Vitreoscilla Haemoglobin: A Tool to Reduce Overflow Metabolism
Abstract
:1. Overflow Metabolism and Bioprocessing
1.1. Causes of Overflow Metabolism
1.1.1. Metabolic Imbalance
1.1.2. Proteome Allocation
1.1.3. Molecular Crowding
2. Vitreoscilla Haemoglobin as a Tool to Reduce Overflow Metabolism
2.1. Aerobic Expression of Vitreoscilla Hemoglobin
2.2. Metabolic Consequences of Aerobic Expression of Vitreoscilla Haemoglobin
Author Contributions
Funding
Conflicts of Interest
References
- PORTER, J.R. Louis PASTEUR; achievements and disappointments, 1861. Bacteriol. Rev. 1961, 25, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, W.M. Lactic acid in amphibian muscle. J. Physiol. 1907, 35, 247–309. [Google Scholar] [CrossRef]
- Warburg, O.; Minami, S. Versuche an überlebendem carcinom-gewebe. Klin. Wochenschr. 1923, 2, 776–777. [Google Scholar] [CrossRef]
- Crabtree, H.G. The carbohydrate metabolism of certain pathological overgrowths. Biochem. J. 1928, 22, 1289–1298. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, A. Overflow Metabolism: From Yeast to Marathon Runners, 1st ed.; Academic Press: London, UK, 2018. [Google Scholar]
- Holms, W.H. The central metabolic pathways of Escherichia coli: Relationship between flux and control at a branch point, efficiency of conversion to biomass, and excretion of acetate. Curr. Top Cell. Regul. 1986, 28, 69–105. [Google Scholar] [CrossRef]
- De Mey, M.; De Maeseneire, S.; Soetaert, W.; Vandamme, E. Minimizing acetate formation in E. coli fermentations. J. Ind. Microbiol. Biotechnol. 2007, 34, 689–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiteman, M.A.; Altman, E. Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol. 2006, 24, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Liu, Y.; Lv, X.; Li, J.; Du, G.; Liu, L. Combinatorial pathway enzyme engineering and host engineering overcomes pyruvate overflow and enhances overproduction of N-acetylglucosamine in Bacillus subtilis. Microb. Cell Fact. 2019, 18, 1. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Vijayasankaran, N.; Autsen, J.; Santuray, R.; Hudson, T.; Amanullah, A.; Li, F. Comparative metabolite analysis to understand lactate metabolism shift in Chinese hamster ovary cell culture process. Biotechnol. Bioeng. 2012, 109, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.A.; Trinh, C.T. Overflow metabolism and growth cessation in Clostridium thermocellum DSM1313 during high cellulose loading fermentations. Biotechnol. Bioeng. 2017, 114, 2592–2604. [Google Scholar] [CrossRef]
- Wittmann, C.; Kiefer, P.; Zelder, O. Metabolic fluxes in Corynebacterium glutamicum during lysine production with sucrose as carbon source. Appl. Environ. Microbiol. 2004, 70, 7277–7287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paczia, N.; Nilgen, A.; Lehmann, T.; Gätgens, J.; Wiechert, W.; Noack, S. Extensive exometabolome analysis reveals extended overflow metabolism in various microorganisms. Microb. Cell Fact. 2012, 11, 122. [Google Scholar] [CrossRef] [Green Version]
- Nanda, P.; Patra, P.; Das, M.; Ghosh, A. Reconstruction and analysis of genome-scale metabolic model of weak Crabtree positive yeast Lachancea kluyveri. Sci. Rep. 2020, 10, 16314. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.; Peter, V.; Meinert, S.; Kornfeld, G.; Hardiman, T.; Wiechert, W.; Noack, S. Simultaneous utilization of glucose and gluconate in Penicillium chrysogenum during overflow metabolism. Biotechnol. Bioeng. 2013, 110, 3235–3243. [Google Scholar] [CrossRef]
- Nocon, J.; Steiger, M.G.; Pfeffer, M.; Sohn, S.B.; Kim, T.Y.; Maurer, M.; Rußmayer, H.; Pflügl, S.; Ask, M.; Haberhauer-Troyer, C.; et al. Model based engineering of Pichia pastoris central metabolism enhances recombinant protein production. Metab. Eng. 2014, 24, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Majewski, R.A.; Domach, M.M. Simple constrained-optimization view of acetate overflow in E. coli. Biotechnol. Bioeng. 1990, 35, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Kayser, A.; Weber, J.; Hecht, V.; Rinas, U. Metabolic flux analysis of Escherichia coli in glucose-limited continuous culture. I. Growth-rate-dependent metabolic efficiency at steady state. Microbiology 2005, 151, 693–706. [Google Scholar] [CrossRef] [Green Version]
- Vemuri, G.N.; Altman, E.; Sangurdekar, D.P.; Khodursky, A.B.; Eiteman, M.A. Overflow metabolism in Escherichia coli during steady-state growth: Transcriptional regulation and effect of the redox ratio. Appl. Environ. Microbiol. 2006, 72, 3653–3661. [Google Scholar] [CrossRef] [Green Version]
- Yeo, H.C.; Hong, J.; Lakshmanan, M.; Lee, D.Y. Enzyme capacity-based genome scale modelling of CHO cells. Metab. Eng. 2020, 60, 138–147. [Google Scholar] [CrossRef]
- Shojaosadati, S.A.; Kolaei, S.M.V.; Babaeipour, V.; Farnoud, A.M. Recent advances in high cell density cultivation for production of recombinant protein. Iran. J. Biotechnol. 2008, 6, 63–84. [Google Scholar]
- De Anda, R.; Lara, A.R.; Hernández, V.; Hernández-Montalvo, V.; Gosset, G.; Bolívar, F.; Ramírez, O.T. Replacement of the glucose phosphotransferase transport system by galactose permease reduces acetate accumulation and improves process performance of Escherichia coli for recombinant protein production without impairment of growth rate. Metab. Eng. 2006, 8, 281–290. [Google Scholar] [CrossRef]
- Fuentes, L.G.; Lara, A.R.; Martínez, L.M.; Ramírez, O.T.; Martínez, A.; Bolívar, F.; Gosset, G. Modification of glucose import capacity in Escherichia coli: Physiologic consequences and utility for improving DNA vaccine production. Microb. Cell Fact. 2013, 12, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrete, A.; Majdalani, N.; Phue, J.N.; Shiloach, J. Reducing acetate excretion from E. coli K-12 by over-expressing the small RNA SgrS. New Biotechnol. 2013, 30, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Bäcklund, E.; Markland, K.; Larsson, G. Cell engineering of Escherichia coli allows high cell density accumulation without fed-batch process control. Bioprocess Biosyst. Eng. 2008, 31, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wlaschin, K.F.; Hu, W.S. Engineering cell metabolism for high-density cell culture via manipulation of sugar transport. J. Biotechnol. 2007, 131, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Veit, A.; Polen, T.; Wendisch, V.F. Global gene expression analysis of glucose overflow metabolism in Escherichia coli and reduction of aerobic acetate formation. Appl. Microbiol. Biotechnol. 2007, 74, 406–421. [Google Scholar] [CrossRef]
- Vemuri, G.N.; Eiteman, M.A.; McEwen, J.E.; Olsson, L.; Nielsen, J. Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2007, 104, 2402–2407. [Google Scholar] [CrossRef] [Green Version]
- Bulté, D.B.; Palomares, L.A.; Parra, C.G.; Martínez, J.A.; Contreras, M.A.; Noriega, L.G.; Ramírez, O.T. Overexpression of the mitochondrial pyruvate carrier reduces lactate production and increases recombinant protein productivity in CHO cells. Biotechnol. Bioeng. 2020, 117, 2633–2647. [Google Scholar] [CrossRef]
- Peebo, K.; Valgepea, K.; Maser, A.; Nahku, R.; Adamberg, K.; Vilu, R. Proteome reallocation in Escherichia coli with increasing specific growth rate. Mol. Biosyst. 2015, 11, 1184–1193. [Google Scholar] [CrossRef]
- Basan, M.; Hui, S.; Okano, H.; Zhang, Z.; Shen, Y.; Williamson, J.R.; Hwa, T. Overflow metabolism in Escherichia coli results from efficient proteome allocation. Nature 2015, 528, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Yang, A. Modelling overflow metabolism in Escherichia coli with flux balance analysis incorporating differential proteomic efficiencies of energy pathways. BMC Syst. Biol. 2019, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nielsen, J. Energy metabolism controls phenotypes by protein efficiency and allocation. Proc. Natl. Acad. Sci. USA 2019, 116, 17592–17597. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Yang, A. Quantification of proteomic and metabolic burdens predicts growth retardation and overflow metabolism in recombinant Escherichia coli. Biotechnol. Bioeng. 2019, 116, 1484–1495. [Google Scholar] [CrossRef]
- Chen, Y.; van Pelt-KleinJan, E.; van Olst, B.; Douwenga, S.; Boeren, S.; Bachmann, H.; Molenaar, D.; Nielsen, J.; Teusink, B. Proteome constraints reveal targets for improving microbial fitness in nutrient-rich environments. Mol. Syst. Biol. 2021, 17, e10093. [Google Scholar] [CrossRef]
- Liu, J.K.; Lloyd, C.; Al-Bassam, M.M.; Ebrahim, A.; Kim, J.N.; Olson, C.; Aksenov, A.; Dorrestein, P.; Zengler, K. Predicting proteome allocation, overflow metabolism, and metal requirements in a model acetogen. PLoS Comput. Biol. 2019, 15, e1006848. [Google Scholar] [CrossRef] [PubMed]
- Lastiri-Pancardo, G.; Mercado-Hernández, J.S.; Kim, J.; Jiménez, J.I.; Utrilla, J. A quantitative method for proteome reallocation using minimal regulatory interventions. Nat. Chem. Biol. 2020, 16, 1026–1033. [Google Scholar] [CrossRef]
- de la Cruz, M.; Ramírez, E.A.; Sigala, J.C.; Utrilla, J.; Lara, A.R. Plasmid DNA Production in Proteome-Reduced. Microorganisms 2020, 8, 1444. [Google Scholar] [CrossRef]
- Brown, G.C. Total cell protein concentration as an evolutionary constraint on the metabolic control distribution in cells. J. Theor. Biol. 1991, 153, 195–203. [Google Scholar] [CrossRef]
- Vazquez, A.; Oltvai, Z.N. Macromolecular crowding explains overflow metabolism in cells. Sci. Rep. 2016, 6, 31007. [Google Scholar] [CrossRef] [Green Version]
- Beg, Q.K.; Vazquez, A.; Ernst, J.; de Menezes, M.A.; Bar-Joseph, Z.; Barabási, A.L.; Oltvai, Z.N. Intracellular crowding defines the mode and sequence of substrate uptake by Escherichia coli and constrains its metabolic activity. Proc. Natl. Acad. Sci. USA 2007, 104, 12663–12668. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, A.; Beg, Q.K.; Demenezes, M.A.; Ernst, J.; Bar-Joseph, Z.; Barabási, A.L.; Boros, L.G.; Oltvai, Z.N. Impact of the solvent capacity constraint on E. coli metabolism. BMC Syst. Biol. 2008, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, A.; Liu, J.; Zhou, Y.; Oltvai, Z.N. Catabolic efficiency of aerobic glycolysis: The Warburg effect revisited. BMC Syst. Biol. 2010, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hoek, M.J.; Merks, R.M. Redox balance is key to explaining full vs. partial switching to low-yield metabolism. BMC Syst. Biol. 2012, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, K.; Vemuri, G.N.; Mahadevan, R. Economics of membrane occupancy and respiro-fermentation. Mol. Syst. Biol. 2011, 7, 500. [Google Scholar] [CrossRef] [PubMed]
- Szenk, M.; Dill, K.A.; de Graff, A.M.R. Why Do Fast-Growing Bacteria Enter Overflow Metabolism? Testing the Membrane Real Estate Hypothesis. Cell Syst. 2017, 5, 95–104. [Google Scholar] [CrossRef]
- Stark, B.C.; Pagilla, K.R.; Dikshit, K.L. Recent applications of Vitreoscilla hemoglobin technology in bioproduct synthesis and bioremediation. Appl. Microbiol. Biotechnol. 2015, 99, 1627–1636. [Google Scholar] [CrossRef]
- Efiok, B.J.; Webster, D.A. A cytochrome that can pump sodium ion. Biochem. Biophys. Res. Commun. 1990, 173, 370–375. [Google Scholar] [CrossRef]
- Stark, B.C.; Dikshit, K.L.; Pagilla, K.R. The Biochemistry of Vitreoscilla hemoglobin. Comput. Struct. Biotechnol. J. 2012, 3, e201210002. [Google Scholar] [CrossRef] [Green Version]
- Stark, B.C.; Dikshit, K.L.; Pagilla, K.R. Recent advances in understanding the structure, function, and biotechnological usefulness of the hemoglobin from the bacterium Vitreoscilla. Biotechnol. Lett. 2011, 33, 1705–1714. [Google Scholar] [CrossRef]
- Yu, F.; Zhao, X.; Wang, Z.; Liu, L.; Yi, L.; Zhou, J.; Li, J.; Chen, J.; Du, G. Recent Advances in the Physicochemical Properties and Biotechnological Application of. Microorganisms 2021, 9, 1445. [Google Scholar] [CrossRef]
- Webster, D.A.; Dikshit, K.L.; Pagilla, K.R.; Stark, B.C. The Discovery of Vitreoscilla Hemoglobin and Early Studies on Its Biochemical Functions, the Control of Its Expression, and Its Use in Practical Applications. Microorganisms 2021, 9, 1637. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, R.P.; Dikshit, K.L.; Liu, Y.X.; Webster, D.A. The bacterial hemoglobin from Vitreoscilla can support the aerobic growth of Escherichia coli lacking terminal oxidases. Arch. Biochem. Biophys. 1992, 293, 241–245. [Google Scholar] [CrossRef]
- Dikshit, K.L.; Webster, D.A. Cloning, characterization and expression of the bacterial globin gene from Vitreoscilla in Escherichia coli. Gene 1988, 70, 377–386. [Google Scholar] [CrossRef]
- Kallio, P.T.; Kim, D.J.; Tsai, P.S.; Bailey, J.E. Intracellular expression of Vitreoscilla hemoglobin alters Escherichia coli energy metabolism under oxygen-limited conditions. Eur. J. Biochem. 1994, 219, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Jaén, K.E.; Velazquez, D.; Delvigne, F.; Sigala, J.C.; Lara, A.R. Engineering, E. coli for improved microaerobic pDNA production. Bioprocess Biosyst. Eng. 2019, 42, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Khosla, C.; Bailey, J.E. Evidence for partial export of Vitreoscilla hemoglobin into the periplasmic space in Escherichia coli. Implications for protein function. J. Mol. Biol. 1989, 210, 79–89. [Google Scholar] [CrossRef]
- Ramandeep; Hwang, K.W.; Raje, M.; Kim, K.J.; Stark, B.C.; Dikshit, K.L.; Webster, D.A. Vitreoscilla hemoglobin. Intracellular localization and binding to membranes. J. Biol. Chem. 2001, 276, 24781–24789. [Google Scholar] [CrossRef] [Green Version]
- Juárez, M.; González-De la Rosa, C.H.; Sigala, J.C.; Lara, A.R. Effect of Vitreoscilla hemoglobin on recombinant protein expression and energy metabolism of CHO cells. Rev. Mex. Ing. Quím. 2021, 20, 281–288. [Google Scholar] [CrossRef]
- Ouyang, P.; Wang, H.; Hajnal, I.; Wu, Q.; Guo, Y.; Chen, G.Q. Increasing oxygen availability for improving poly(3-hydroxybutyrate) production by Halomonas. Metab. Eng. 2018, 45, 20–31. [Google Scholar] [CrossRef]
- Suen, Y.L.; Tang, H.; Huang, J.; Chen, F. Enhanced production of fatty acids and astaxanthin in Aurantiochytrium sp. by the expression of Vitreoscilla hemoglobin. J. Agric. Food Chem. 2014, 62, 12392–12398. [Google Scholar] [CrossRef]
- Juárez, M.; González-De la Rosa, C.H.; Memún, E.; Sigala, J.C.; Lara, A.R. Aerobic expression of Vitreoscilla hemoglobin improves the growth performance of CHO-K1 cells. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J.; Wei, X.X.; Ouyang, S.P.; Wu, Q.; Chen, G.Q. Microbial production of L -glutamate and L -glutamine by recombinant Corynebacterium glutamicum harboring Vitreoscilla hemoglobin gene vgb. Appl. Microbiol. Biotechnol. 2008, 77, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.B.; Guo, X.L.; Zhang, M.L.; Huang, Q.G.; Qi, F.; Huang, J.Z. Enhancement of l-phenylalanine production in Escherichia coli by heterologous expression of Vitreoscilla hemoglobin. Biotechnol. Appl. Biochem. 2018, 65, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Pablos, T.E.; Sigala, J.C.; Le Borgne, S.; Lara, A.R. Aerobic expression of Vitreoscilla hemoglobin efficiently reduces overflow metabolism in Escherichia coli. Biotechnol. J. 2014, 9, 791–799. [Google Scholar] [CrossRef]
- Lara, A.R.; Galindo, J.; Jaén, K.E.; Juárez, M.; Sigala, J.C. Physiological Response of. J. Microbiol. Biotechnol. 2020, 30, 1592–1596. [Google Scholar] [CrossRef]
- Liu, D.; Ke, X.; Hu, Z.C.; Zheng, Y.G. Improvement of pyrroloquinoline quinone-dependent d-sorbitol dehydrogenase activity from Gluconobacter oxydans via expression of Vitreoscilla hemoglobin and regulation of dissolved oxygen tension for the biosynthesis of 6-(N-hydroxyethyl)-amino-6-deoxy-α-l-sorbofuranose. J. Biosci. Bioeng. 2021, 131, 518–524. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Y.; Cui, Q.; Song, X. Expression of Vitreoscilla hemoglobin enhances production of arachidonic acid and lipids in Mortierella alpina. BMC Biotechnol. 2017, 17, 68. [Google Scholar] [CrossRef]
- Suthar, D.H.; Chattoo, B.B. Expression of Vitreoscilla hemoglobin enhances growth and levels of alpha-amylase in Schwanniomyces occidentalis. Appl. Microbiol. Biotechnol. 2006, 72, 94–102. [Google Scholar] [CrossRef]
- Wu, J.M.; Hsu, T.A.; Lee, C.K. Expression of the gene coding for bacterial hemoglobin improves beta-galactosidase production in a recombinant Pichia pastoris. Biotechnol. Lett. 2003, 25, 1457–1462. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Shen, X.; Ke, F.; Zhao, H.; Liu, Y.; Xu, L.; Yan, Y. Intracellular expression of Vitreoscilla hemoglobin improves production of Yarrowia lipolytica lipase LIP2 in a recombinant Pichia pastoris. Enzyme. Microb. Technol. 2012, 50, 22–28. [Google Scholar] [CrossRef]
- Bhave, S.L.; Chattoo, B.B. Expression of vitreoscilla hemoglobin improves growth and levels of extracellular enzyme in Yarrowia lipolytica. Biotechnol. Bioeng. 2003, 84, 658–666. [Google Scholar] [CrossRef]
- Huberts, D.H.; Niebel, B.; Heinemann, M. A flux-sensing mechanism could regulate the switch between respiration and fermentation. FEMS Yeast Res. 2012, 12, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Pinu, F.R.; Granucci, N.; Daniell, J.; Han, T.L.; Carneiro, S.; Rocha, I.; Nielsen, J.; Villas-Boas, S.G. Metabolite secretion in microorganisms: The theory of metabolic overflow put to the test. Metabolomics 2018, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, H.; Zhao, G.; Caiyin, Q.; Qiao, J. Redox cofactor engineering in industrial microorganisms: Strategies, recent applications and future directions. J. Ind. Microbiol. Biotechnol. 2018, 45, 313–327. [Google Scholar] [CrossRef]
- Niebel, B.; Leupold, S.; Heinemann, M. An upper limit on Gibbs energy dissipation governs cellular metabolism. Nat. Metab. 2019, 1, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millard, P.; Enjalbert, B.; Uttenweiler-Joseph, S.; Portais, J.C.; Létisse, F. Control and regulation of acetate overflow in Escherichia coli. Elife 2021, 10, e63661. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Marinari, E.; De Martino, A. A yield-cost tradeoff governs Escherichia coli’s decision between fermentation and respiration in carbon-limited growth. NPJ Syst. Biol. Appl. 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Groot, D.H.; Lischke, J.; Muolo, R.; Planqué, R.; Bruggeman, F.J.; Teusink, B. The common message of constraint-based optimization approaches: Overflow metabolism is caused by two growth-limiting constraints. Cell Mol. Life Sci. 2020, 77, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Matsuoka, Y. Redox rebalance against genetic perturbations and modulation of central carbon metabolism by the oxidative stress regulation. Biotechnol. Adv. 2019, 37, 107441. [Google Scholar] [CrossRef]
- Cheng, C.; O’Brien, E.J.; McCloskey, D.; Utrilla, J.; Olson, C.; LaCroix, R.A.; Sandberg, T.E.; Feist, A.M.; Palsson, B.O.; King, Z.A. Laboratory evolution reveals a two-dimensional rate-yield tradeoff in microbial metabolism. PLoS Comput. Biol. 2019, 15, e1007066. [Google Scholar] [CrossRef] [Green Version]
- Tsai, P.S.; Nägeli, M.; Bailey, J.E. Intracellular expression of Vitreoscilla hemoglobin modifies microaerobic Escherichia coli metabolism through elevated concentration and specific activity of cytochrome o. Biotechnol. Bioeng. 1996, 49, 151–160. [Google Scholar] [CrossRef]
- Puustinen, A.; Finel, M.; Haltia, T.; Gennis, R.B.; Wikström, M. Properties of the two terminal oxidases of Escherichia coli. Biochemistry 1991, 30, 3936–3942. [Google Scholar] [CrossRef]
- Orth, J.D.; Conrad, T.M.; Na, J.; Lerman, J.A.; Nam, H.; Feist, A.M.; Palsson, B. A comprehensive genome-scale reconstruction of Escherichia coli metabolism--2011. Mol. Syst. Biol. 2011, 7, 535. [Google Scholar] [CrossRef]
- Schellenberger, J.; Que, R.; Fleming, R.; Thiele, I.; Orth, J.; Feist, A.; Zielinski, D.; Bordbar, A.; Lewis, N.; Rahmanian, S.; et al. Quantitative prediction of cellular metabolism with constraint-based models: The COBRA Toolbox v2.0. Nat. Protoc. 2011, 6, 1290–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taymaz-Nikerel, H.; Borujeni, A.E.; Verheijen, P.J.; Heijnen, J.J.; van Gulik, W.M. Genome-derived minimal metabolic models for Escherichia coli MG1655 with estimated in vivo respiratory ATP stoichiometry. Biotechnol. Bioeng. 2010, 107, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Kalnenieks, U.; Balodite, E.; Rutkis, R. Metabolic Engineering of Bacterial Respiration: High vs. Low P/O and the Case of. Front. Bioeng. Biotechnol. 2019, 7, 327. [Google Scholar] [CrossRef] [Green Version]
- Fischer, E.; Zamboni, N.; Sauer, U. High-throughput metabolic flux analysis based on gas chromatography-mass spectrometry derived 13C constraints. Anal. Biochem. 2004, 325, 308–316. [Google Scholar] [CrossRef]
- Carlson, R.; Srienc, F. Fundamental Escherichia coli biochemical pathways for biomass and energy production: Creation of overall flux states. Biotechnol. Bioeng. 2004, 86, 149–162. [Google Scholar] [CrossRef]
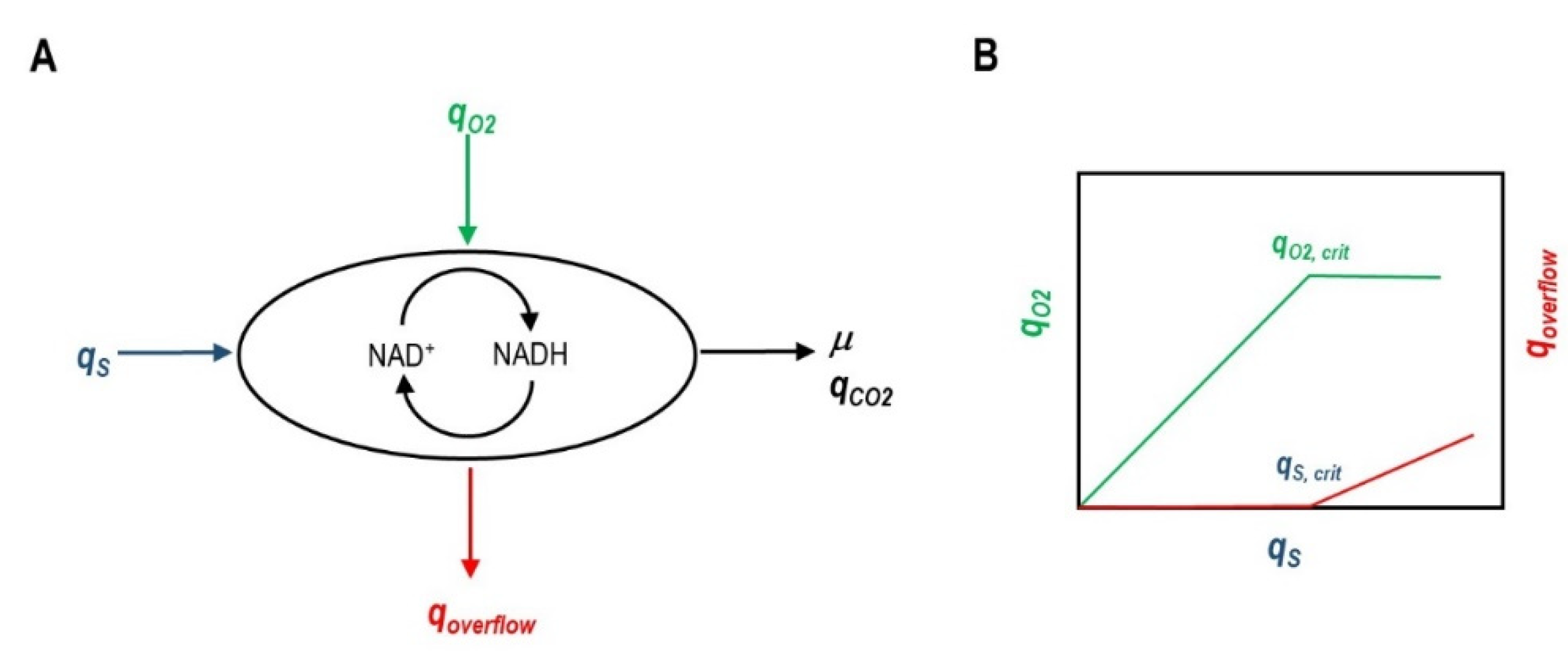

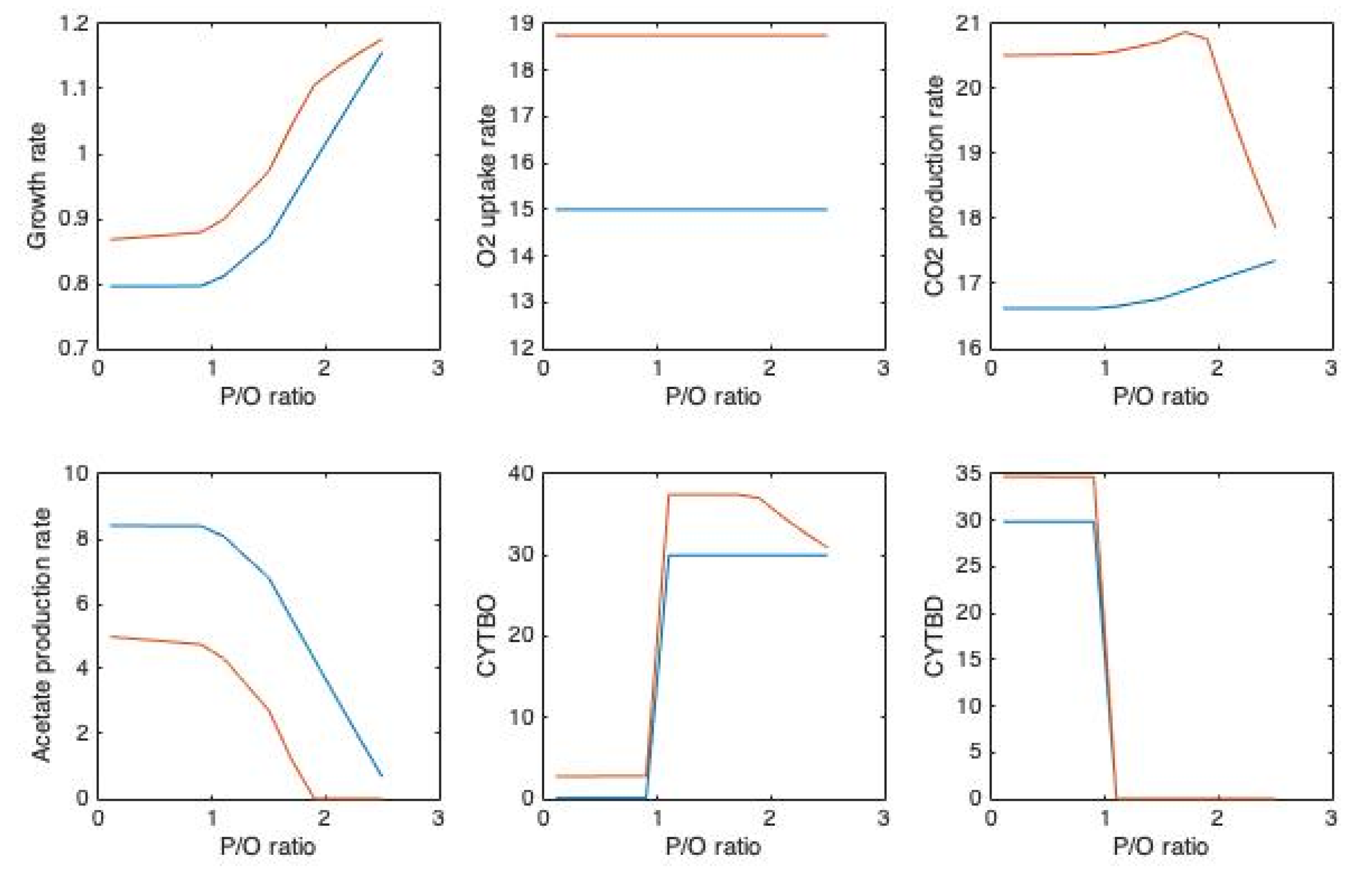
| Organism | Main Overflow Metabolites |
|---|---|
| Bacillus subtilis | Acetoin, acetate [9] |
| CHO cells | Lactate [10] |
| Clostridium thermocellum | Lactate, acetate, ethanol [11] |
| Corynebacterium glutamicum | Dihydroxyacetone, acetate [12] |
| Escherichia coli | Acetate [13] |
| Lachance kluyveri | Ethylacetate [14] |
| Saccharomyces cerevisiae | Ethanol [13] |
| Penicillium chrysogenum | Gluconate [15] |
| Pichia pastoris | Ethanol, acetate [16] |
| Organism | Reported Effect |
|---|---|
| Aurantiochytrium sp. | 44% higher total fatty acid and 9-fold higher astaxanthin contents [61] |
| CHO cells | 40–100% increase of tissue plasminogen activator production [62] μ and biomass yields increase, lactate production per cell decreased by 40% [62] NAD+/NADH ratio and ATP cell content decreased, NADP+/NADPH ratio increased [59] |
| Corynebacterium glutamicum | Synthesis of biomass increased 10% and L-glutamate production increased 30% [63] |
| Escherichia coli | Increased qO2, μ and L-phenylalanine production [64] 60% decrease of acetate accumulation when VHb was expressed from a plasmid. Two-fold increase of plasmid DNA yield from biomass in strain W3110 [65] 37% and 50% reduction in acetate production rate in strains W3110 and BL21, respectively, when VHb was expressed from the chromosome. Different impact on the expression of genes from the TCA cycle and cytochromes, depending on the strain (W3110 or BL21) [66] |
| Gluconobacter oxydans | 8% increase of volumetric oxidation activity of N-2-hydroxyethyl glucamine [67] |
| Mortierella alpina | Increased μ and 1.6-fold higher arachidonic acid production [68] |
| Schwanniomyces occidentalis | μ and alpha-amylase production increased [69] |
| Pichia pastoris | 4-fold higher β-galactosidase activity [70] 31.5% higher expression of Y. lipolytica LIP2 lipase [71] |
| Yarrowia lipolytica | 23% higher μ, 2.6-fold higher biomass formation, 92% higher RNase production [72] |
| Name of the Reaction | Stoichiometry | |
|---|---|---|
| NADH dehydrogenase | NADH16pp | (2×P/O+1) h[c] + nadh[c] + q8[c] → nad[c] + q8h2[c] +(2×P/O) h[p] |
| FADH dehydrogenase | FDH4pp | (2×P/O+1) h[c] + q8[c] + for[p] → q8h2[c] + co2[p] + (2×P/O) h[p] |
| Cytochrome oxidase bd-type | CYTBD2pp | (P/O) h[c] + 0.5 o2[c] + mql8[c] → h2o[c] + mqn8[c] + (P/O) h[p] |
| Cytochrome oxidase bd-type | CYTBDpp | (P/O) h[c] + 0.5 o2[c] + q8h2[c] → h2o[c] + q8[c] + (P/O) h[p] |
| Cytochrome oxidase bo-type | CYTBO3_4pp | (2×P/O) h[c] + 0.5 o2[c] + q8h2[c] → h2o[c] + q8[c] +(2×P/O) h[p] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taymaz-Nikerel, H.; Lara, A.R. Vitreoscilla Haemoglobin: A Tool to Reduce Overflow Metabolism. Microorganisms 2022, 10, 43. https://doi.org/10.3390/microorganisms10010043
Taymaz-Nikerel H, Lara AR. Vitreoscilla Haemoglobin: A Tool to Reduce Overflow Metabolism. Microorganisms. 2022; 10(1):43. https://doi.org/10.3390/microorganisms10010043
Chicago/Turabian StyleTaymaz-Nikerel, Hilal, and Alvaro R. Lara. 2022. "Vitreoscilla Haemoglobin: A Tool to Reduce Overflow Metabolism" Microorganisms 10, no. 1: 43. https://doi.org/10.3390/microorganisms10010043
APA StyleTaymaz-Nikerel, H., & Lara, A. R. (2022). Vitreoscilla Haemoglobin: A Tool to Reduce Overflow Metabolism. Microorganisms, 10(1), 43. https://doi.org/10.3390/microorganisms10010043







