Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds
Abstract
:1. Introduction
1.1. Natural Products
1.2. Endophytes
1.3. Classification of Endophytic Fungi
1.4. Plant Selection
2. Antimicrobial Potential of Endophytic Fungi
2.1. Endophyte Culture Extracts with Antimicrobial Properties
2.2. Antimicrobial Compounds of Different Chemical Classes Produced by Endophyte
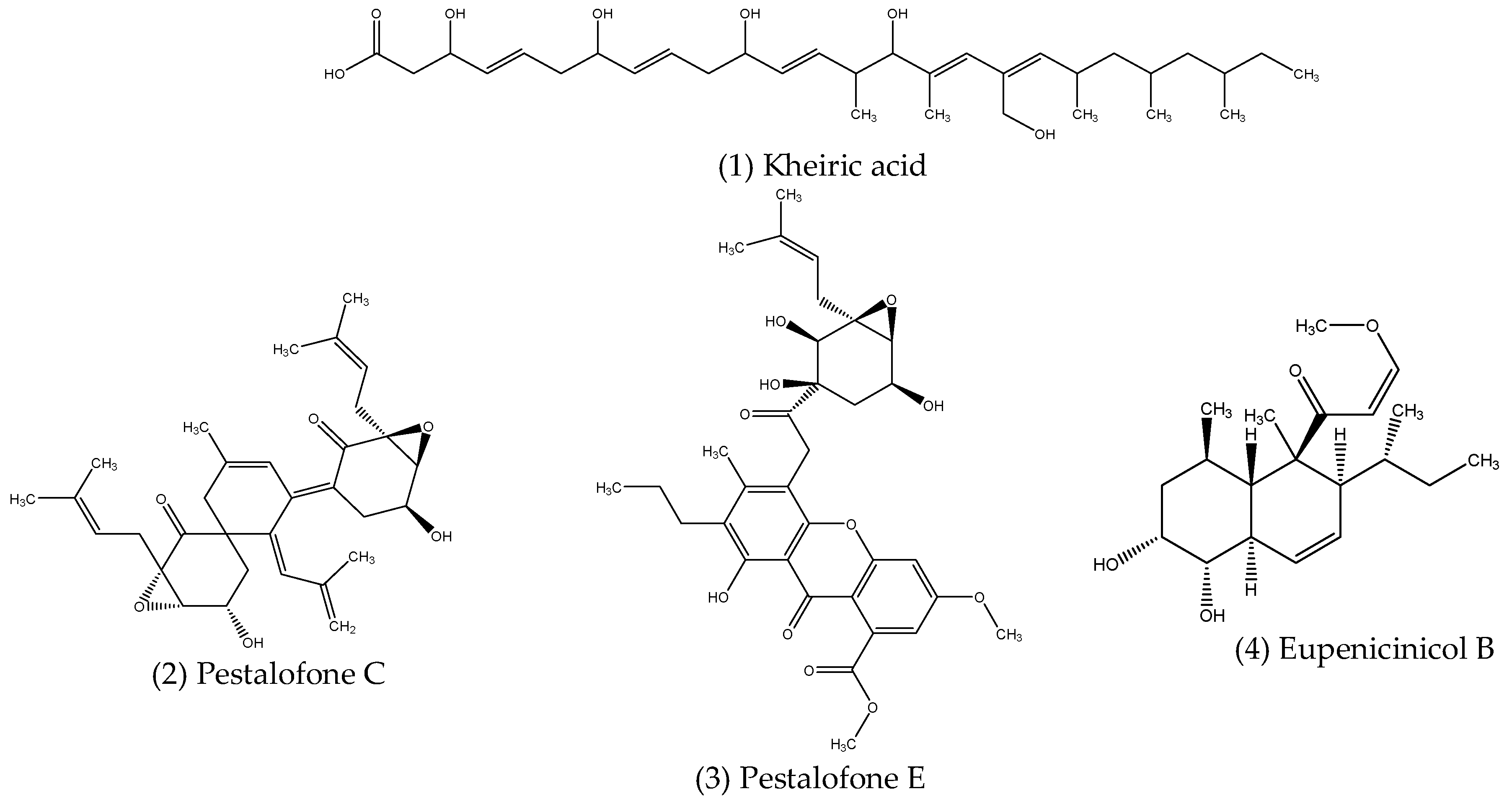
2.2.1. Aliphatic Compounds
2.2.2. Alkaloids and Other Nitrogenous Compounds
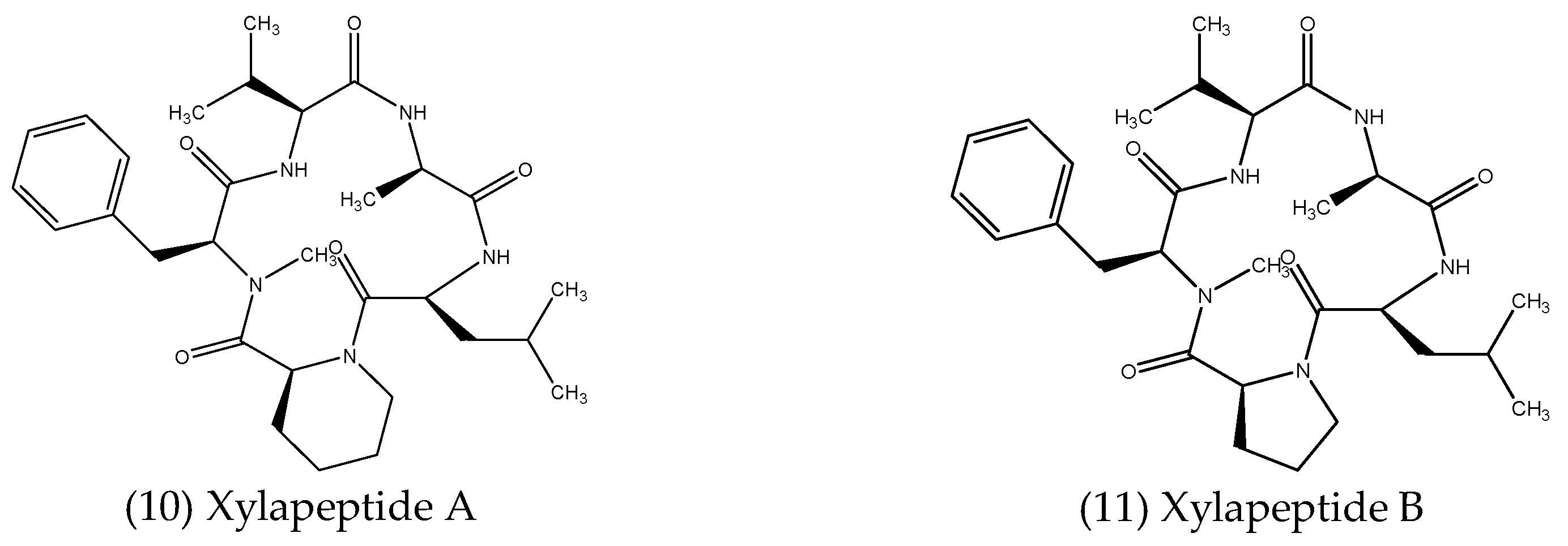
2.2.3. Peptides
2.2.4. Phenolic Compounds
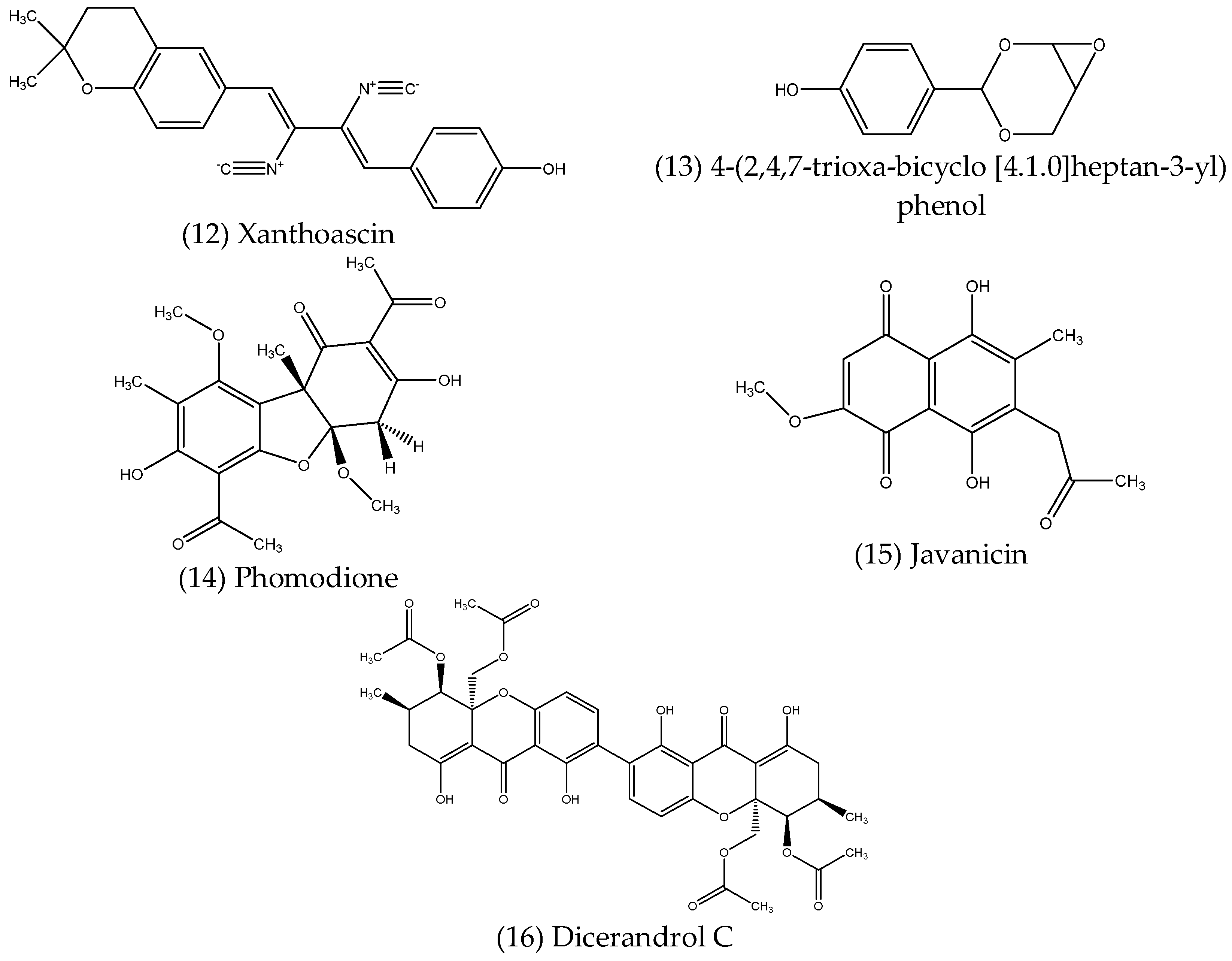
2.2.5. Polyketides
2.2.6. Terpenoid Compounds
| Chemical Class | Endophyte | Host Plant | Activity | Reference |
|---|---|---|---|---|
| Aliphatic compounds | Curvularia papendorfii | Vernonia amygdalina | Antibacterial | [84] |
| Pestalotiopsis fici | Unidentified | Antifungal | [85] | |
| Eupenicillium sp. LG41 | Xanthium sibiricum | Antibacterial | [86] | |
| Alkaloids | Phomopsis sp. PSU-D15 | Garcinia dulcis (Roxb.) | Antimycobacterial | [87] |
| Penicillium brocae MA-231 | Mangroves | Antibacterial Antifungal | [88] | |
| Penicillium sp. CPCC 400817 | Mangroves | Antibacterial | [89] | |
| Penicillium janthinellum | Panax notoginseng | Antibacterial | [90] | |
| Peptides | Xylaria sp. | Sophora tonkinensis | Antibacterial Antifungal | [92] |
| Phenolics | Aspergillus sp. IFB-YXS | Ginkgo biloba L. | Antibacterial | [94] |
| Pestalotiopsis mangiferae | Mangifera indica Linn. | Antibacterial Antifungal | [95] | |
| Phoma sp. | Saurauia scaberrinae | Antibacterial Antifungal | [96] | |
| Chloridium sp. | Azadirachta indica A. Juss. | Antibacterial | [97] | |
| Phomopsis longicolla | Bostrychia radicans | Antibacterial | [98] | |
| Polyketides | Talaromyces funiculosus | Salicornia bigelovii | Antibacterial | [100] |
| Trichoderma koningiopsis QA-3 | Artemisia argyi | Antibacterial Antifungal | [101] | |
| Penicillium sp. | Garcinia nobilis | Antibacterial | [102] | |
| Terpenoids | Trichoderma virens QA-8 | Artemisia argyi | Antibacterial Antifungal | [103] |
| Leptosphaeria sp. XL026 | P. notoginseng | Antibacterial | [104] | |
| Emericella sp. XL 029 | P. notoginseng | Antibacterial Antifungal | [105] | |
| Trichoderma atroviride | Colquhounia coccinea var. mollis | Antibacterial | [107] | |
| Bipolaris sp. TJ403-B1 | Wheat | Antibacterial | [107] | |
| Aspergillus sp. TJ23 | Hypericum perforatum | Antibacterial | [108,109] |
2.3. Antibiofilm Compounds Produced by Endophytic Fungi
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation (WHO). Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 16 September 2022).
- Grace, D. Infectious diseases and agriculture. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 439–447. [Google Scholar]
- Badawy, B.; Gwida, M.; Sadat, A.; EL-Toukhy, M.; Sayed-Ahmed, M.; Alam, N.; Ahmad, S.; Ali, M.S.; Elafify, M. Prevalence and Antimicrobial Resistance of Virulent Listeria monocytogenes and Cronobacter sakazakii in Dairy Cattle, the Environment, and Dried Milk with the In Vitro Application of Natural Alternative Control. Antibiotics 2022, 11, 1087. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B. Pharmaceuticals: Natural products and natural product models. In Natural Products in Chemical Biology; Civjan, N., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 287–324. [Google Scholar]
- Kingston, D.G.I.; Newman, D.J. Natural products as anticancer agents. In Natural Products in Chemical Biology; Civjan, N., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 325–349. [Google Scholar]
- Zaferanloo, B.; Pepper, S.A.; Coulthard, S.A.; Redfern, C.P.F.; Palombo, E.A. Metabolites of endophytic fungi from Australian native plants as potential anticancer agents. FEMS Microbiol. Lett. 2018, 365, fny078. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G. The Emergence of Endophytic Microbes and Their Biological Promise. J. Fungi 2018, 4, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.G.; Griffin, E.A. The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: How far have we come and where do we go from here? Environ. Microbiol. 2020, 22, 2107–2123. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.K.; Verekar, S.A.; Bhave, S.V. Endophytic fungi: A reservoir of antibacterials. Front. Microbiol. 2015, 5, 715. [Google Scholar] [CrossRef] [Green Version]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef]
- Mosunova, O.; Navarro-Muñoz, J.C.; Collemare, J. The biosynthesis of fungal secondary metabolites: From fundamentals to biotechnological applications. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, Y.S.; Williams, L.L. A glimpse of Ayurveda—The forgotten history and principles of Indian traditional medicine. J. Tradit. Comp. Med. 2017, 7, 50–53. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Biodiversity: A continuing source of novel drug leads. Pure Appl. Chem. 2005, 77, 7–24. [Google Scholar] [CrossRef]
- Chain, E.; Florey, H.W.; Gardner, A.D.; Heatley, N.G.; Jennings, M.A.; Orr-Ewing, J.; Sanders, A.G. Penicillin as a chemotherapeutic agent. Lancet 1940, 236, 226–228. [Google Scholar] [CrossRef]
- Clardy, J.; Fischbach, M.A.; Currie, C.R. The natural history of antibiotics. Curr. Biol. 2009, 19, R437–R441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef] [Green Version]
- Endo, A. A historical perspective on the discovery of statins. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 2010, 86, 484–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crump, A. Ivermectin: Enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, G. Natural products in drug discovery: Present status and perspectives. In Pharmaceutical Biotechnology; Guzmán, C.A., Feuerstein, G.Z., Eds.; Springer: New York, NY, USA, 2009; pp. 13–27. [Google Scholar]
- Wright, G.D. Unlocking the potential of natural products in drug discovery. Microb. Biotechnol. 2019, 12, 55–57. [Google Scholar] [CrossRef] [Green Version]
- De Bary, A. Morphologie und Physiologie der Pilze, Flechten und Myxomyceten; Engelmann: Leipzig, Germany, 1866. [Google Scholar]
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Eds.; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar]
- Wennström, A. Endophyte: The Misuse of an Old Term. Oikos 1994, 71, 535. [Google Scholar] [CrossRef]
- Wilson, D. Endophyte: The Evolution of a Term, and Clarification of Its Use and Definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Stone, J.K.; Bacon, C.W.; White, J.F., Jr. An overview of endophytic microbes: Endophytism defined. In Microbial Endophytes; CRC Press: Boca Raton, FL, USA, 2000; pp. 17–44. [Google Scholar]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [Green Version]
- Krings, M.; Taylor, T.N.; Dotzler, N. Fungal endophytes as a driving force in land plant evolution: Evidence from the fossil record. In Biocomplexity of Plant-Fungal Interactions; Southworth, D., Ed.; John Wiley & Sons, Inc.: West Sussex, UK, 2012; pp. 5–28. [Google Scholar]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Boil. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef]
- Hassan, S.E.-D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.-Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 2017, 8, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef]
- Chhabra, S.; Dowling, D.N. Endophyte-promoted nutrient acquisition: Phosphorus and iron. In Functional Importance of the Plant Microbiome: Implications for Agriculture, Forestry and Bioenergy; Doty, S.L., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 21–42. [Google Scholar]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities with Multifunctional Prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.-O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, M.; Germida, J.J.; Vujanovic, V. Fungal endophytes enhance wheat heat and drought tolerance in terms of grain yield and second-generation seed viability. J. Appl. Microbiol. 2014, 116, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Abdelrahman, M.; Radwan, U.; El-Zayat, S.; El-Sayed, M.A. Effect of Thermomyces fungal endophyte isolated from extreme hot desert-adapted plant on heat stress tolerance of cucumber. Appl. Soil Ecol. 2018, 124, 155–162. [Google Scholar] [CrossRef]
- Sangamesh, M.B.; Jambagi, S.; Vasanthakumari, M.M.; Shetty, N.J.; Kolte, H.; Ravikanth, G.; Nataraja, K.N.; Uma Shaanker, R. Thermotolerance of fungal endophytes isolated from plants adapted to the Thar Desert, India. Symbiosis 2018, 75, 135–147. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.-J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Ind. Crops Prod. 2020, 143, 111931. [Google Scholar] [CrossRef]
- Ding, S.; Huang, C.-L.; Sheng, H.-M.; Song, C.-L.; Li, Y.-B.; An, L.-Z. Effect of inoculation with the endophyte Clavibacter sp. strain Enf12 on chilling tolerance in Chorispora bungeana. Physiol. Plant. 2011, 141, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; He, R.; Chai, Q.; Li, C.; Nan, Z. Transcriptomic analyses giving insights into molecular regulation mechanisms involved in cold tolerance by Epichloë endophyte in seed germination of Achnatherum inebrians. Plant Growth Regul. 2016, 80, 367–375. [Google Scholar] [CrossRef]
- Li, T.; Liu, M.J.; Zhang, X.T.; Zhang, H.B.; Sha, T.; Zhao, Z.W. Improved tolerance of maize (Zea mays L.) to heavy metals by colonization of a dark septate endophyte (DSE) Exophiala pisciphila. Sci. Total Environ. 2011, 409, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Passari, A.K.; Upadhyaya, K.; Singh, G.; Abdel-Azeem, A.M.; Thankappan, S.; Uthandi, S.; Hashem, A.; Abd_Allah, E.F.; Malik, J.A.; As, A. Enhancement of disease resistance, growth potential, and photosynthesis in tomato (Solanum lycopersicum) by inoculation with an endophytic actinobacterium, Streptomyces thermocarboxydus strain BPSAC147. PLoS ONE 2019, 14, e0219014. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ji, X.-L.; Shen, T.; Tang, W.-T.; Li, S.-S.; Zhu, Z.-Q.; Kumar, J.; Li, H.-Y. Fungal endophytic communities of two wild Rosa varieties and the role of an endophytic Seimatosporium sp. in enhancing host plant powdery mildew resistance. Plant Soil 2020, 447, 553–564. [Google Scholar] [CrossRef]
- Thakur, A.; Kaur, S.; Kaur, A.; Singh, V. Enhanced resistance to Spodoptera litura in endophyte infected cauliflower plants. Environ. Entomol. 2013, 42, 240–246. [Google Scholar] [CrossRef]
- Mousa, W.K.; Schwan, A.; Davidson, J.; Strange, P.; Liu, H.; Zhou, T.; Auzanneau, F.-I.; Raizada, M.N. An endophytic fungus isolated from finger millet (Eleusine coracana) produces anti-fungal natural products. Front. Microbiol. 2015, 6, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, S.; Santamaria, O.; Halecker, S.; Lledó, S.; Stadler, M. Antagonism between Byssochlamys spectabilis (anamorph Paecilomyces variotii) and plant pathogens: Involvement of the bioactive compounds produced by the endophyte. Ann. Appl. Biol. 2017, 171, 464–476. [Google Scholar] [CrossRef]
- Soliman, S.; Greenwood, J.S.; Bombarely, A.; Mueller, L.A.; Tsao, R.; Mosser, D.D.; Raizada, M.N. An Endophyte Constructs Fungicide-Containing Extracellular Barriers for Its Host Plant. Curr. Biol. 2015, 25, 2570–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, W.K.; Shearer, C.; Limay-Rios, V.; Ettinger, C.L.; Eisen, J.A.; Raizada, M.N. Root-hair endophyte stacking in finger millet creates a physicochemical barrier to trap the fungal pathogen Fusarium graminearum. Nat. Microbiol. 2016, 1, 16167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejía, L.C.; Herre, E.A.; Sparks, J.P.; Winter, K.; García, M.N.; Van Bael, S.A.; Stitt, J.; Shi, Z.; Zhang, Y.; Guiltinan, M.J.; et al. Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front. Microbiol. 2014, 5, 479. [Google Scholar] [CrossRef]
- Rao, G.; Nageswara, N.; Neelapu, N.R.R.; Surekha, C. Induction of plant systemic resistance in legumes Cajanus cajan, Vignaradiata, Vigna mungo against plant pathogens Fusarium oxysporum and Alternaria alternata—A Trichoderma viride mediated reprogramming of plant defense mechanism. Int. J. Recent Sci. Res. 2015, 6, 4270–4280. [Google Scholar]
- Abdel-Lateif, K. Trichoderma as biological control weapon against soil borne plant pathogens. Afr. J. Biotechnol. 2017, 16, 2299–2306. [Google Scholar]
- Bacilio-Jiménez, M.; Aguilar-Flores, S.; del Valle, M.V.; Pérez, A.; Zepeda, A.; Zenteno, E. Endophytic bacteria in rice seeds inhibit early colonization of roots by Azospirillum brasilense. Soil Biol. Biochem. 2001, 33, 167–172. [Google Scholar] [CrossRef]
- Fuchs, B.; Krischke, M.; Mueller, M.J.; Krauss, J. Herbivore-specific induction of defence metabolites in a grass–endophyte association. Funct. Ecol. 2017, 31, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, B.; Krischke, M.; Mueller, M.J.; Krauss, J. Plant age and seasonal timing determine endophyte growth and alkaloid biosynthesis. Fungal Ecol. 2017, 29, 52–58. [Google Scholar] [CrossRef]
- Bae, H.; Roberts, D.P.; Lim, H.-S.; Strem, M.D.; Park, S.-C.; Ryu, C.-M.; Melnick, R.L.; Bailey, B.A. Endophytic Trichoderma Isolates from Tropical Environments Delay Disease Onset and Induce Resistance Against Phytophthora capsici in Hot Pepper Using Multiple Mechanisms. Mol. Plant Microbe Interact. 2011, 24, 336–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.-R.; Tian, X.-L.; Shen, B.-M.; Mao, Z.-C.; Chen, G.-h.; Xie, B.-Y. Transformation of the endophytic fungus Acremonium implicatum with GFP and evaluation of its biocontrol effect against Meloidogyne incognita. World J. Microb. Biot. 2015, 31, 549–556. [Google Scholar] [CrossRef]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The Potential Application of Endophytes in Management of Stress from Drought and Salinity in Crop Plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.C.; de Paula, S.; Torres, A.G.; de Souza, V.H.M.; Pascholati, S.F.; Schmidt, D.; Dourado Neto, D. Endophytic Fungi: Biological Control and Induced Resistance to Phytopathogens and Abiotic Stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef]
- Lacava, P.T.; Bogas, A.C.; Cruz, F.d.P.N. Plant Growth Promotion and Biocontrol by Endophytic and Rhizospheric Microorganisms from the Tropics: A Review and Perspectives. Front. Sustain. Food Syst. 2022, 6, 796113. [Google Scholar] [CrossRef]
- Rodriguez, R.; White, J., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Nazir, A.; Rahman, H.A. Secrets of plants: Endophytes. Int. J. Plant Biol. 2018, 9, 7810. [Google Scholar] [CrossRef]
- Aamir, M.; Rai, K.K.; Zehra, A.; Kumar, S.; Yadav, M.; Shukla, V.; Upadhyay, R.S. 12—Fungal endophytes: Classification, diversity, ecological role, and their relevance in sustainable agriculture. In Microbial Endophytes; Kumar, A., Singh, V.K., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 291–323. [Google Scholar]
- Nair, D.N.; Padmavathy, S. Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. 2014, 2014, 250693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Ghadge, V.; Kumar, P.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Nalli, Y.; Rathod, M.R.; Shinde, P.B. Biodiversity and Antimicrobial Potential of Bacterial Endophytes from Halophyte Salicornia brachiata. Antonie Van Leeuwenhoek 2021, 114, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Strobel, G.; Stierle, D.; Grothaus, P.; Bignami, G. The Search for a Taxol-Producing Microorganism Among the Endophytic Fungi of the Pacific Yew, Taxus brevifolia. J. Nat. Prod. 1995, 58, 1315–1324. [Google Scholar] [CrossRef]
- Castillo, U.F.; Strobel, G.A.; Ford, E.J.; Hess, W.M.; Porter, H.; Jensen, J.B.; Albert, H.; Robison, R.; Condron, M.A.; Teplow, D.B. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscansa. Microbiology 2002, 148, 2675–2685. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhang, Q.-Y.; Jia, M.; Ming, Q.-L.; Yue, W.; Rahman, K.; Qin, L.-P.; Han, T. Endophytic fungi with antitumor activities: Their occurrence and anticancer compounds. Crit. Rev. Microbiol. 2016, 42, 454–473. [Google Scholar] [CrossRef] [PubMed]
- Nalini, M.; Prakash, H. Diversity and bioprospecting of actinomycete endophytes from the medicinal plants. Lett. Appl. Microbiol. 2017, 64, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Raimi, A.; Adeleke, R. Bioprospecting of endophytic microorganisms for bioactive compounds of therapeutic importance. Arch. Microbiol. 2021, 203, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.P.; Braun, G.H.; Pupo, M.T.; Said, S. Antimicrobial activity from endophytic fungi Arthrinium state of Apiospora montagnei Sacc. and Papulaspora immersa. Braz. Arch. Biol. Techn. 2010, 53, 629–632. [Google Scholar] [CrossRef]
- Arivudainambi, U.E.; Anand, T.D.; Shanmugaiah, V.; Karunakaran, C.; Rajendran, A. Novel bioactive metabolites producing endophytic fungus Colletotrichum gloeosporioides against multidrug-resistant Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 2011, 61, 340–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.-M.; Chen, J.; Cui, J.-L.; Chen, X.-M.; Guo, S.-X. Antimicrobial activity and biodiversity of endophytic fungi in Dendrobium devonianum and Dendrobium thyrsiflorum from Vietman. Curr. Microbiol. 2011, 62, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, A.; Nagl, M.; Schwendinger, K.; Kreiseder, B.; Wiederstein, M.; Pretsch, D.; Genov, M.; Hollaus, R.; Zinssmeister, D.; Debbab, A. Antimicrobial and anti-inflammatory activities of endophytic fungi Talaromyces wortmannii extracts against acne-inducing bacteria. PLoS ONE 2014, 9, e97929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Long, J.; Li, W.J.; Yang, A.M.; Yang, Z.D.; Liu, X.F.; Zou, R.X. Evaluation of in vitro antibacterial activity of endophytic fungus from Anemone tomentosa. Adv. Mater. Res. 2014, 881–883, 484–487. [Google Scholar]
- Tonial, F.; Maia, B.H.; Gomes-Figueiredo, J.A.; Sobottka, A.M.; Bertol, C.D.; Nepel, A.; Savi, D.C.; Vicente, V.A.; Gomes, R.R.; Glienke, C. Influence of culturing conditions on bioprospecting and the antimicrobial potential of endophytic fungi from Schinus terebinthifolius. Curr. Microbiol. 2016, 72, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Passari, A.K.; Chandra, P.; Leo, V.V.; Kumar, B.; Uthandi, S.; Thankappan, S.; Gupta, V.K.; Singh, B.P. Determination and production of antimicrobial compounds by Aspergillus clavatonanicus strain MJ31, an endophytic fungus from Mirabilis jalapa L. using UPLC-ESI-MS/MS and TD-GC-MS analysis. PLoS ONE 2017, 12, e0186234. [Google Scholar] [CrossRef] [Green Version]
- Atiphasaworn, P.; Monggoot, S.; Gentekaki, E.; Brooks, S.; Pripdeevech, P. Antibacterial and Antioxidant Constituents of Extracts of Endophytic Fungi Isolated from Ocimum basilicum var. thyrsiflora Leaves. Curr. Microbiol. 2017, 74, 1185–1193. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Hamayun, M.; Jan, F.G.; Iqbal, A. Novel antimicrobial and antioxidative activity by endophytic Penicillium roqueforti and Trichoderma reesei isolated from Solanum surattense. Acta Physiol. Plant. 2019, 41, 164. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Ma, J.; Zhou, D.; Xu, J. Phylogenetic Diversity, Antimicrobial and Antioxidant Potential and Identification of Bioactive Compounds from Culturable Endophytic Fungi Associated with Mangrove Bruguiera sexangula (Lour.) Poir. Curr. Microbiol. 2021, 78, 479–489. [Google Scholar] [CrossRef]
- Mousa, W.; Raizada, M. The Diversity of Anti-Microbial Secondary Metabolites Produced by Fungal Endophytes: An Interdisciplinary Perspective. Front. Microbiol. 2013, 4, 65. [Google Scholar] [CrossRef] [Green Version]
- Sarethy, I.P.; Srivastava, N.; Pan, S. Endophytes: The unmapped repository for natural products. In Natural Bio-Active Compounds: Volume 1: Production and Applications; Akhtar, M.S., Swamy, M.K., Sinniah, U.R., Eds.; Springer: Singapore, 2019; pp. 41–70. [Google Scholar]
- Khiralla, A.; Spina, R.; Varbanov, M.; Philippot, S.; Lemiere, P.; Slezack-Deschaumes, S.; André, P.; Mohamed, I.; Yagi, S.M.; Laurain-Mattar, D. Evaluation of Antiviral, Antibacterial and Antiproliferative Activities of the Endophytic Fungus Curvularia papendorfii, and Isolation of a New Polyhydroxyacid. Microorganisms 2020, 8, 1353. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Chen, X.; Guo, L.; Che, Y. Pestalofones A–E, bioactive cyclohexanone derivatives from the plant endophytic fungus Pestalotiopsis fici. Bioorgan. Med. Chem. 2009, 17, 606–613. [Google Scholar] [CrossRef]
- Li, G.; Kusari, S.; Lamshöft, M.; Schüffler, A.; Laatsch, H.; Spiteller, M. Antibacterial Secondary Metabolites from an Endophytic Fungus, Eupenicillium sp. LG41. J. Nat. Prod. 2014, 77, 2335–2341. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Sommart, U.; Phongpaichit, S.; Sakayaroj, J.; Kirtikara, K. Metabolites from the endophytic fungus Phomopsis sp. PSU-D15. Phytochemistry 2008, 69, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Li, X.-M.; Liu, Y.; Xu, G.-M.; Wang, B.-G. Antimicrobial alkaloids produced by the mangrove endophyte Penicillium brocae MA-231 using the OSMAC approach. RSC Adv. 2017, 7, 55026–55033. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Li, X.; Zhao, J.; He, N.; Li, Y.; Zhang, T.; Wang, S.; Yu, L.; Xie, Y. GKK1032C, a new alkaloid compound from the endophytic fungus Penicillium sp. CPCC 400817 with activity against methicillin-resistant S. aureus. J. Antibiot. 2019, 72, 237–240. [Google Scholar] [CrossRef]
- Xie, J.; Wu, Y.-Y.; Zhang, T.-Y.; Zhang, M.-Y.; Peng, F.; Lin, B.; Zhang, Y.-X. New antimicrobial compounds produced by endophytic Penicillium janthinellum isolated from Panax notoginseng as potential inhibitors of FtsZ. Fitoterapia 2018, 131, 35–43. [Google Scholar] [CrossRef]
- Oide, S.; Turgeon, B.G. Natural roles of nonribosomal peptide metabolites in fungi. Mycoscience 2020, 61, 101–110. [Google Scholar] [CrossRef]
- Xu, W.-F.; Hou, X.-M.; Yao, F.-H.; Zheng, N.; Li, J.; Wang, C.-Y.; Yang, R.-Y.; Shao, C.-L. Xylapeptide A, an Antibacterial Cyclopentapeptide with an Uncommon L-Pipecolinic Acid Moiety from the Associated Fungus Xylaria sp. (GDG-102). Sci. Rep. 2017, 7, 6937. [Google Scholar]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wei, W.; Shi, J.; Chen, C.; Zhao, G.; Jiao, R.; Tan, R. Natural phenolic metabolites from endophytic Aspergillus sp. IFB-YXS with antimicrobial activity. Bioorg. Med. Chem. Lett. 2015, 25, 2698–2701. [Google Scholar] [CrossRef]
- Subban, K.; Subramani, R.; Johnpaul, M. A novel antibacterial and antifungal phenolic compound from the endophytic fungus Pestalotiopsis mangiferae. Nat. Prod. Res. 2013, 27, 1445–1449. [Google Scholar] [CrossRef]
- Hoffman, A.M.; Mayer, S.G.; Strobel, G.A.; Hess, W.M.; Sovocool, G.W.; Grange, A.H.; Harper, J.K.; Arif, A.M.; Grant, D.M.; Kelley-Swift, E.G. Purification, identification and activity of phomodione, a furandione from an endophytic Phoma species. Phytochemistry 2008, 69, 1049–1056. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Verma, V.C.; Kumar, A.; Gond, S.K.; Harper, J.K.; Hess, W.M.; Lobkovosky, E.; Ma, C.; Ren, Y.; Strobel, G.A. Javanicin, an Antibacterial Naphthaquinone from an Endophytic Fungus of Neem, Chloridium sp. Curr. Microbiol. 2009, 58, 233–238. [Google Scholar] [CrossRef]
- Erbert, C.; Lopes, A.A.; Yokoya, N.S.; Furtado, N.A.; Conti, R.; Pupo, M.T.; Lopes, J.L.C.; Debonsi, H.M. Antibacterial compound from the endophytic fungus Phomopsis longicolla isolated from the tropical red seaweed Bostrychia radicans. Bot. Mar. 2012, 55, 435–440. [Google Scholar] [CrossRef]
- Korman, T.P.; Ames, B.; Tsai, S.-C. 1.08—Structural enzymology of polyketide synthase: The structure–sequence–function correlation. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 305–345. [Google Scholar]
- Guo, J.; Ran, H.; Zeng, J.; Liu, D.; Xin, Z. Tafuketide, a phylogeny-guided discovery of a new polyketide from Talaromyces funiculosus Salicorn 58. Appl. Microbiol. Biot. 2016, 100, 5323–5338. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-S.; Wang, D.-J.; Li, X.-M.; Li, H.-L.; Meng, L.-H.; Li, X.; Pi, Y.; Zhou, X.-W.; Wang, B.-G. Antimicrobial polyketides from Trichoderma koningiopsis QA-3, an endophytic fungus obtained from the medicinal plant Artemisia argyi. RSC Adv. 2017, 7, 51335–51342. [Google Scholar] [CrossRef] [Green Version]
- Jouda, J.-B.; Kusari, S.; Lamshöft, M.; Mouafo Talontsi, F.; Douala Meli, C.; Wandji, J.; Spiteller, M. Penialidins A–C with strong antibacterial activities from Penicillium sp., an endophytic fungus harboring leaves of Garcinia nobilis. Fitoterapia 2014, 98, 209–214. [Google Scholar] [CrossRef]
- Shi, X.-S.; Meng, L.-H.; Li, X.-M.; Li, X.; Wang, D.-J.; Li, H.-L.; Zhou, X.-W.; Wang, B.-G. Trichocadinins B–G: Antimicrobial Cadinane Sesquiterpenes from Trichoderma virens QA-8, an Endophytic Fungus Obtained from the Medicinal Plant Artemisia argyi. J. Nat. Prod. 2019, 82, 2470–2476. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Liu, T.-K.; Shi, Q.; Yang, X.-L. Sesquiterpenoids and diterpenes with antimicrobial activity from Leptosphaeria sp. XL026, an endophytic fungus in Panax notoginseng. Fitoterapia 2019, 137, 104243. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.-J.; Zhang, S.-B.; Xian, P.-J.; Wu, X.; Yang, D.-F.; Fu, H.-Y.; Yang, X.-L. Emericellins A and B: Two sesquiterpenoids with an unprecedented tricyclo[4,4,2,1]hendecane scaffold from the liquid cultures of endophytic fungus Emericella sp. XL 029. Fitoterapia 2018, 131, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Y.; Liu, Y.; Lin, Y.-T.; Liu, Y.-C.; Guo, K.; Li, X.-N.; Luo, S.-H.; Li, S.-H. Antibacterial harziane diterpenoids from a fungal symbiont Trichoderma atroviride isolated from Colquhounia coccinea var. mollis. Phytochemistry 2020, 170, 112198. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-T.; He, Y.; Shen, L.; Hu, Z.-X.; Zhang, Y.-H. Bipolarins A–H, eight new ophiobolin-type sesterterpenes with antimicrobial activity from fungus Bipolaris sp. TJ403-B1. Chi. J. Nat. Med. 2019, 17, 935–944. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Sun, W.; Li, Q.; Li, X.-N.; Zhu, H.; Huang, J.; Liu, J.; Wang, J.; Xue, Y.; et al. Spiroaspertrione A, a Bridged Spirocyclic Meroterpenoid, as a Potent Potentiator of Oxacillin against Methicillin-Resistant Staphylococcus aureus from Aspergillus sp. TJ23. J. Org. Chem. 2017, 82, 3125–3131. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, X.; He, Y.; Sun, W.; Feng, W.; Liu, J.; Hu, Z.; Xu, Q.; Zhu, H.; Zhang, J.; et al. Aspermerodione, a novel fungal metabolite with an unusual 2,6-dioxabicyclo[2.2.1]heptane skeleton, as an inhibitor of penicillin-binding protein 2a. Sci. Rep. 2018, 8, 5454. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Document M26-A; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999. [Google Scholar]
- Qvortrup, K.; Hultqvist, L.D.; Nilsson, M.; Jakobsen, T.H.; Jansen, C.U.; Uhd, J.; Andersen, J.B.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Small Molecule Anti-biofilm Agents Developed on the Basis of Mechanistic Understanding of Biofilm Formation. Front. Chem. 2019, 7, 742. [Google Scholar] [CrossRef] [Green Version]
- Meenambiga, S.; Rajagopal, K. Antibiofilm activity and molecular docking studies of bioactive secondary metabolites from endophytic fungus Aspergillus nidulans on oral Candida albicans. J. Appl. Pharm. Sci. 2018, 8, 37–45. [Google Scholar]
- Rashmi, M.; Meena, H.; Meena, C.; Kushveer, J.S.; Busi, S.; Murali, A.; Sarma, V.V. Anti-quorum sensing and antibiofilm potential of Alternaria alternata, a foliar endophyte of Carica papaya, evidenced by QS assays and in-silico analysis. Fungal Biol. 2018, 122, 998–1012. [Google Scholar] [CrossRef]
- Fathallah, N.; Raafat, M.M.; Issa, M.Y.; Abdel-Aziz, M.M.; Bishr, M.; Abdelkawy, M.A.; Salama, O. Bio-Guided Fractionation of Prenylated Benzaldehyde Derivatives as Potent Antimicrobial and Antibiofilm from Ammi majus L. Fruits-Associated Aspergillus amstelodami. Molecules 2019, 24, 4118. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.-Q.; Lin, X.; Wang, Y.; Wang, J.; Zhou, X.; Yang, B.; Liu, J.; Yang, X.; Wang, Y.; Liu, Y. New phenyl derivatives from endophytic fungus Aspergillus flavipes AIL8 derived of mangrove plant Acanthus ilicifolius. Fitoterapia 2014, 95, 194–202. [Google Scholar] [CrossRef]




| Host Plant | Endophyte | Extract | Active Against | Reference |
|---|---|---|---|---|
| Smallanthus sonchifolius | Papulaspora immersa, Apiospora montagnei Sacc. | Ethyl acetate, n-butanol and water | E. coli, K. rhizophila, P. aeruginosa and S. aureus | [72] |
| Vitex negundo L. | Colletotrichum gloeosporioides | Methanol | B. subtilis, C. albicans, E. coli, P. aeruginosa and S. aureus (including several strains of MRSA) | [73] |
| Dendrobium devonianum and Dendrobium thyrsiflorum | Epicoccum nigrum, Fusarium tricinctum, Phoma sp. | Ethanol | B. subtilis, C. albicans, E. coli and S. aureus | [74] |
| Aloe vera | Talaromyces wortmannii | Ethyl acetate | E. faecalis, MRSA, P.acnes, S. epidermidis and S. pneumoniae | [75] |
| Anemone tomentosa | Unidentified | Ethyl acetate, n-butanol and methanol | B. licheniformis, E. coli, K. pneumoniae, P. aeruginosa, S. aureus and S. uberis | [76] |
| Schinus terebinthifolius | Alternaria sp., Bjerkandera sp., Diaporthe sp., Penicillium sp., Xylaria sp. | Ethyl acetate and methanol | C. albicans, P. aeruginosa and S. aureus | [77] |
| Mirabilis jalapa L. | Aspergillus clavatonanicus | Ethyl acetate | B.subtilis, E. coli, M. luteus and S. aureus | [78] |
| Ocimum basilicum var. thyrsiflora | Nigrospora sp. | Ethyl acetate | B. cereus, E. coli, S. epidermidis and V. cholerae | [79] |
| Solanum surattense | Penicillium roqueforti, Trichoderma reesei | Ethyl acetate | A. tumefaciens, X. oryzae, P. syringae and R. solanacearum | [80] |
| Bruguiera sexangula | Gelasinospora endodonta | Ethyl acetate | E. coli | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, D.J.; Palombo, E.A.; Moulton, S.E.; Zaferanloo, B. Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds. Microorganisms 2022, 10, 1990. https://doi.org/10.3390/microorganisms10101990
Caruso DJ, Palombo EA, Moulton SE, Zaferanloo B. Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds. Microorganisms. 2022; 10(10):1990. https://doi.org/10.3390/microorganisms10101990
Chicago/Turabian StyleCaruso, Daniel J., Enzo A. Palombo, Simon E. Moulton, and Bita Zaferanloo. 2022. "Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds" Microorganisms 10, no. 10: 1990. https://doi.org/10.3390/microorganisms10101990
APA StyleCaruso, D. J., Palombo, E. A., Moulton, S. E., & Zaferanloo, B. (2022). Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds. Microorganisms, 10(10), 1990. https://doi.org/10.3390/microorganisms10101990







