‘Candidatus Mycoplasma Haemoalbiventris’ and Tick-Borne Pathogens in Black-Eared Opossum (Didelphis aurita) from Southeastern Brazil
Abstract
:1. Introduction
2. Material and Methods
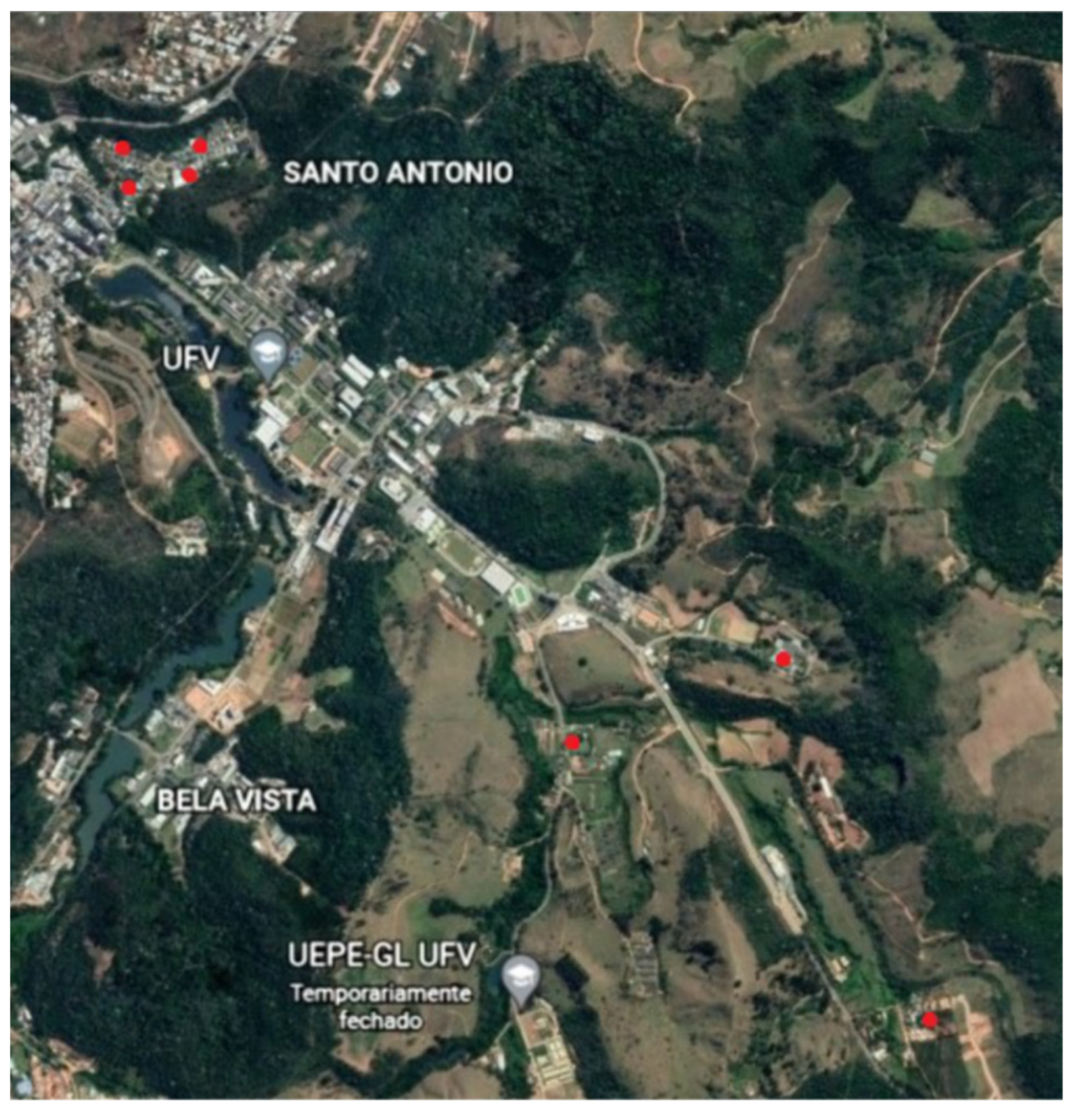
2.1. Study Area
2.2. Sampling
2.3. Hematological and Biochemical Analyses
2.4. Morphometry
2.5. DNA Extraction
2.6. PCR Assays
2.7. Sequencing and Phylogenetic Analysis
2.8. Statistical Analysis
2.9. Ethical Aspects
3. Results
3.1. Hematological and Biochemical Analyses
3.2. PCR Assays
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardner, A.L. Mammals of South America, Volume 1: Marsupials, Xenarthrans, Shrews, and Bats; University of Chicago Press: Chicago, IL, USA, 2008; ISBN 978-0-226-28242-8. [Google Scholar]
- Rossi, R.; Bianconi, G.; Pedro, W.A. Ordem Didelphimorphia. In Mamíferos do Brasil; Universidade Estadual de Londrina: Londrina, Brazil, 2006; pp. 34–37. [Google Scholar]
- Bezerra-Santos, M.A.; Fontes, C.S.; Nogueira, B.C.F.; Yamatogi, R.S.; Ramos, R.A.N.; Galhardo, J.A.; Furtado, L.F.V.; Rabelo, É.M.L.; de Araújo, J.V.; Campos, A.K. Gastrointestinal Parasites in the Opossum Didelphis aurita: Are They a Potential Threat to Human Health? J. Parasit. Dis. 2020, 44, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, S.E.; Gottdenker, N.; Krishnavajhala, A.; Fox, A.; Wilder, H.K.; González, K.; Smith, D.; López, M.; Perea, M.; Rigg, C.; et al. Correction: Synanthropic Mammals as Potential Hosts of Tick-Borne Pathogens in Panama. PLoS ONE 2019, 14, e0226195. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, M.M.; Bezerra, A.M.R. Infection Agents of Didelphidae (Didelphimorphia) of Brazil: An Underestimated Matter in Zoonoses Research. Mammalia 2022, 86, 105–122. [Google Scholar] [CrossRef]
- Messick, J.B.; Walker, P.G.; Raphael, W.; Berent, L.; Shi, X. “Candidatus Mycoplasma haemodidelphidis” sp. nov., “Candidatus Mycoplasma haemolamae” sp. nov. and Mycoplasma haemocanis comb. nov., Haemotrophic Parasites from a Naturally Infected Opossum (Didelphis virginiana), Alpaca (Lama pacos) and Dog (Canis familiaris): Phylogenetic and Secondary Structural Relatedness of Their 16S RRNA Genes to Other Mycoplasmas. Int. J. Syst. Evol. Microbiol. 2002, 52, 693–698. [Google Scholar] [CrossRef]
- de Oliveira, R.P.A.; Collere, F.C.M.; Ferrari, L.D.R.; Coradi, V.d.S.; Soares, N.d.A.; Leandro, A.d.S.; de Oliveira, W.F.; Galvão, S.R.; Kafka, R.; Delai, R.M.; et al. ‘Candidatus Mycoplasma haemoalbiventris’ and Tick-Borne Pathogens Screening in White-Eared Opossums (Didelphis albiventris) from Curitiba and Foz Do Iguaçu Cities, Paraná State, Southern Brazil. Rev. Bras. Parasitol. Vet. 2021, 30, e009721. [Google Scholar] [CrossRef] [PubMed]
- Pontarolo, G.H.; Kühl, L.F.; Pedrassani, D.; Campos, M.; Borges Figuereido, F.; Valente Marinho, J.D.; Gonçalves, L.R.; André, M.R.; Wischral, T.S.; Vieira, J.; et al. ‘Candidatus Mycoplasma Haemoalbiventris’, a Novel Hemoplasma Species in White-eared Opossums (Didelphis Albiventris) from Brazil. Transbound Emerg. Dis. 2021, 68, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Massini, P.F.; Drozino, R.N.; Otomura, F.H.; Mongruel, A.C.B.; Valente, J.D.M.; Toledo, M.J.d.O.; Martins, T.F.; Vidotto, O.; Vieira, T.S.W.J.; Vieira, R.F.d.C. Detection of Hemotropic Mycoplasma sp. in White-Eared Opossums (Didelphis albiventris) from Southern Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 797–801. [Google Scholar] [CrossRef]
- Gonçalves, L.R.; Herrera, H.M.; Nantes, W.A.G.; Santos, F.M.; Porfírio, G.E.d.O.; Barreto, W.T.G.; de Macedo, G.C.; Assis, W.d.O.; Campos, J.B.V.; da Silva, T.M.V.; et al. Genetic Diversity and Lack of Molecular Evidence for Hemoplasma Cross-Species Transmission between Wild and Synanthropic Mammals from Central-Western Brazil. Acta Trop. 2020, 203, 105303. [Google Scholar] [CrossRef]
- Ayala, S.C.; D’Alessandro, A.; Mackenzie, R.; Angel, D. Hemoparasite Infections in 830 Wild Animals from the Eastern Llanos of Colombia. J. Parasitol. 1973, 59, 52–59. [Google Scholar] [CrossRef]
- de Thoisy, B.; Michel, J.C.; Vogel, I.; Vié, J.C. A Survey of Hemoparasite Infections in Free-Ranging Mammals and Reptiles in French Guiana. J. Parasitol. 2000, 86, 1035–1040. [Google Scholar] [CrossRef]
- Allen, K.E.; Yabsley, M.J.; Johnson, E.M.; Reichard, M.V.; Panciera, R.J.; Ewing, S.A.; Little, S.E. Novel Hepatozoon in Vertebrates from the Southern United States. J. Parasitol. 2011, 97, 648–653. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.R.L.; Fornazari, F.; Demoner, L.d.C.; Teixeira, C.R.; Langoni, H.; O’Dwyer, L.H. Didelphis albiventris Naturally Infected with Hepatozoon canis in Southeastern Brazil. Ticks Tick Borne Dis. 2017, 8, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Colle, A.C.; de Mendonça, R.F.B.; Maia, M.O.; Freitas, L.d.C.; Witter, R.; Marcili, A.; de Aguiar, D.M.; Muñoz-Leal, S.; Labruna, M.B.; Rossi, R.V.; et al. Molecular Survey of Tick-Borne Pathogens in Small Mammals from Brazilian Amazonia. Rev. Bras. Parasitol. Vet. 2019, 28, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.R.; Paludo, G.; Bisol, T.B.; Perles, L.; de Oliveira, L.B.; de Oliveira, C.M.; da Silva, T.M.V.; Nantes, W.A.G.; Duarte, M.A.; Santos, F.M.; et al. Molecular Detection of Piroplasmids in Synanthropic Rodents, Marsupials, and Associated Ticks from Brazil, with Phylogenetic Inference of a Putative Novel Babesia sp. from White-Eared Opossum (Didelphis albiventris). Parasitol. Res. 2021, 120, 3537–3546. [Google Scholar] [CrossRef]
- Soares, H.S.; Marcili, A.; Barbieri, A.R.M.; Minervino, A.H.H.; Moreira, T.R.; Gennari, S.M.; Labruna, M.B. Novel Piroplasmid and Hepatozoon Organisms Infecting the Wildlife of Two Regions of the Brazilian Amazon. Int. J. Parasitol. Parasites Wildl. 2017, 6, 115–121. [Google Scholar] [CrossRef]
- Lopes, M.G.; Muñoz-Leal, S.; de Lima, J.T.R.; Fournier, G.F.d.S.R.; Acosta, I.d.C.L.; Martins, T.F.; Ramirez, D.G.; Gennari, S.M.; Labruna, M.B. Ticks, Rickettsial and Erlichial Infection in Small Mammals from Atlantic Forest Remnants in Northeastern Brazil. Int. J. Parasitol. Parasites Wildl. 2018, 7, 380–385. [Google Scholar] [CrossRef]
- André, M.R.; Calchi, A.C.; Perles, L.; Gonçalves, L.R.; Uccella, L.; Lemes, J.R.B.; Nantes, W.A.G.; Santos, F.M.; Porfírio, G.E.d.O.; Barros-Battesti, D.M.; et al. Novel Ehrlichia and Hepatozoon Genotypes in White-Eared Opossums (Didelphis albiventris) and Associated Ticks from Brazil. Ticks Tick-Borne Dis. 2022, 13, 102022. [Google Scholar] [CrossRef]
- Miziara, S.R.; Paiva, F.; Andreotti, R.; Koller, W.W.; Lopes, V.A.; Pontes, N.T.; Bitencourt, K. Ocorrência de Ixodes loricatus Neumann, 1899 (Acari: Ixodidae) parasitando Didelphis albiventris (Lund, 1841), (Didelphimorphia: Didelphidae), em Campo Grande, MS. Rev. Bras. Parasitol. Vet. 2008, 17, 158–160. [Google Scholar] [CrossRef]
- Horta, M.C.; Labruna, M.B.; Pinter, A.; Linardi, P.M.; Schumaker, T.T.S. Rickettsia Infection in Five Areas of the State of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz 2007, 102, 793–801. [Google Scholar] [CrossRef]
- Bezerra-Santos, M.A.; Nogueira, B.C.F.; Yamatogi, R.S.; Ramos, R.A.N.; Galhardo, J.A.; Campos, A.K. Ticks, Fleas and Endosymbionts in the Ectoparasite Fauna of the Black-Eared Opossum Dipelphis aurita in Brazil. Exp. Appl. Acarol. 2020, 80, 329–338. [Google Scholar] [CrossRef]
- Sousa, K.C.M.D.; Calchi, A.C.; Herrera, H.M.; Dumler, J.S.; Barros-Battesti, D.M.; Machado, R.Z.; André, M.R. Anaplasmataceae Agents among Wild Mammals and Ectoparasites in Brazil. Epidemiol. Infect. 2017, 145, 3424–3437. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Raimundo, J.M.; da Silva, A.T.; Carpintero, F.M.; Pires, J.R.; Benevenute, J.L.; Machado, R.Z.; André, M.R.; Baldani, C.D. Detection of a Putative Novel Genotype of Ehrlichia sp. from Opossums (Didelphis aurita) from Brazil. Rev. Bras. Parasitol. Vet. 2018, 28, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Climate-Data.Org. Viçosa Climate: Average Temperature, Weather by Month, Viçosa Weather Averages. Available online: https://en.climate-data.org/south-america/brazil/minas-gerais/vicosa-25021/ (accessed on 16 August 2022).
- Inokuma, H.; Okuda, M.; Ohno, K.; Shimoda, K.; Onishi, T. Analysis of the 18S rRNA Gene Sequence of a Hepatozoon Detected in Two Japanese Dogs. Vet. Parasitol. 2002, 106, 265–271. [Google Scholar] [CrossRef]
- Carvalho do Nascimento, C.; Horta, M.C. Didelphimorphia (Gambá e Cuíca). In Tratado de Animais Selvagens: Medicina Veterinária; Roca: São Paulo, Brazil, 2014; Volume 1, ISBN 978-85-277-2648-1. [Google Scholar]
- Barros-Battesti, D.M.; Arzua, M.; Bechara, G.H. Carrapatos de importância médico-veterinária da região neotropical: Um guia ilustrado para identificação de espécies; Vox/ICTTD-3/Butantan: São Paulo, Brezil, 2006; 223p, ISBN 85-99909-01-0. [Google Scholar]
- Casagrande, R.A.; Cesar, M.d.O.; Horta, M.C.; Rossi, S.; Teixieira, R.H.; Matushima, E.R. Perfil hematológico de gambás Didelphis aurita e D. albiventris do Estado de São Paulo, Brasil. Acta Scientiarum. Biol. Sci. 2009, 31, 185–189. [Google Scholar] [CrossRef]
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Development and Evaluation of a Seminested PCR for Detection and Differentiation of Babesia gibsoni (Asian Genotype) and B. canis DNA in Canine Blood Samples. J. Clin. Microbiol. 2003, 41, 4172–4177. [Google Scholar] [CrossRef]
- Willi, B.; Meli, M.L.; Lüthy, R.; Honegger, H.; Wengi, N.; Hoelzle, L.E.; Reusch, C.E.; Lutz, H.; Hofmann-Lehmann, R. Development and Application of a Universal Hemoplasma Screening Assay Based on the SYBR Green PCR Principle. J. Clin. Microbiol. 2009, 47, 4049–4054. [Google Scholar] [CrossRef]
- Vieira, R.F.d.C.; Vidotto, O.; Vieira, T.S.W.J.; Guimaraes, A.M.S.; dos Santos, A.P.; Nascimento, N.C.; dos Santos, N.J.R.; Martins, T.F.; Labruna, M.B.; Marcondes, M.; et al. Molecular Investigation of Hemotropic Mycoplasmas in Human Beings, Dogs and Horses in a Rural Settlement in Southern Brazil. Rev. Inst. Med. Trop. São Paulo 2015, 57, 353–357. [Google Scholar] [CrossRef]
- Machado, C.A.L.; Vidotto, O.; Conrado, F.O.; Santos, N.J.R.; Valente, J.D.M.; Barbosa, I.C.; Trindade, P.W.S.; Garcia, J.L.; Biondo, A.W.; Vieira, T.S.W.J.; et al. Mycoplasma ovis Infection in Goat Farms from Northeastern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2017, 55, 1–5. [Google Scholar] [CrossRef]
- Hoelzle, K.; Winkler, M.; Kramer, M.M.; Wittenbrink, M.M.; Dieckmann, S.M.; Hoelzle, L.E. Detection of ‘Candidatus Mycoplasma haemobos’ in Cattle with Anaemia. Vet. J. 2011, 187, 408–410. [Google Scholar] [CrossRef]
- Mongruel, A.C.B.; Spanhol, V.C.; Valente, J.D.M.; Porto, P.P.; Ogawa, L.; Otomura, F.H.; Marquez, E.d.S.; André, M.R.; Vieira, T.S.W.J.; Vieira, R.F.d.C. Survey of Vector-Borne and Nematode Parasites Involved in the Etiology of Anemic Syndrome in Sheep from Southern Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, 1–12. [Google Scholar] [CrossRef]
- Parola, P.; Roux, V.; Camicas, J.-L.; Baradji, I.; Brouqui, P.; Raoult, D. Detection of Ehrlichiae in African Ticks by Polymerase Chain Reaction. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 707–708. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Sela, I.; Ashkenazy, H.; Katoh, K.; Pupko, T. GUIDANCE2: Accurate Detection of Unreliable Alignment Regions Accounting for the Uncertainty of Multiple Parameters. Nucleic Acids Res. 2015, 43, W7–W14. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree; University of Edinburgh, Institute of Evolutionary Biology: Edinburgh, UK, 2016. [Google Scholar]
- Leigh, J.W.; Bryant, D. Popart: Full-Feature Software for Haplotype Network Construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-Joining Networks for Inferring Intraspecific Phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Lewis, J.H. Comparative Hematology: Studies on Opossums Didelphis Marsupialis (virginianus). Comp. Biochem. Physiol. Part A Physiol. 1975, 51, 275–280. [Google Scholar] [CrossRef]
- Aviner, S.; Miskin, H.; London, D.; Horowitz, S.; Schlesinger, M. Mycoplasma Pneumonia Infection: A Possible Trigger for Immune Thrombocytopenia. Indian J. Hematol. Blood Transfus. 2011, 27, 46–50. [Google Scholar] [CrossRef]
- Scott, M.A.; Stockham, S.L. Fundamentals of Veterinary Clinical Pathology; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-118-68607-2. [Google Scholar]
- Thrall, M.A.; Weiser, G.; Allison, R.W.; Campbell, T.W. Veterinary Hematology and Clinical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-118-37840-3. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals; Academic Press: Cambridge, MA, USA, 2008; ISBN 978-0-08-056882-9. [Google Scholar]
- Wicks, R.M.; Spencer, P.B.S.; Moolhuijzen, P.; Clark, P. Morphological and Molecular Characteristics of a Species of Hepatozoon Miller, 1908 (Apicomplexa: Adeleorina) from the Blood of Isoodon Obesulus (Marsupialia: Peramelidae) in Western Australia. Syst. Parasitol. 2006, 65, 19–25. [Google Scholar] [CrossRef]
- Merino, S.; Vásquez, R.A.; Martínez, J.; Celis-Diez, J.L.; Gutiérrez-Jiménez, L.; Ippi, S.; Sánchez-Monsalvez, I.; Martínez-De La Puente, J. Molecular Characterization of an Ancient Hepatozoon Species Parasitizing the ‘Living Fossil’ Marsupial ‘Monito Del Monte’ Dromiciops Gliroides from Chile. Biol. J. Linn. Soc 2009, 98, 568–576. [Google Scholar] [CrossRef]
- Silva, O.d.’U.; Arantes, J.B. Sobre Uma Hemogregarina Da Gamba. Memórias Do Inst. Oswaldo Cruz 1918, 8, 61–63. [Google Scholar] [CrossRef]
- Baneth, G.; Mathew, J.S.; Shkap, V.; Macintire, D.K.; Barta, J.R.; Ewing, S.A. Canine Hepatozoonosis: Two Disease Syndromes Caused by Separate Hepatozoon spp. Trends Parasitol. 2003, 19, 27–31. [Google Scholar] [CrossRef]





| Erythrocytes | ||
| Analyte | Mean ± SD | Range (Min-Max) |
| Red blood cells (×106/µL) | 4.31 ± 1.06 | 1.68–7.33 |
| Hemoglobin (g/dL) | 11.77 ± 1.99 | 5.80–15.60 |
| PCV (%) | 38.15 ± 6.47 | 19.00–50.30 |
| Total protein (g/dL) | 7.41 ± 0.85 | 5.80–9.60 |
| MVC (fl) | 84.00 ± 8.17 | 65.50–110.10 |
| MCH (pg) | 28.47 ± 5.28 | 16.23–39.82 |
| MCHC (%) | 30.91 ± 1.84 | 26.90–34.23 |
| RDW (%) | 10.02 ± 0.69 | 8.70–11.40 |
| Fibrinogen (g/dL) | 0.23 ± 0.09 | 0.20–0.60 |
| Leukocytes | ||
| Analyte | Mean ± SD | Range (Min-Max) |
| Leukocytes /µL | 14,596.67 ± 6200.58 | 5800–36,700 |
| Neutrophils /µL | 6755.37 ± 3614.52 | 2300–17,616 |
| Lymphocytes /µL | 5306.87 ± 2499.46 | 456–11,233 |
| Monocytes /µL | 416.43 ± 493.10 | 73–2202 |
| Band /µL | 60.33 ± 158 | 0–734 |
| Eosinophils /µL | 1790.77 ± 1603.63 | 0–6606 |
| Basophils /µL | 202.30 ± 246.69 | 0–1088 |
| Platelets | ||
| Analyte | Mean ± SD | Range (Min-Max) |
| Platelets ×103/µL | 284.96 ± 173.23 | 32–716 |
| Serum biochemical analyses | ||
| Analyte | Mean ± SD | Range (Min-Max) |
| Albumin (g/dL) | 3.02 ± 0.44 | 1.85–3.75 |
| Total protein (g/dL) | 7.58 ± 1.12 | 5.49–9.33 |
| Globulins (g/dL) | 4.56 ± 1.20 | 2.77–7.48 |
| Albumin:globulin ratio | 0.70 ± 0.20 | 0.24–1.09 |
| AP (U/L) | 1009.30 ± 855.14 | 223.00–3345.00 |
| ALT (U/L) | 65.82 ± 48.19 | 29.80–226.40 |
| AST (U/L) | 192.93 ± 162.55 | 3.00–994.00 |
| Urea (mg/dL) | 71.37 ± 17.44 | 50.00–292.00 |
| Creatinine (mg/dL) | 0.49 ± 0.19 | 0.00–0.76 |
| GGT (U/L) | 31.00 ± 11.58 | 18.00–65.00 |
| Glucose (mg/dL) | 120.71 ± 46.76 | 44.80–256.60 |
| Hematology | |||
|---|---|---|---|
| Analyte | Negative | Positive | p-Value |
| Red blood cells (×106/µL) | 4.596 ± 1.259 | 4.199 ± 0.989 | 0.3734 |
| Hemoglobin (g/dL) | 11.65 ± 0.89 | 11.81 ± 2.28 | 0.8465 |
| PCV (%) | 38.51 ± 2.90 | 38.02 ± 7.41 | 0.8570 |
| Total protein (g/dL) | 6.800 ± 0.59 | 7.800 ± 0.79 | 0.0083 * |
| MVC (fl) | 85.75 ± 7.8 | 84.40 ± 8.34 | 0.9542 |
| MCH (pg) | 27.53 ± 6.67 | 28.81± 4.81 | 0.5642 |
| MCHC (%) | 30.34 ± 2.51 | 31.12 ± 1.55 | 0.3111 |
| RDW (%) | 10.36 ± 0.64 | 9.900 ± 0.67 | 0.1034 |
| Fibrinogen (g/dL) | 0.2000 ± 0.07 | 0.2000 ± 0.09 | >0.9999 |
| Leukocytes /µL | 13,250 ± 4724.69 | 13,000 ± 6751.73 | 0.9817 |
| Neutrophils /µL | 5636 ± 3729.82 | 6341± 3647.43 | 0.6622 |
| Lymphocytes /µL | 4655 ± 1857.24 | 5265 ± 2733.69 | >0.9999 |
| Monocytes /µL | 187.0 ± 235.15 | 243.5 ± 551.48 | 0.3156 |
| Band /µL | 72.75 ± 148.51 | 55.81 ± 164.58 | 0.8620 |
| Eosinophils /µL | 1514 ± 1729.28 | 1320 ± 1598.27 | 0.9451 |
| Basophils /µL | 167.0 ± 227.87 | 165.5 ± 258.27 | >0.9999 |
| Platelets ×103/µL | 452,500 ± 184,945 | 224,045 ± 124,344 | 0.0006 * |
| Serum biochemical analyses | |||
| Albumin (g/dL) | 2.779 ± 0.15 | 3.005 ± 0.39 | 0.1271 |
| Total protein (g/dL) | 6.370 ± 0.56 | 7.559 ± 1.03 | 0.0048 * |
| Globulins (g/dL) | 3.591 ± 0.60 | 4.555 ± 1.11 | 0.0285 * |
| Albumin:globulin ratio | 0.7935 ± 0.13 | 0.7035 ± 0.20 | 0.2579 |
| AP (U/L) | 1932 ± 827.10 | 857.0 ± 706.91 | 0.0021 * |
| ALT (U/L) | 46.90 ± 11.76 | 55.00 ± 48.09 | 0.1154 |
| AST (U/L) | 117.5 ± 152.61 | 233.5 ± 229.33 | 0.2371 |
| Urea (mg/dL) | 78.65 ± 19.19 | 73.50 ± 59.99 | 0.7214 |
| Creatinine (mg/dL | 0.4600 ± 0.15 | 0.4591 ± 0.19 | 0.9905 |
| GGT (U/L) | 27.50 ± 7.34 | 27.00 ± 12.46 | 0.6525 |
| Glucose (mg/dL) | 126.4 ± 51.13 | 126.4 ± 69.34 | 0.9994 |
| Hemotropic Mycoplasmas | ||||
| Variable | +/n | OR (95% CI) | p-Value | |
| Gender | ||||
| Male | 7/11 (63.33) | 0.4667 (0.1066–2.036) | 0.4172 | |
| Female | 15/19 (78.94) | |||
| Age group | ||||
| Adult | 17/23 (73.91) | 1.1333 (0.1858–5.956) | >0.9999 | |
| Sub adult | 5/7 (71.42) | |||
| Presence of ectoparasites | ||||
| Ectoparasite | 17/21 (80.95) | 1.7000 (0.2578–11,58) | 0.6219 | |
| Without ectoparasite | 5/9 (55.55) | |||
| Hepatozoon sp. | ||||
| Variable | +/n | OR (95% CI) | p-Value | |
| Gender | ||||
| Male | 3/10 (30.00) | 2.0000 (0.3769–10.13) | 0.6382 | |
| Female | 3/17 (17.64) | |||
| Age group | ||||
| Adult | 5/20 (25.00) | 2.0000 (0.2494–27.07) | >0.9999 | |
| Sub adult | 1/7 (14.28) | |||
| Presence of ectoparasites | ||||
| Ectoparasite | 4/19 (21.05) | 0.8000 (0.1267–5.119) | >0.9999 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco, A.M.O.; Bento, L.D.; Souto, P.C.; Girardi, F.M.; Nogueira, B.C.F.; Yamatogi, R.S.; Campos, A.K.; Cray, C.; Montiani-Ferreira, F.; Collere, F.C.M.; et al. ‘Candidatus Mycoplasma Haemoalbiventris’ and Tick-Borne Pathogens in Black-Eared Opossum (Didelphis aurita) from Southeastern Brazil. Microorganisms 2022, 10, 1955. https://doi.org/10.3390/microorganisms10101955
Orozco AMO, Bento LD, Souto PC, Girardi FM, Nogueira BCF, Yamatogi RS, Campos AK, Cray C, Montiani-Ferreira F, Collere FCM, et al. ‘Candidatus Mycoplasma Haemoalbiventris’ and Tick-Borne Pathogens in Black-Eared Opossum (Didelphis aurita) from Southeastern Brazil. Microorganisms. 2022; 10(10):1955. https://doi.org/10.3390/microorganisms10101955
Chicago/Turabian StyleOrozco, Andrés Maurício Ortega, Lucas Drumond Bento, Pollyanna Cordeiro Souto, Fabricia Modolo Girardi, Bárbara Cristina Félix Nogueira, Ricardo Seiti Yamatogi, Artur Kanadani Campos, Carolyn Cray, Fabiano Montiani-Ferreira, Flávia Carolina Meira Collere, and et al. 2022. "‘Candidatus Mycoplasma Haemoalbiventris’ and Tick-Borne Pathogens in Black-Eared Opossum (Didelphis aurita) from Southeastern Brazil" Microorganisms 10, no. 10: 1955. https://doi.org/10.3390/microorganisms10101955





