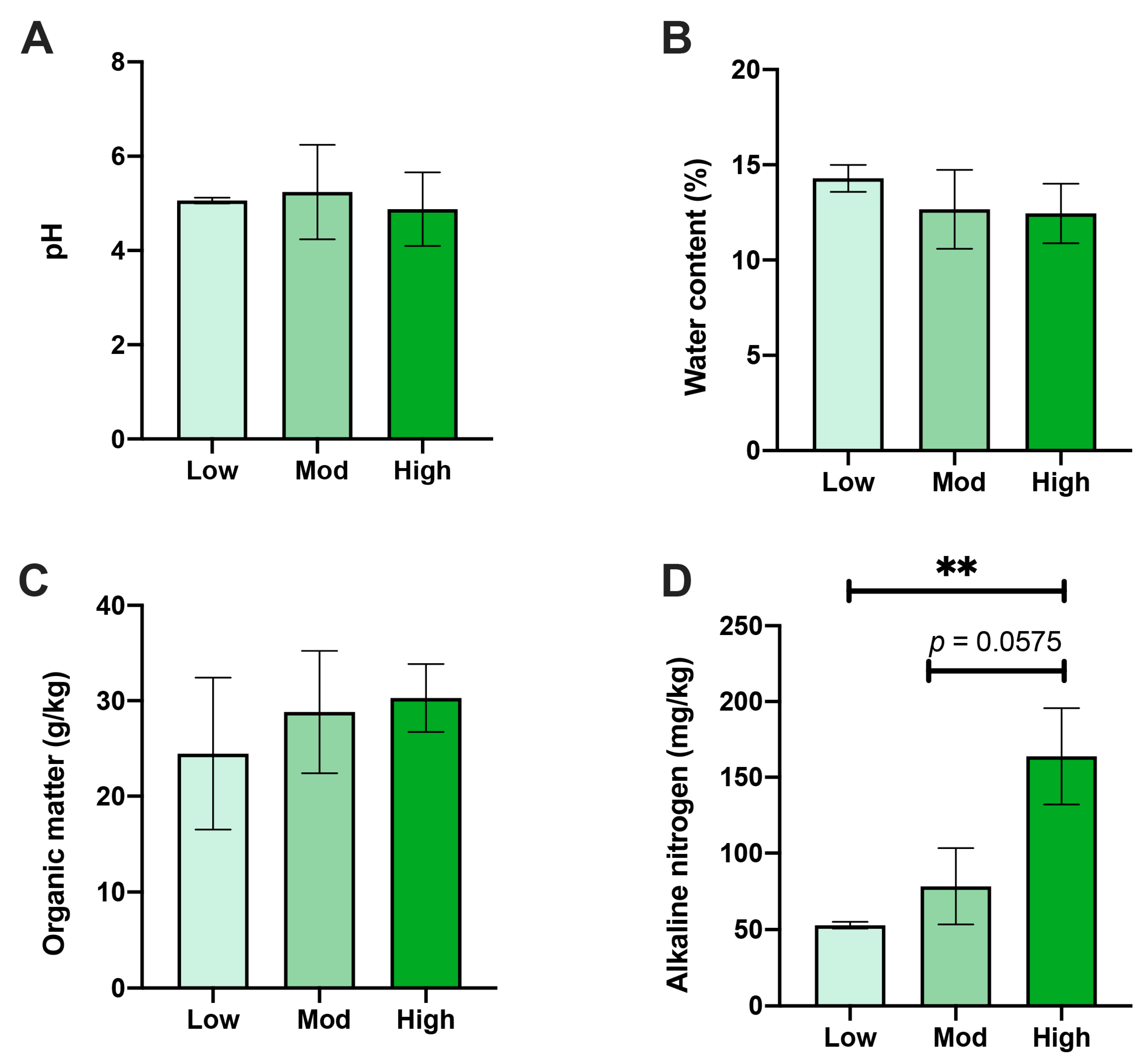
The rhizospheric soil physical and chemical properties of A. spinulosa were affected by different degrees of P. pubescens invasion. Notably, AN content in the high-invasive group was distinctively higher than that of the low-invasive group (p < 0.01) and slightly higher than that of the moderate group (p < 0.1) (Figure 2A). Nonetheless, no alterations were identified in pH, WC, or OM between the three comparisons (Figure 2B–D).
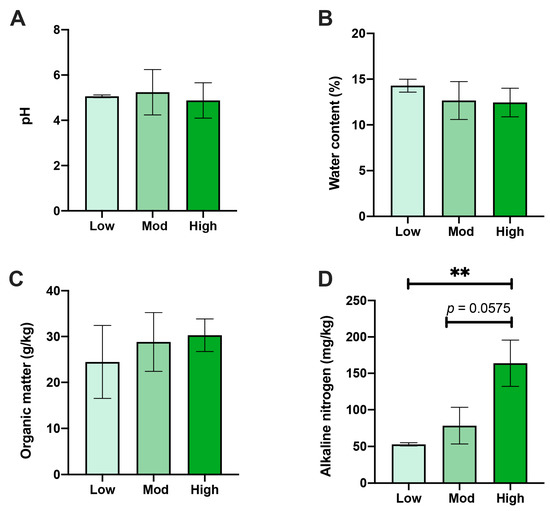
Figure 2.
Effects of moso bamboo invasion on rhizospheric soil properties of A. spinulosa. Alterations of (A) soil pH, (B) water content, (C) organic matter, and (D) available nitrogen under low, moderate, and high moso bamboo invasions. ** Asterisk indicates the significant difference (p < 0.01).
3.2. Effects of Invasion on Denitrifying Bacterial Community Composition
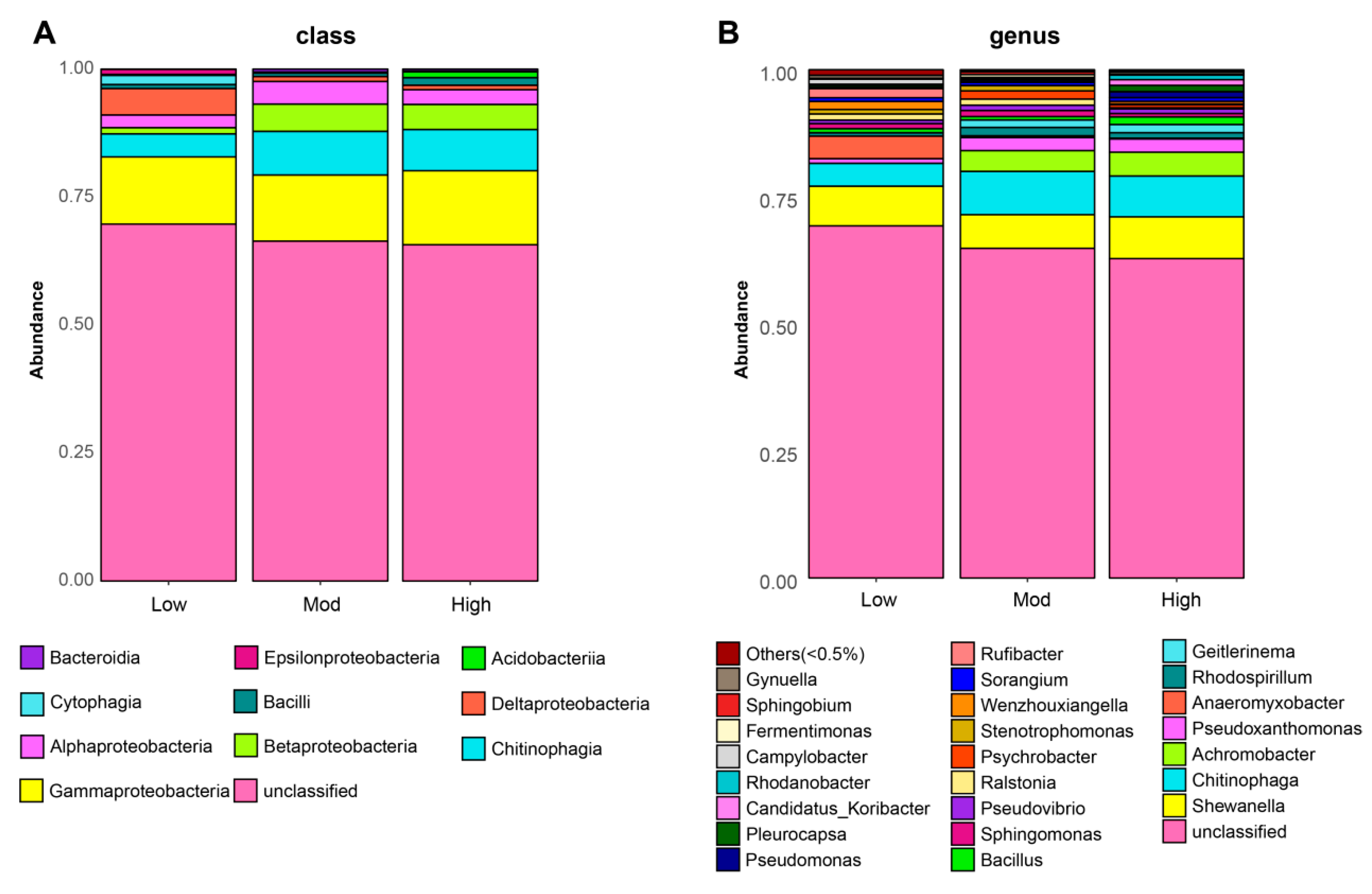
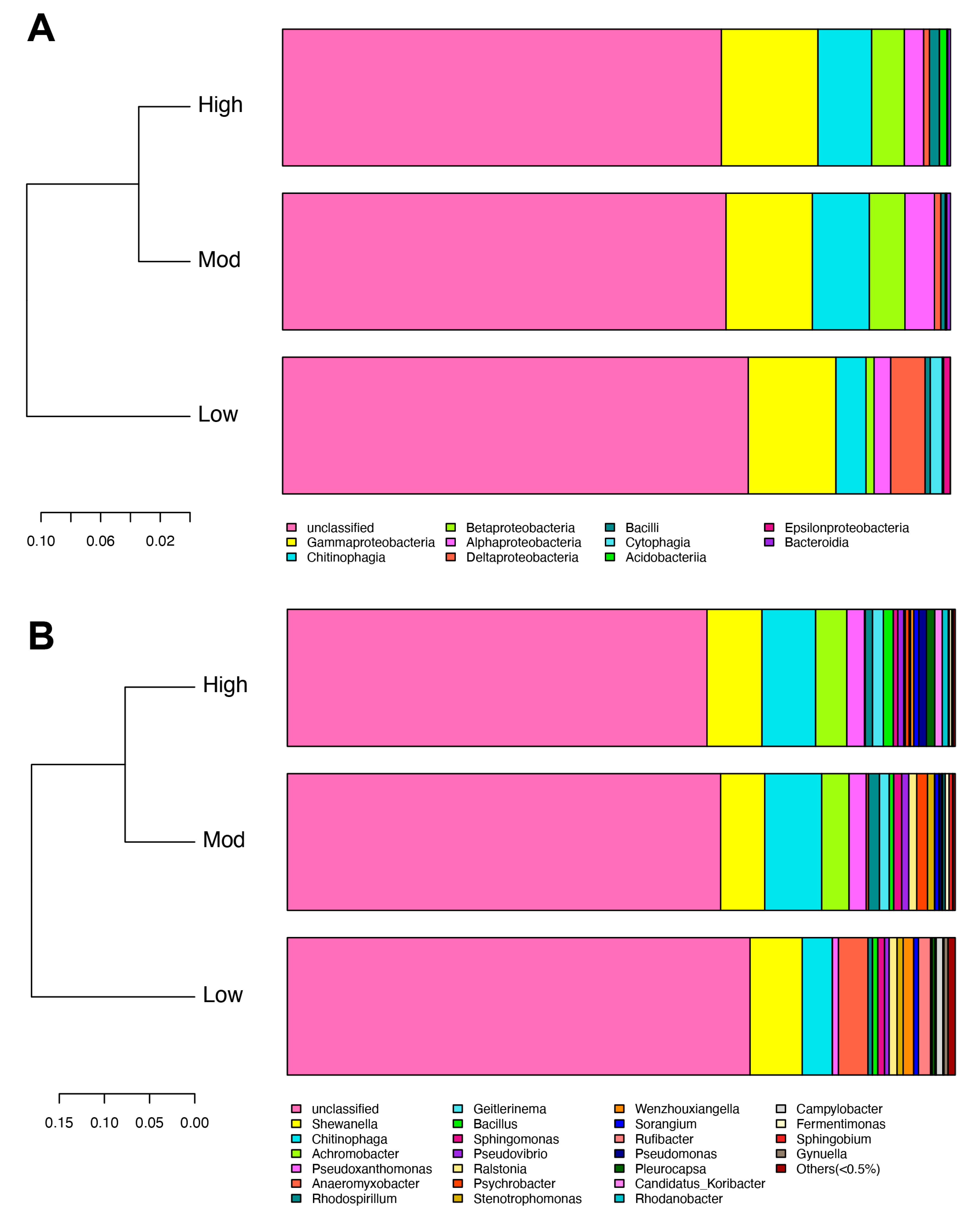
After sequencing, a total of 201,641 raw reads and 119,600 clean reads were collected from all nine soil samples. The number of sequences ranged from 36,918 to 43,944 per group, with a mean of 39,867 sequences. To avoid any bias in the distribution of taxa, the soil bacterial diversity of each sample was obtained according to rarefaction curves. Finally, we yielded a total of 5 phyla, 10 classes, 21 orders, 28 families, 32 genera, and 41 species of denitrifying bacterial taxa. Specifically, denitrifying bacterial sequences were primarily composed of the phyla Proteobacteria (27.53%), Bacteroidetes (5.26%), Firmicutes (1.61%), Cyanobacteria (1.21%), and Acidobacteria (0.81%). Additionally, Gammaproteobacteria dominated in all three groups, followed by Chitinophagia, Betaproteobacteria, and Alphaproteobacteria (Figure 3A). In addition, Shewanella, Chitinophaga, and Achromobacter were the primary genera found in three groups (Figure 3B). Notably, the top three genera found in the study showed the highest relative abundances in the high invasive group. The abundance in the moderate group was also higher than that of the low group (Table 2).
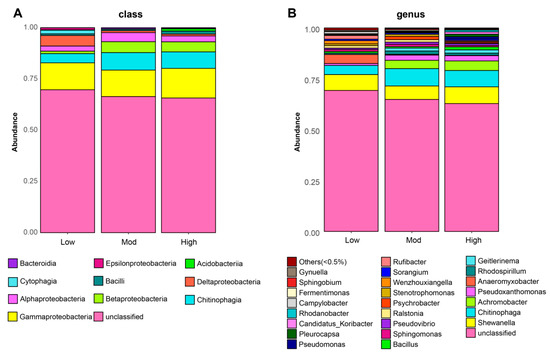
Figure 3.
Effects of moso bamboo invasion on denitrifying bacterial community composition. Barplots showing the denitrifying bacterial community composition at class (A) and genus (B) levels.

Table 2.
The relative abundance of dominant denitrifying bacteria in the rhizosphere of three sites.
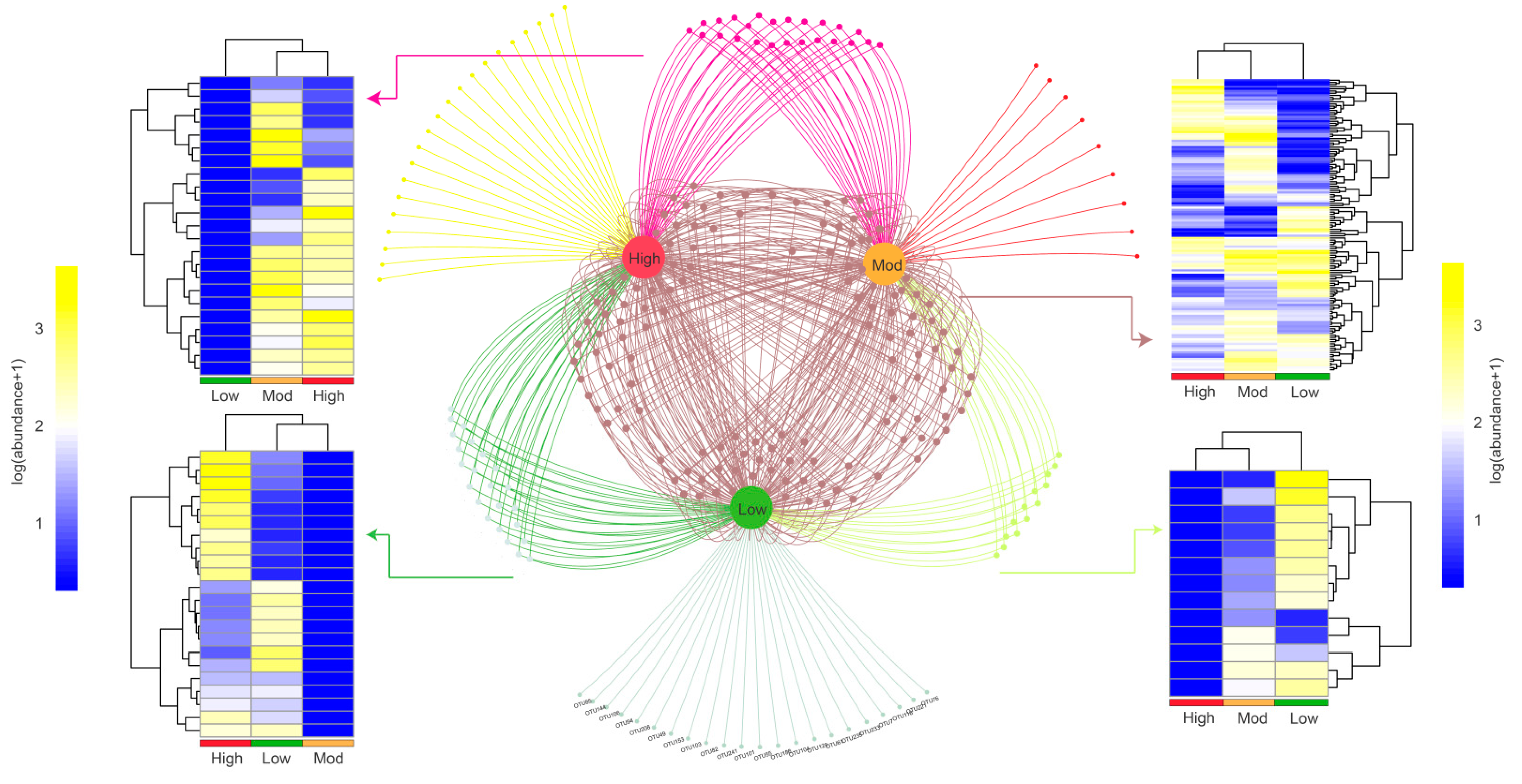
We then explored the shared and specific OTUs based on the Venn network (Figure 4). The results showed that a total of 22, 9, and 21 OTUs were identified as unique to low-, moderate-, and high-invasive groups, respectively. Additionally, a total of 136 OTUs existed in all samples, and most OTUs belonged to Chitinophaga (2.94%), Shewanella (2.21%), and Stenotrophomonas (2.21%). For those OTUs showed both in two groups, we found that 12, 22, and 24 OTUs were represented in low + moderate, low + high, and moderate + high groups, respectively. The clustering heatmap showed that moderate- and high-invasive groups were highly clustered, indicating that P. pubescens invasion could reshape the compositional structure of denitrifying bacteria.
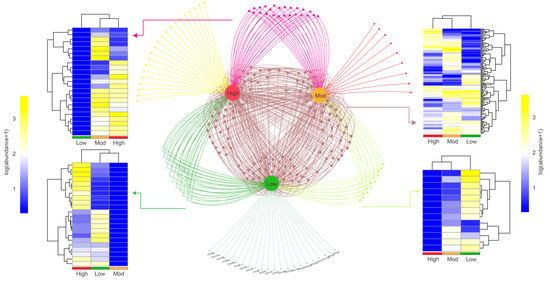
Figure 4.
OTUs association network showing interactions between different treatments. The nodes represent OTUs shared or specialized by low, moderate, and high moso bamboo invasions. Heatmap showing the clustering patterns and abundances of OTUs identified in the three groups.
3.3. Effects of Invasion on Denitrifying Bacterial Community Diversity
Diversity indexes, Ace, Chao1, Shannon, and Simpson were used to evaluate the diversity of soil denitrifying bacterial community in the rhizospheric soil of A. spinulosa. The mean coverage values in the three groups were larger than 0.999, indicating that the obtained OTUs could comprehensively evaluate the alterations of denitrifying bacterial diversity (Table 3). The Ace and Chao1 indexes in low- (190.18 and 191.16) and moderate- (205.49 and 204.50) invasive groups were lower than those of the high- (207.69 and 206.75) invasive group, and the two indexes increased with invasive degree. Similarly, the Shannon index was ranked as follows: high invasion (4.29) > moderate invasion (4.19) > low invasion (4.15). Contrarily, the ranking of the Simpson index was moderate invasion (0.030) > low invasion (0.029) > high invasion (0.024). We then analyzed beta diversity to further evaluate the similarities and differences of different invasive degrees on the denitrifying bacterial community. Weighted unifrac clustering trees based on taxonomy showed that moderate- and high-invasive groups were highly clustered at both the class and genus levels (Figure 5), which was consistent with the clustering heatmap described above.

Table 3.
Diversity index of various groups of denitrifying bacteria.
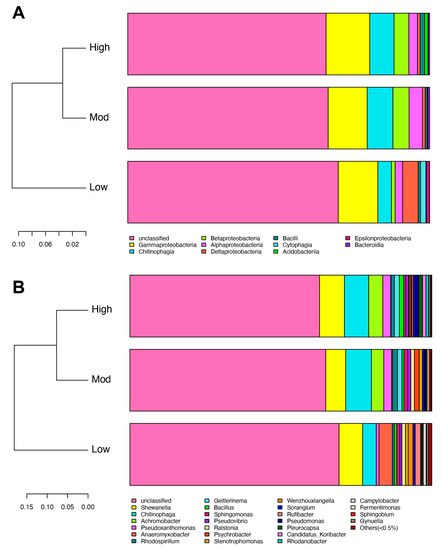
Figure 5.
Effects of moso bamboo invasion on denitrifying bacterial community beta diversity. Barplots showing the denitrifying bacterial community composition at the class (A) and genus (B) levels. Weighted unifrac tree showing the distances of the identified denitrifying bacteria.
3.4. Relationships of Denitrifying Bacteria with Soil Properties and the Growth of A. spinulosa
Spearman correlation analysis was performed on the soil properties and the denitrifying bacterial community abundance at the genus level (Table 4). The results showed that most denitrifying bacterial genera (e.g., Shewanella, Chitinophaga, and Achromobacter) were positively correlated with soil OM and AN but negatively correlated with soil pH and WC. Notably, soil OM was significantly altered by Shewanella (R2 = 1.000, p < 0.05), soil WC was altered by Chitinophaga (R2 = −1.000, p < 0.05), Achromobacter (R2 = −1.000, p < 0.05), and Anaeromyxobacter (R2 = −0.998, p < 0.05). When considering the growth of A. spinulosa, we found that Chitinophaga was distinctively correlated with height (R2 = −0.984, p < 0.05) and Sorangium was dramatically correlated with crown width (R2 = −0.999, p < 0.05).

Table 4.
The relationships of denitrifying bacteria with plant and soil properties based on Spearman rank correlation analysis.
4. Discussion
Plant invasion has distinctive impacts on plant characteristics and soil microbial communities [25,26]. Growing evidence has shown that microbial communities plays an essential role in inducing the successful expansion of invasive species. As a result, the physical and chemical properties of the colonized soil are altered, making it more suitable for the expansion of invasive species and further strengthening the situation of invasion. In addition, it has been demonstrated that soil N cycling can be an essential driver in forest succession [27,28]. Notably, altered total N content, ammonification rates (soil NH4+-N), and nitrification rates (soil NO3--N) following the P. pubescens expansion into broad-leaved forests have been shown by many researchers in various areas [29]. Hence, the objectives of this study were to evaluate the invasive effects and mechanisms of bamboo on an endangered plant’s habitat and demonstrate the role of denitrifying bacteria on the successful expansion of P. pubescens.
4.1. Successful Moso Bamboo Invasion Altered Root Biomass and Soil N Content
In this study, we identified three moso bamboo invasive forests with low (0.0008), moderate (0.6551), and high (0.9326) IMP values. Successful invasions dramatically altered the underground root biomass of both P. pubescens and A. spinulosa. Specifically, the root biomass of P. pubescens was increased with the degree of invasion at the soil layers of 10 cm, 20 cm, and 30 cm. Bamboo has a capacity for carbon sequestration, owing to its fast growth and dense rooting system, dominating the soil layer of 0~20 cm [15,30]. Hence, P. pubescens can spread to other forestlands through the growth of roots and stems in the underground part, so that root biomass can be significantly increased [31]. Our findings are consistent with a previous study that reported the total root mass of an invasive species, Imperata cylindrica (cogongrass), increased at the 5–15 cm depth after invading longleaf pine (Pinus palustris) forests [32,33]. Interestingly, the root biomass of A. spinulosa was maximal at depths of 10, 20, and 30 cm under moderate disturbance. The intermediate disturbance hypothesis suggests that high species diversity can be maintained under moderate disturbance, and our findings proved that moderate disturbance of P. pubescens increased the root biomass of A. spinulosa and might have indirectly contributed to the growth and survival of the colonized plant [34,35]. This observation is also in agreement with a previous study that reported that the biomass harvesting of invaded Populus tremuloides and Pinus Bansksiana forests was greatest at intermediate disturbance severities [36].
The expansion of invasive plants can affect other plants’ rhizosphere soil’s physical and chemical properties [37]. Studies have demonstrated that the soil in Chishui Alsophila National Nature Reserve is acidic soil that permits the expansion of moso bamboo. Additionally, suitable OM content and WC help moso bamboo easily invade the original habitat of A. spinulosa [15]. These soil properties were not altered under different invasive groups, indicating they might not be the primary driver of the expansion of moso bamboo. Soil N accumulation primarily relies on N input through root mortality and N output by decomposition [38]. Hence, the increased soil AN under high disturbance was mainly due to an extensive underground bamboo root system and microorganisms, which entailed a highly dynamic N cycling process. These findings agree with other reports that the fluxes of N in the bamboo-dominant forest were 37.5% larger than that of a neighboring secondary, evergreen, broadleaved forest [29]. In addition, increased N uptake could prevent primitive tree growth, seed germination, and seedling establishment, thereby threatening plant biodiversity [2,29]. Taken together, the successful invasion of P. pubescens distinctively altered the N content in the rhizosphere soil of A. spinulosa, making it more suitable for the expansion of P. pubescens.
4.2. Moso Bamboo Invasion Increased Denitrifying Bacterial Diversity and Reshaped Its Composition
This study found that the invasion of P. pubescens exerted a distinctive effect on denitrifying bacterial community diversity of A. spinulosa rhizospheric soil. Based on the Ace and Chao1 indexes, the study identified that the community richness under moderate and high invasion was greater than under low invasion. Similarly, high invasion harbored the highest denitrifying bacterial community diversity, according to the Shannon and Simpson indexes. Similar findings illustrated that moso bamboo invasion of broadleaf forests increased soil fungal alpha diversity and community composition closely involved in soil carbon and N production [8]. Additionally, high Rhynchelytrum repens invasion and Wedelia trilobata invasion increased the associated bacterial diversity involved in nutrients uptake, thereby permitting a successful expansion into the native habitat [39,40]. Besides, the weighted unifrac tree supported our viewpoint that successful moso bamboo invasion showed distinctive denitrifying bacterial community structure compared with the low-invasive group. This finding is in agreement with a previous report that illustrated reed canary grass invasion increased the ratio of denitrifying to total bacteria [41]. Hence, the increased abundance and diversity of denitrifying bacteria entailed a well-developed N cycling process, which was consistent with the observation about AN content described above.
The bacteria with denitrification ability are distributed in more than 50 genera, most of which belong to Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria [42]. Shewanella is a dominant denitrifying genus in Gammaproteobacteria and has been found in various habitats, such as oceans, lake sediments, and humid environments [43]. Hence, it is speculated that the denitrifying bacteria play essential roles in the Chishui Alsophila National Nature Reserve due to the mid-subtropical humid monsoon climate and well-developed river system [44]. Shewanella can maintain its own metabolism as a carbon source by fermentation products and can be utilized as electron acceptors, making it more viable in different ecosystem environments. In addition, Chitinophaga and Achromobacter were also the primary genera found in the study, and their abundances increased with invasive degree. Our observations are in line with a previous soybean study showing the abundance of Chitinophaga and Achromobacter changed with the addition of soybean residues and increased with exposure time [45]. Hence, the increased Chitinophaga and Achromobacter abundances were presumed to be due to the increasing P. pubescens litter residues, but further validation will strengthen and echo our hypothesis. In addition, the increase in denitrifying bacteria must necessarily be correlated with the increase in nitrogen-fixing bacteria, so future studies should investigate the alterations of nitrifying bacteria.
4.3. Relationships of Denitrifying Bacteria with Soil Properties and the Growth of A. spinulosa
The results of soil physical and chemical properties showed that the soil AN content was increased with P. pubescens invasion, which would inevitably promote soil N cycling and strengthen denitrification. In addition, denitrification, as an essential part of N cycling, can reduce nitrate in soil and alleviate the toxicity induced by nitrate accumulation [46,47]. According to the Spearman correlation analysis, the abundances of most denitrifying bacteria were positively correlated with soil OM and AN. The complex rhizomes and roots of P. pubescens could fill up the soil N and carbon pools, to some extent, and provide enough N and carbon sources for denitrifying bacteria, as described previously [21]. In addition, the decomposition of bamboo litter also increased the content of soluble carbon and nitrogen, which accelerated the nutrient cycling of soil, thereby increasing the abundance of denitrifying bacteria, such as Shewanella, and creating an invasive habitat for P. pubescens in turn. Interestingly, the abundances of Chitinophaga, Achromobacter, and Anaeromyxobacter were negatively correlated with WC. Growing evidence has shown that a high moisture content decreases rates of OM decomposition and that soil WC decreases microbial activity by altering the diffusion of soluble substrates, bacterial movement, and intracellular water potential [48,49]. Hence, soil WC might determine Chitinophaga, Achromobacter, and Anaeromyxobacter structures by controlling nutrient availability and cell movement, which is in line with a previous study by Zhou et al. [50], who pointed out that soil WC connecting soil particles may influence soil bacterial diversity patterns.
Tree height and crown width are important indicators for photosynthesis and the respiration of trees, directly influencing the viability and productivity of plants [51]. Evidence has been found that bamboo invasion reduced mean tree height (11.7 m) compared with native broadleaved forest (13.1 m) [29]. Contrarily, certain invasive vegetation removal can increase basal diameter, tree height, and phylogenetic diversity of wetland plant communities [52]. Our Spearman correlation analysis showed that tree height and crown width of A. spinulosa were negatively correlated with two denitrifying bacteria, Chitinophaga and Sorangium, respectively. It is reasonable to assume that the high abundances of Chitinophaga and Sorangium promoted active N cycling suitable for bamboo expansion, whereas the bamboo expansion might further threaten the growth of the invaded plant A. spinulosa. Hence, Chitinophaga and Sorangium might be the putative indicators for evaluating the effects of bamboo invasion on the growth of A. spinulosa.
To better understand this putative mechanism of invasion by P. pubescens, it would be a priority to compare its rhizospheric soil denitrifying bacterial communities in its native and non-native habitats in future research, which can assist in estimating the enemy expansion and enhanced mutualism hypotheses. To further explore the abiotic and biotic responses of A. spinulosa, investigations of the diversity and richness of other microorganisms (e.g., nirS and nosZ denitrifying bacteria, nitrifying bacteria, azotobacteria, arbuscular mycorrhizal fungi, and ectomycorrhizal fungi) in natural/rhizosphere soils should be included in our future studies, as they are also crucial in participating in soil N cycles and plant growth and survival.
5. Conclusions
Our study found that low, moderate, and high P. pubescens invasions altered the underground root biomass of P. pubescens and A. spinulosa. Interestingly, we also observed that moderate invasion might play a positive role in the growth and survival of A. spinulosa. We further identified that bamboo invasion increased the soil N cycling of A. spinulosa rhizospheric soil with the help of denitrifying bacteria (e.g., Shewanella, Chitinophaga, and Achromobacter). The enhanced N cycling process in the rhizosphere soil of A. spinulosa made it more suitable for the expansion of P. pubescens. Futhermore, our observations illustrated that two denitrifying bacteria genera might be critical indicators for evaluating the effects of bamboo invasion on the growth of A. spinulosa. To sum up, this study was the first to analyze the effects of moso bamboo on the community structure of denitrifying bacteria in the colonized plant’s rhizospheric soil, providing essential contributions for the understanding of bamboo’s invasive mechanisms and the habitat protection of the A.spinulosa community.
Author Contributions
Y.Z., H.D. and H.Q. conceived and designed the study. J.Z., C.X. and H.Z. helped with the experiment design. Y.Z. and H.Q. collected the samples. Y.Z. and H.Q. analyzed the data and prepared the figures and table. H.D. and J.Z. helped with the improvement of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Chongqing Technology Innovation and Application Development Special Key Project-Research and Application of Typical Damaged Ecosystem Restoration Technology in Nature Reserve (No. cstc2019jscx-tjsbX0005), the Chongqing Science and Technology Forest Project-Investigation of Invasive Species in Chongqing Daba Mountain-Wuling Mountain Biodiversity Conservation Priority Area and Research on Control Countermeasures (No. TD2021-4).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
Our deepest gratitude goes to the anonymous reviewer(s) for their professional suggestions and careful work that helped improve this paper substantially. We would like to thank Bo Lv (Hunan Normal University) and Jianping Xie (Southwest University) for their technical support.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Song, Q.N.; Ouyang, M.; Yang, Q.P.; Lu, H.; Yang, G.Y.; Chen, F.S.; Shi, J.M. Degradation of litter quality and decline of soil nitrogen mineralization after moso bamboo (Phyllostachys pubscens) expansion to neighboring broadleaved forest in subtropical China. Plant Soil 2016, 404, 113–124. [Google Scholar] [CrossRef]
- Song, Q.N.; Yang, Q.P.; Liu, J.; Yu, D.K.; He, Y.J. Effects of Phyllostachys edulis expansion on soil nitrogen mineralization and its availability in evergreen broadleaf forest. Chin. J. Appl. Ecol. 2013, 24, 338–344. [Google Scholar]
- Song, X.; Jiang, H.; Zhang, Z.; Zhou, G.; Zhang, S.; Peng, C. Interactive effects of elevated UV-B radiation and N deposition on decomposition of Moso bamboo litter. Soil Biol. Biochem. 2014, 69, 11–16. [Google Scholar] [CrossRef]
- Okutomi, K.; Shinoda, S.; Fukuda, H. Causal analysis of the invasion of broad-leaved forest by bamboo in Japan. J. Veg. Sci. 1996, 7, 723–728. [Google Scholar] [CrossRef]
- Larpkern, P.; Moe, S.R.; Totland, R. Bamboo dominance reduces tree regeneration in a disturbed tropical forest. Oecologia 2011, 165, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, L.; Deng, B.; Liu, Y.; Kong, F.; Huang, G.; Zou, Q.; Liu, Q.; Guo, X.; Fu, Y. Effects of moso bamboo (Phyllostachys edulis) invasions on soil nitrogen cycles depend on invasion stage and warming. Environ. Sci. Pollut. Res. 2017, 24, 24989–24999. [Google Scholar] [CrossRef]
- Zou, N.; Shi, W.; Hou, L.; Kronzucker, H.J.; Huang, L.; Gu, H.; Yang, Q.; Deng, G.; Yang, G. Superior growth, N uptake and NH4+ tolerance in the giant bamboo Phyllostachys edulis over the broad-leaved tree Castanopsis fargesii at elevated NH4+ may underlie community succession and favor the expansion of bamboo. Tree Physiol. 2020, 11, 1606–1622. [Google Scholar] [CrossRef]
- Li, Y.; Chang, Y.; Xu, S.; Guo, Q.; Gao, Z. Bamboo invasion of broadleaf forests altered soil fungal community closely linked to changes in soil organic C chemical composition and mineral N production. Plant Soil 2017, 418, 507–512. [Google Scholar] [CrossRef]
- Chang, E.H.; Chiu, C.Y. Changes in soil microbial community structure and activity in a cedar plantation invaded by moso bamboo. Appl. Soil Ecol. 2015, 91, 1–7. [Google Scholar] [CrossRef]
- Wu, J.S.; Jiang, P.K.; Wang, Z.L. The Effects of Phyllostachys pubescens Expansion on Soil Fertility in National Nature Reserve of Mount Tianmu. Acta Agric. Univ. Jiangxiensis 2008, 30, 689–692, (Chinese with English Abstract). [Google Scholar]
- Ma, Y.; Jiao, P.; Qi, Z.; Jiang, Z.; Guan, S. Characterization of the complete chloroplast genome of Alsophila spinulosa, an endangered species endemic to China. Mitochondrial DNA Part B 2020, 5, 2262–2263. [Google Scholar] [CrossRef]
- Smith, A.R.; Tryon, R.M.; Tryon, A.F. Ferns and allied plants with special reference to tropical America. Am. Fern J. 1983, 73, 94. [Google Scholar] [CrossRef]
- Wang, T.; Su, Y.; Li, Y. Population Genetic Variation in the Tree Fern Alsophila spinulosa (Cyatheaceae): Effects of Reproductive Strategy. PLoS ONE 2012, 7, e41780. [Google Scholar] [CrossRef]
- Su, Y.J.; Wang, T.; Bo, Z.; Jiang, Y.; Chen, G.P.; Ouyang, P.Y.; Sun, Y.F. Genetic differentiation of relictual populations of Alsophila spinulosa in southern China inferred from cpDNA trnL-F noncoding sequences. Mol. Phylogenetics Evol. 2005, 34, 323–333. [Google Scholar] [CrossRef]
- Qu, H.; Deng, H.; Sheng, L. Effects of Phyllostachys heterocycla expansion on morphological plasticity of endangered plant Alsophila spinulosa root system. Acta Ecol. Sin. 2020, 40, 1219–1227, (Chinese with English Abstract). [Google Scholar]
- Brettar, I.; Christen, R.; Höfle, M. Shewanella denitrificans sp. nov., a vigorously denitrifying bacterium isolated from the oxic-anoxic interface of the Gotland Deep in the central Baltic Sea. Int. J. Syst. Evol. Microbiol. 2002, 52, 2211. [Google Scholar]
- Kathiravan, V.; Krishnani, K.K. Pseudomonas aeruginosa and Achromobacter sp.: Nitrifying aerobic denitrifiers have a plasmid encoding for denitrifying functional genes. World J. Microbiol. Biotechnol. 2014, 30, 1187–1198. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Zhong, S.; Wang, T.; Ji, M.; Wu, X.; Zhang, X. Antibiotic-induced role interchange between rare and predominant bacteria retained the function of a bacterial community for denitrifying quinoline degradation. J. Appl. Microbiol. 2020, 129, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Velho, V.F.; Magnus, B.S.; Daudt, G.C.; Xavier, J.A.; Guimarães, L.B.; Costa, R.H.R. Effect of COD/N ratio on N2O production during nitrogen removal by aerobic granular sludge. Water Sci. Technol. 2017, 76, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Q.P.; Yu, D.K.; Song, Q.N.; Wang, B. Contribution of fine root to soil nutrient heterogeneity at two sides of the bamboo and broad-leaved forest interface. Chin. J. Plant Ecol. 2013, 37, 739–749. [Google Scholar] [CrossRef]
- Fukushima, K.; Usui, N.; Ogawa, R.; Tokuchi, N. Impacts of moso bamboo (Phyllostachys pubescens) invasion on dry matter and carbon and nitrogen stocks in a broad-leaved secondary forest located in Kyoto, western Japan. Plant Species Biol. 2015, 30, 81–95. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, F.S.; Wu, X.Q.; Luan, F.G.; Zhang, L.P.; Fang, X.M.; Wan, S.Z.; Hu, X.F.; Ye, J.R.; Daniel, C. Isolation and characterization of two phosphate-solubilizing fungi from rhizosphere soil of moso bamboo and their functional capacities when exposed to different phosphorus sources and pH environments. PLoS ONE 2018, 13, e0199625. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Agricultural Publisher: Beijing, China, 2000; pp. 355–356. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef]
- Craig, M.E.; Fraterrigo, J.M. Plant-microbial competition for nitrogen increases microbial activities and carbon loss in invaded soils. Oecologia 2017, 184, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, J.; He, J.Z.; Yu, F.H.; Ge, Y. Plant diversity enhances soil fungal diversity and microbial resistance to plant invasion. Appl. Environ. Microbiol. 2021, 87, e00251-21. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 1997, 385, 59–61. [Google Scholar] [CrossRef]
- Krozucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M.; Britto, D.T. Root ammonium transport efficiency as a determinant in forest colonization patterns: An hypothesis. Physiol. Plant. 2010, 117, 164–170. [Google Scholar] [CrossRef]
- Song, Q.N.; Hui, L.; Liu, J.; Yang, J.; Yang, Q.P. Accessing the impacts of bamboo expansion on NPP and N cycling in evergreen broadleaved forest in subtropical China. Sci. Rep. 2017, 7, 40383. [Google Scholar] [CrossRef]
- Schomakers, J.; Jien, S.H.; Lee, T.Y.; Huang, J.C.; Hseu, Z.Y.; Lin, Z.L.; Lee, L.C.; Hein, T.; Mentler, A.; Zehetner, F. Soil and biomass carbon re-accumulation after landslide disturbances. Geomorphology 2019, 288, 164–174. [Google Scholar] [CrossRef]
- Kleinhenz, V.; Midmore, D.J. Aspects of bamboo agronomy. Adv. Agron. 2001, 74, 99–153. [Google Scholar]
- Fahey, C.; Koyama, A.; Antunes, P.M.; Dunfield, K.E.; Flory, L. Plant communities mediate the interactive effects of invasion and drought on soil microbial communities. ISME J. 2020, 14, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Estrada, S.; Luke, F. Cogongrass (Imperata cylindrica) invasions in the US: Mechanisms, impacts, and threats to biodiversity-Science Direct. Glob. Ecol. Conserv. 2015, 3, 1–10. [Google Scholar]
- Catford, J.A.; Daehler, C.C.; Murphy, H.T.; Sheppard, A.W.; Hardesty, B.D.; Westcott, D.A.; Rejmánek, M.; Bellingham, P.J.; Pergl, J.; Horvitz, C.C. The intermediate disturbance hypothesis and plant invasions: Implications for species richness and management. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 231–241. [Google Scholar] [CrossRef]
- Tremolieres, M. Plant response strategies to stress and disturbance: The case of aquatic plants. J. Biosci. 2004, 29, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Kurth, V.J.; D’Amato, A.W.; Bradford, J.B.; Palik, B.J.; Looney, C.E. Assessing the ecological impacts of biomass harvesting along a disturbance severity gradient. Ecol. Appl. 2020, 30, e02042. [Google Scholar] [CrossRef] [PubMed]
- Philippe, H.; Claude, P.; Benoît, J. Rhizosphere: A new frontier for soil biogeochemistry. J. Geochem. Explor. 2006, 88, 210–213. [Google Scholar]
- Castro-Díez, P.; Godoy, O.; Alonso, A.; Gallardo, A.; Saldaña, A. What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecol. Lett. 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Wang, J.; Chen, W.; Chen, X.; Zhu, H. Effect of invasive degree of Rhynchelytrum repens on microorganisms in rhizosphere soil. J. Northwest A F Univ. -Nat. Sci. Ed. 2017, 45, 165–172, (Chinese with English Abstract). [Google Scholar]
- Si, C.C.; Dai, Z.C.; Lin, Y.; Qi, S.S.; Huang, P.; Miao, S.L.; Du, D.L. Local adaptation and phenotypic plasticity both occurred in Wedelia trilobata invasion across a tropical island. Biol. Invasions 2014, 16, 2323–2337. [Google Scholar] [CrossRef]
- Gordon, B.A.; Lenhart, C.; LaPara, T.M. A comparison of nitrate removal and denitrifying bacteria populations among three wetland plant communities. J. Environ. Qual. 2020, 49, 210–219. [Google Scholar] [CrossRef]
- Verbaendert, I.; De Vos, P.; Boon, N.; Heylen, K. Denitrification in Gram-positive bacteria: An underexplored trait. Biochem. Soc. Trans. 2011, 39, 254–258. [Google Scholar] [CrossRef][Green Version]
- Middleton, S.S.; Latmani, R.B.; Mackey, M.R.; Ellisman, M.H.; Tebo, B.M.; Criddle, C.S. Cometabolism of Cr(VI) by Shewanella oneidensis MR-1 produces cell-associated reduced chromium and inhibits growth. Biotechnol. Bioeng. 2003, 83, 627–637. [Google Scholar] [CrossRef]
- Qin, L.; Hongping, D.; Zongfeng, L.; Sheng, L.; Qiulin, L.; Dongping, N. Characteristics of plant community in the Guizhou Chishui Alsophila spinulata National Nature Reserve, southwestern China. J. Beijing For. Univ. 2019, 41, 19–31, (Chinese with English Abstract). [Google Scholar]
- Lian, T.X. The turn over of Photosynthetic Carbon of Soybean and Relevant Bacterial Community Characteristics in the Mollisols. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2016. (In Chinese). [Google Scholar]
- Anjana, S.U.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A Review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Noguchi, K. Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in Arabidopsis thaliana Shoots. Plant Cell Physiol. 2012, 53, 577–591. [Google Scholar]
- Killham, K.; Amato, M.; Ladd, J.N. Effect of substrate location in soil and soil pore-water regime on carbon turnover. Soil Biol. Biochem. 1993, 25, 57–62. [Google Scholar] [CrossRef]
- Schjnning, P.; Thomsen, I.K.; Moldrup, P.; Christensen, B.T. Linking soil microbial activity to water- and air-phase contents and diffusivities. Soil Sci. Soc. Am. J. 2003, 67, 156–165. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, B.; Treves, D.S.; Wu, L.Y.; Marsh, T.L.; O’Neill, R.V.; Palumbo, A.V.; Tiedje, J.M. Spatial and resource factors influencing high microbial diversity in soil. Appl. Environ. Microbiol. 2002, 68, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Biging, G.S.; Matthias, D. A comparison of distance-dependent competition measures for height and basal area growth of individual conifer trees. Forestence 1992, 38, 695–720. [Google Scholar]
- Lishawa, S.C.; Lawrence, B.A.; Albert, D.A.; Larkin, D.J.; Tuchman, N.C. Invasive species removal increases species and phylogenetic diversity of wetland plant communities. Ecol. Evol. 2019, 9, 6231–6244. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).