Abstract
Fermentation is an ancient method used worldwide to process and preserve food while enhancing its nutraceutical profile. Alga-based fermented products have recently been developed and tested due to growing interest in healthy sustainable diets, which demands the development of innovative practices in food production, operating for both human health and Earth sustainability. Algae, particularly microalgae such as Arthrospira platensis, Chlorella vulgaris, and Dunaliella salina, are already cultivated as sources of food due to their valuable compounds, including proteins, pigments, lipids, carotenoids, polyunsaturated fatty acids, steroids, and vitamins. Due to their nutritional composition, functional diversity, and flexible metabolism, microalgae represent good fermentation substrates for lactic acid bacteria (LAB) and yeasts. This review presents an overview of the scientific studies on microalga fermentation underlining microalgae’s properties and health benefits coupled with the advantages of LAB and yeast fermentation. The potential applications of and future perspectives on such functional foods are discussed.
1. Introduction
Conventional food production is no longer sustainable and places a heavy burden on the environment as the global food system is causing greenhouse gas emissions, biodiversity loss, terrestrial ecosystem destruction, freshwater consumption, and nutrient loading [1]. Since the world’s population will reach almost 10 billion in 2050 [1,2], a current planetary challenge is feeding the global population while preserving natural ecosystems. Drastic changes are required to move towards healthy diets, regenerative production practices, and waste valorization.
In the search for new or alternative crops to meet the growing demand for food (and feed), algae represent an emerging biological resource for sustainable innovative transformation [3,4]. Indeed, with respect to environmentally friendly food production, algae can be produced on non-arable land, allowing for a high rate of production per square meter compared to plants; intensive alga cultivation systems require only a minimal use of freshwater, and can even use seawater [5]. Food-processing wastewater also has the potential to be valorized as an algal growth medium once its biomass safety has been assessed [4].
1.1. Microalgae’s Properties and Health Benefits
Algae are a functional group of organisms able to use light as a source of energy to fuel cell metabolism and growth while releasing oxygen; they actually include species that are very phylogenetically distant, including eukaryotes and cyanobacteria, performing oxygenic photosynthesis [6].
Algae range in size from micrometers (for microalgae) to meters (for macroalgae or seaweeds). Microalgae are unicellular and belong to several taxonomic groups: Rhodophyta (red algae), Chlorophyta (green algae), Charophyta, Glaucophyta, Chlorarachniophyta, Euglenozoa (Euglenoids), Bacillariophyceae (Diatoms), Dinophyta (Dinoflagellate), Eustigmatophyceae, and Cryptophyta [7].
Moreover, in addition to oxygenic photosynthesis, many microalgae perform heterotrophic or mixotrophic metabolism. Such a unique ability, which cannot be found in plants [8,9], enables them to thrive in various extreme environmental conditions, even in the absence of light. Accordingly, microalgae are globally distributed and may be found in all types of environments, from deserts to arctic ice [10].
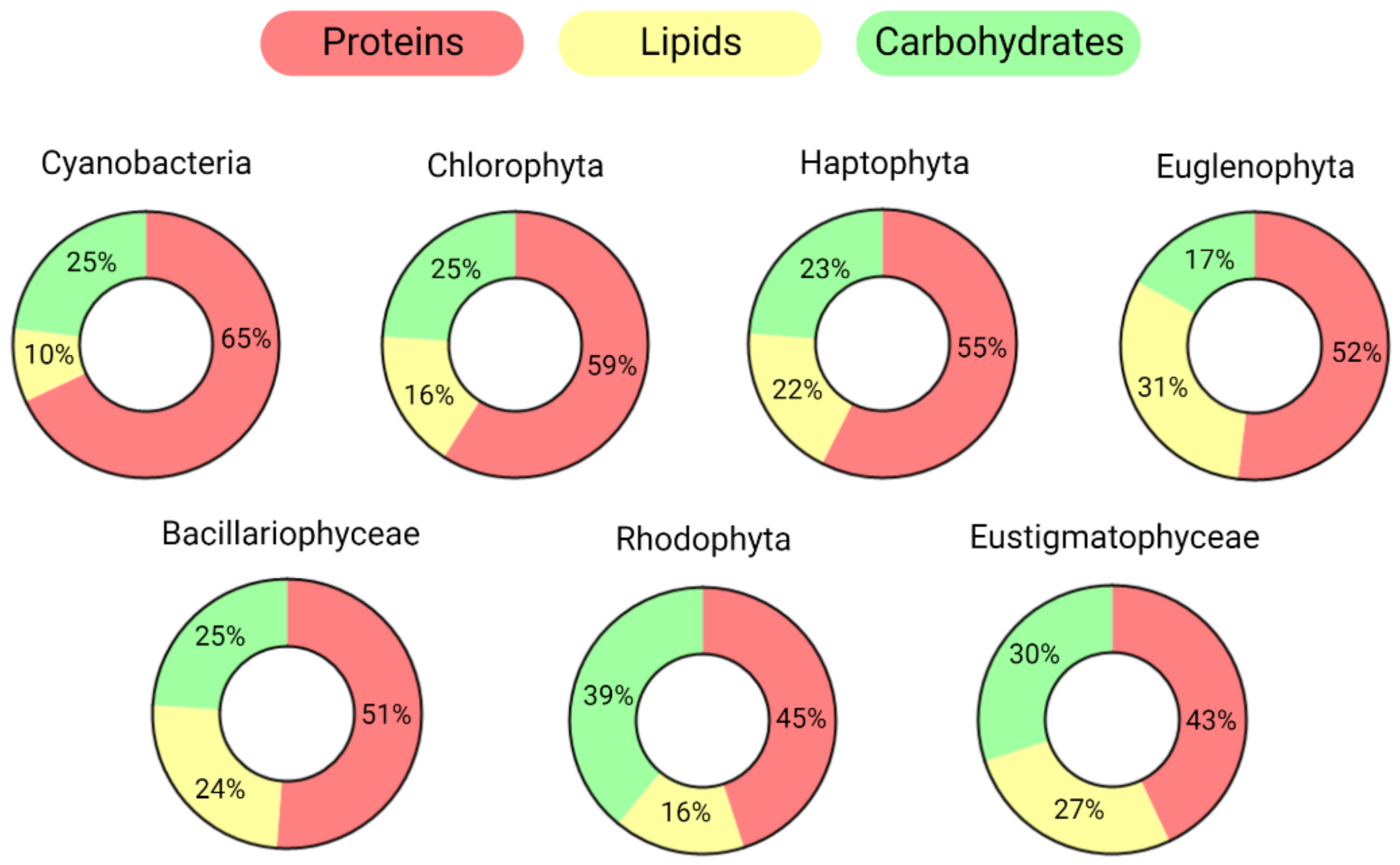
Due to their metabolic flexibility and diversity in terms of genetics, morphology, and habitat, the average macromolecular composition (regarding lipids, proteins, and carbohydrates) varies widely among distinct species. In nutrient-rich conditions, cyanobacteria usually accumulate higher concentrations of proteins than other phyla, while Rhodophyta and Euglenophyta store more carbohydrates and lipids, respectively (Figure 1).
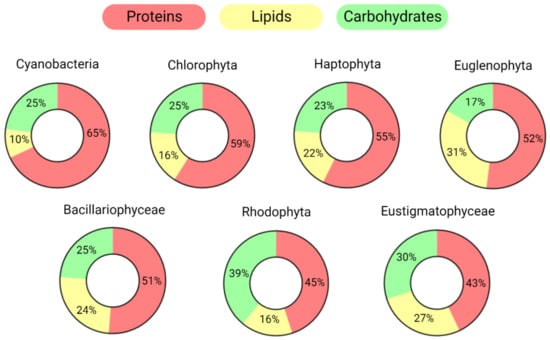
Figure 1.
Average macromolecular compositions of the main microalgal taxonomic groups. Groups are ordered based on their protein abundance. Only the three main pools are reported: proteins, lipids, and carbohydrates. The values are normalized, excluding other main components of the dry weight such as ash, moisture, and pigments.
The values reported in Figure 1 were calculated by averaging the organic compositions of species belonging to the same phylum/taxonomic group based on the scientific literature [3,11,12,13,14,15,16]; they refer to the composition determined during the exponential phase when extracellular nutrients were replete [17]. Both the phylogenetic history, which refers to the evolutive process of host and plastid endosymbiosis, and the environmental conditions are crucial in determining the macromolecular composition of algae [18,19].
Indeed, when nutrients become scant, algal acclimation strategies may lead to significant changes in the proportions of the main macromolecular pools [13,14,19].
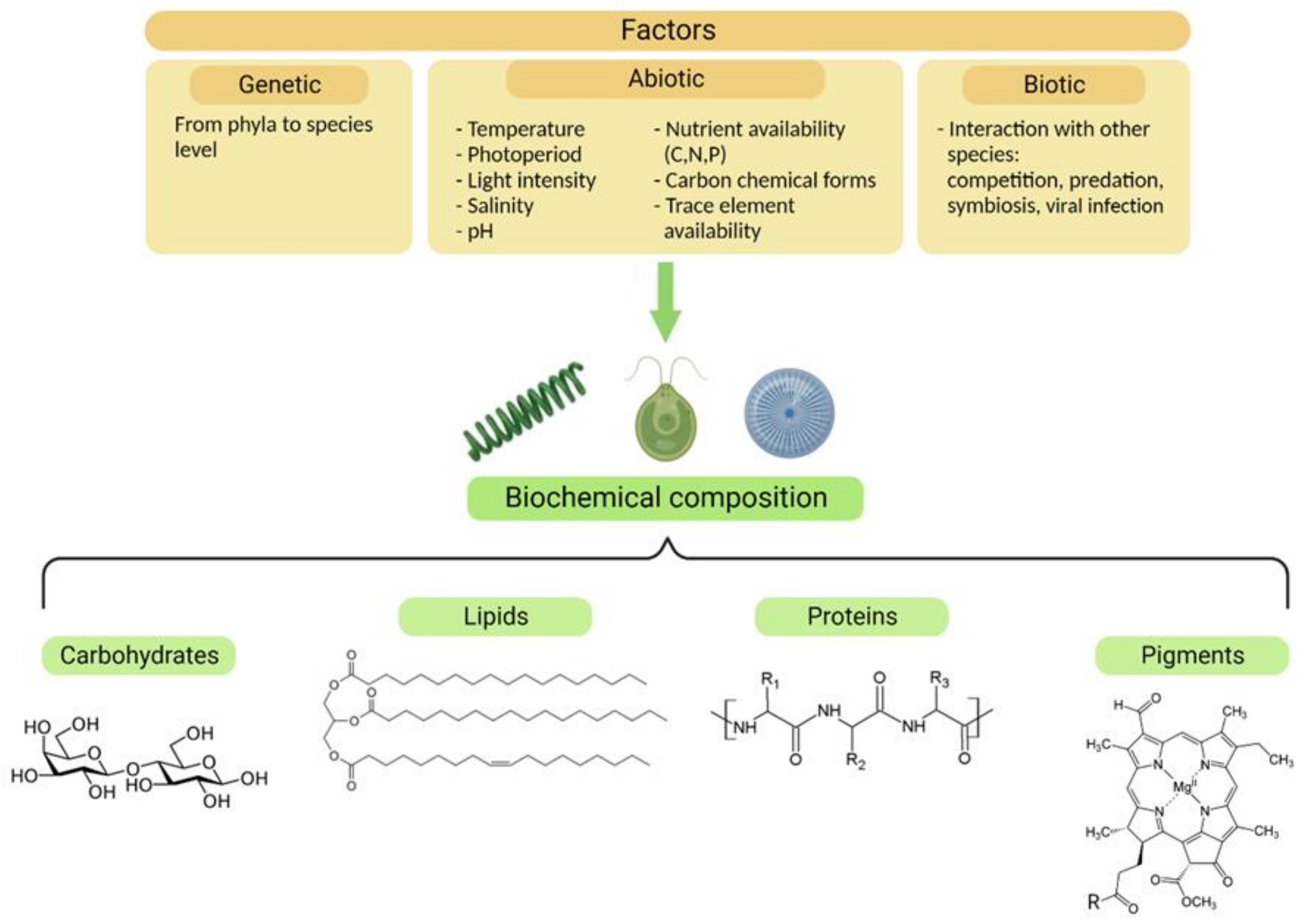
Akin to nutrient starvation or limitation, many other abiotic factors contribute to the biochemistry of microalgae (Figure 2). Additionally, biotic factors such as competition for resources, predation, and symbiosis can drastically affect the behavior and composition of microalgae [18,20,21]. The presence of predators affects algal palatability, modulating their organic composition, and activating their chemical defenses [20].
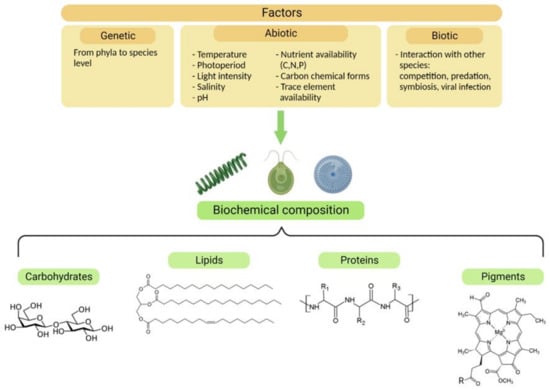
Figure 2.
Factors influencing the biochemical composition of microalgae. Genetic, abiotic, and biotic factors can modify the concentrations and proportions of the four classes of molecules represented in the figure, which are desirable components for nutraceuticals.
From a biotechnological point of view, the easiest and most scalable way to modify the biochemistry of microalgae is through the manipulation of abiotic growth conditions, such as the light intensity and quality, photoperiod, pH, salinity, temperature, nutrient concentration, and chemical form [17,22]. Despite the consequent physiological and biochemical responses being species-specific, a common trend exists across microalgae: for instance, when nitrogen is not available, C-rich molecules, i.e., lipids and carbohydrates, accumulate inside the cell at the expense of the protein pool [13,16,17]. Usually, just one type of energy and C-storage molecular pool is preferred, since the precursor for fatty acid synthesis, glycerol-3-phosphate, is a catabolite derived from glucose [23,24], which is also the starting point for carbohydrate production.
Carbohydrates play two main roles in algae: as structural components of the cell wall and as energy-storage compounds [11]. Glycogen, amylose, amylopectin, floridean starch, and chrysolaminarin are just a few of the storage compounds found in microalgae [14]. Lipids play similar roles in cells, and in addition to their concentrations, their biochemical properties are also modulated by abiotic factors. Light and temperature play major roles in the unsaturation rates of algal fatty acids [15,22]. In particular, low temperatures increase the degree of unsaturation of lipids and increase the percentages of PUFAs (polyunsaturated fatty acids) [25].
Among macromolecules, pigments play an essential role in microalgae, and, apart from chlorophyll a, which is the major photosynthetic pigment, many other accessory compounds play roles in light capture and protection from oxidative damage [12]. Widely used in aquaculture and medicine, β-carotene, astaxanthin, lutein, fucoxanthin, and phycobilins are only a few of the accessory pigments found in the algal plastids. Unlike chlorophylls, most of them are positively affected by irradiance [25]. Moreover, nitrogen-limited conditions may contribute to the accumulation of pigments such as astaxanthin and β-carotene in the green algae Haematococcus pluvialis and Dunaliella salina, respectively [25].
Microalgae are known to have been consumed by humans for thousands of years [3]; historical records report the consumption of the cyanobacteria Nostoc, Aphanizomenon flos-aquae (Klamath algae), and Arthrospira spp. (known commercially as Spirulina) [26]. The last is currently gathered when it naturally blooms in alkaline lakes by the Kanembu people in Chad and by the Myanmar people in the Republic of the Union of Myanmar [13], but archeological data also suggest that Aztecs commonly used Spirulina as a food during the 14th century [27].
Today, microalgal biomass is primarily sold for nutraceutical and food applications (Table 1). The microalgal species currently approved for EU and Italian markets are regulated and reported for the European Community by the Food Safety Regulation (EC 178/2002) and the Novel Food Regulation (EC 258/97; EC 2015/2283) [9,16,28]. Due to their high-quality nutritional protein value and beneficial amounts of fatty acids, vitamins, antioxidants, and many other bioactive molecules, microalgae are labeled with the nonscientific term “superfood” [3,26], and their health benefits are well recognized and documented [14,15]. As primary producers, algae are responsible for the accumulation of bioactive and bioavailable molecules in the entire food chain [27]. Thus, health benefits can be transferred to humans through the direct consumption of algae or indirectly by eating animals fed with algae; in aquaculture, algae are used as feed and supplements for fish farming [3].
Spirulina and Chlorella spp. are worthy of more detailed discussion, due to their widespread and historical use. The two main commercial species of Spirulina are Arthrospira platensis and Arthrospira maxima, which are filamentous, brackish cyanobacteria. Spirulina was found to have several properties useful for human health, including antioxidant and anti-hypertensive activity [29]. Moreover, the efficacy of Spirulina extract in combating foodborne pathogenic bacteria, either in vitro or in food matrices, has been demonstrated, suggesting perspectives for its further application as a new food preservative [30]. Chlorella, which includes C. vulgaris and C. sorokiniana, among others, is a unicellular eukaryotic spherical green alga belonging to the phylum Chlorophyta [31]. Chlorella has an appreciable content of arginine, a precursor involved in immune system functions, and sterols such as vitamin D2, involved in the absorption of calcium and phosphate [31].
Except for the aforementioned species, which are generally sold as dried whole biomass for the nutraceutical sector and formulated as tablets or powder, the algal market is dominated by high-value components extracted and purified from microalgae [9,27]. For instance, astaxanthin from H. pluvialis has antioxidant and anti-inflammatory effects, and its market price can reach USD 2000 per kg (total production of 300 tons per year) [3,14]. PUFAs (Ω3-6), mostly extracted from Cryptothecodininum cohnii and Schizochytrium limacinum, act at the cardiovascular and nervous system levels; their estimated value is USD 140 per kg [14].
Besides their beneficial properties, algae can also have adverse effects, which need to be considered. Some cyanobacteria can produce toxins under certain environmental conditions (e.g., microcystins, which can affect the nervous system). Contamination with toxic species may occur in algal cultivation systems for GRAS (generally recognized as safe) species [32]. Moreover, heavy metals (such as mercury, cadmium, and arsenic) can bioaccumulate inside algal cells. Additionally, the presence of a cell wall can restrict the access of human digestive enzymes to cell components, resulting in low biomass digestibility [13,14]. The cell wall is silicified in diatoms, calcified in some haptophytes, and organic in cyanobacteria and other eukaryotes [11]; therefore, in vitro digestion models are required to provide useful information about the nutrient bioavailability of microalgal organic matter [13].

Table 1.
Bioactive compounds from microalgae, their applications, and their potential health benefits.
Table 1.
Bioactive compounds from microalgae, their applications, and their potential health benefits.
| Microalgae | Product | Application | Effect on Human Health | Reference |
|---|---|---|---|---|
| Arthrospira platensis (Spirulina) | Biomass | Nutritional supplements and food ingredient | High protein content and rich in essential amino-acids. Rich in Fe, mineral elements, and vitamins | [33,34] |
| Phycocyanin | Nutritional supplements | Antioxidant, anti-inflammatory | [35] | |
| EPS | Medical applications | Anti-thrombotic and anti-tumoral | [6] | |
| Aphanizomenon flos-aquae | Biomass | Nutritional supplements | High protein content and essential fatty acids (Ω3) | [36] |
| Chlorella vulgaris | Biomass | Nutritional supplements and food ingredient | High protein and β-glucan content. Anti-inflammatory and anti-oxidant. | [37] |
| Dunaliella salina | β-carotene | Nutritional supplements and food ingredient | Antioxidant, pro-vitamin A, anti-allergic, anti-inflammatory | [38] |
| Dunaliella tertioletca | Vitamin A, B, E | Nutritional supplements | Maintenance of effective vision, protection against anemia and support of brain function, anti-oxidant | [39] |
| Haematococcus pluvialis | Astaxanthin | Nutritional supplements or supplements in aquaculture | Antioxidant, anti-inflammatory | [40] |
| Isochrysis galbana | EPA and DHA (Ω3) | Nutritional supplements or supplements in aquaculture | Anti-inflammatory, cardiovascular benefits, improves nervous system, atherosclerosis protection | [41,42,43] |
| Cryptothecodininum cohnii | DHA (Ω3) | Nutritional supplements or supplements in aquaculture | Cardiovascular benefits and improves nervous system | |
| Schizochytriumlimacinum | DHA (Ω3) | Nutritional supplements or supplements in aquaculture | Cardiovascular benefits and improves nervous system | |
| Nannochloropsis oceanica | EPA (Ω3) | Nutritional supplements or supplements in aquaculture | Cardiovascular benefits and protection against atherosclerosis, anti-inflammatory | |
| Porphyridium cruentum | ARA (Ω6) | Nutritional supplements or supplements in aquaculture | Improve functional development in infants | [44] |
| Porphyridium purpureum | EPS | Medical application | Anti-thrombotic and anti-tumoral | [6] |
| Phaeodactylum tricornutum | Fucoxanthin | Medical application | Anti-tumoral and beneficial effect against obesity | [45] |
| Euglena gracilis | Paramylon | Medical application | Anti-inflammatory and anti-tumoral | [27] |
EPS, exopolysaccharides; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ARA, arachidonic acid.
1.2. Advantages of Fermentation
Fermentation is an ancient method used worldwide for food processing and preservation [46,47,48,49]. From a biochemical point of view, fermentation has been defined as the breakdown of organic compounds by microorganisms, in either the presence (aerobic) or absence (anaerobic) of oxygen for energy production in terms of ATP production, coupled with other products that may be used as valuable commercial molecules within the food industry and other applications [46]. Alcoholic fermentation and lactic acid fermentation are the most common fermentation types.
Alcoholic fermentation is a well-known process that is mainly carried out by yeasts that transform sugars into ethanol and CO2. S. cerevisiae is the main organism responsible for alcoholic fermentation, and it is frequently used as a starter culture in the transformation of sugar-rich matrices, such as grapes and other fruits or vegetables. To date, the alcoholic fermentation of algae by yeasts has been mainly applied in the biofuel industry [46].
Lactic acid fermentation is carried out by lactic acid bacteria (LAB), a group of Gram-positive, non-spore-forming bacteria that ferment carbohydrates as the main carbon source to produce lactic acid (homofermentation), or lactic acid along with ethanol or acetate and carbon dioxide (heterofermentation) as the major end-product [46,48]. LAB are widely used in the food industry due to their metabolic characteristics of being able to successfully transform and enhance the stability and quality of food products [47]. Indeed, the rapid acidification of food matrices is the main desired LAB activity; hydrolyzing activity, antagonistic activity, and biosynthetic activity are also present [49]. In addition to the production of lactic acid and other organic acids, LAB are able to promote the degradation of polymers such as proteins, lipids, and indigestible polysaccharides (e.g., starch, cellulose, hemicellulose, and others) by synthesizing enzymes that hydrolyze such polymers into smaller units, improving their digestibility and bioavailability [46]. These molecules may also act as flavor substances (as amino acids), thus influencing the aromatic profiles of food products, or show antioxidant, anti-inflammatory, antimicrobial, neuroprotective, anticoagulant, and immunomodulatory effects, thus enhancing the nutraceutical profiles of food [46]. It is worth noting that all the degradation and enzymatic processes that occur during fermentation are natural processes carried out by microorganisms without the use of solvents or acid–base hydrolyses; the latter are commonly applied for the extraction of bioactive molecules from complex matrices, causing the eventual production of large amounts of toxic wastes [46]. LAB are also able to excrete several metabolites that enhance food’s organoleptic and nutritional profiles [46,48]. Indeed, LAB may produce (i) functional molecules, such as gamma-aminobutyric acid (GABA), which is an important neurotransmitter in the nervous system and is involved in many human diseases; (ii) valuable nutritional compounds, such as polyphenols and polyunsaturated fatty acids; (iii) a wide range of organic acids with probiotic roles within the human intestine; (iv) antimicrobial molecules (e.g., bacteriocins) that increase the shelf life of foods; (v) antioxidant molecules, such as vitamins, polysaccharides, and phenolic substances; vi) exopolysaccharides (EPS) that increase the viscosity of foods; vii) a wide range of volatile compounds (e.g., organic acids, heterocyclic compounds, aldehydes, and ketones) that impact the aromatic profiles of a food; and viii) enzymes that reduce harmful and toxic molecules, such as mycotoxins and antinutritional factors, including phytic acid [46,48]. Overall, LAB’s properties and metabolism provide a wide range of marketable products differentiated in terms of their sensory, nutritional, and safety properties [49].
Moreover, some LAB strains can be added directly to food due to their probiotic functions, promoting human health. Indeed, probiotics are defined as “live microorganisms which when administered in adequate amounts, confer a health benefit on the host” [49]. Probiotics are resistant to the acidity of the gastrointestinal tract and to the bile environment; they are able to adhere to the intestinal mucosa by competing with pathogenic bacteria, improving the intestinal microbiota and producing health benefits, such as reducing cholesterol, inflammation, and lactose intolerance, as well as diarrhea [49].
The specific objective of this review is to provide an overview of microalgal fermentation for developing potential added-value food products.
2. Methodology
2.1. Search Procedure
The literature search was restricted to scientific publications dealing with microalga fermentation available in the PubMed (http://www.ncbi.nlm.nih.gov/pubmed accessed on 14 September 2022) and ScienceDirect (http://www.sciencedirect.com/ accessed on 14 September 2022) databases using the following keywords: ‘microalgae fermentation,’ ‘Spirulina fermentation,’ ‘Chlorella fermentation,’ ‘microalgae food fermentation,’ and ‘microalgae beverages fermentation.’ A cross-referencing approach was used to find other scientific studies. The inclusion criteria used for article selection were as follows: (i) publication in a peer-reviewed journal; (ii) published in English. The exclusion criteria were as follows: proceedings, project documents, theses, and papers on (a) microalgae’s nutritional aspects, (b) non-fermented foods based on microalgae, (c) microalga fermentation for purposes other than food production (e.g., cosmetics or environmental and bioenergetic applications), and (d) economic aspects related to algae.
The identified papers were first screened by title and abstract, and then, the full articles of the selected abstracts were retrieved and read. The literature search ultimately yielded 46 documents, including 5 reviews and 41 scientific articles.
2.2. Database Generation
The following information was extracted from the 41 scientific articles: (i) the publication identification: authors, year of publication, and journal; (ii) the microalga species; (iii) the formulation and concentration of the microalga species; (iv) the microbial inoculum employed as the starter culture; (v) the fermentation conditions; (vi) the storage conditions for the novel fermented product, if applied; and (vii) the novel food product, if specified.
3. Results and Discussion
3.1. Literature Review of Microalgal Fermentation for Novel Food Production
Table 2 shows a chronological distribution of the identified papers focused on the innovative application of microalgal fermentation in food production. In most of the papers, Arthrospira spp. (A. platensis or A. maxima) was chosen as a substrate for fermentation (35/41); two papers dealt with both A. platensis and C. vulgaris; in two papers, only C. vulgaris was the experimental organism; in one paper, Pavlova lutheri was used; and in one article, 10 different cyanobacterial and microalgal species, namely, Nostochopsis lobatus, Nostoc commune, Nostoc flagelliforme, Nostoc verrucosum, A. platensis, Dunaliella tertiolecta, Chlorogonium spp., Porphyridium purpureum, Pleurochrysis carterae, and Euglena spp., were selected for food fermentation.
According to Table 2, the microalgal powder/spray-dried/lyophilized/dry/dehydrated biomass was the most common microalgal formulation to be incorporated into the novel fermented food or beverage (33 out of 41 papers), while spirulina grains were reported in 1 paper, the use of fresh vs. oven-dried Spirulina biomass was compared in 1 paper, and the remaining 6 papers cited the microalgal biomass in filtrate/extract/wet/fresh form. The microalga concentration within each novel fermented product mainly ranged from 0.25% to 10%. Concerning the microbial inoculum used to ferment the microalgal species, most of the studies employed pure LAB cultures, mainly the yogurt starter cultures Lactobacillus delbrueckii spp. bulgaricus and Streptococcus thermophilus, and lattococci (Lactococcus lactis subsp. lactis, Lactococcus lactis subsp cremoris, and Lactococcus casei), as well as Lacticaseibacillus casei (basonym Lactobacillus casei), Lactobacillus acidophilus, Lactiplantibacillus plantarum (basonym Lactobacillus plantarum), Lacticaseibacillus paracasei (basonym Lactobacillus paracasei), Levilactobacillus brevis (basonym Lactobacillus brevis), Lacticaseibacillus rhamnosus (basonym Lactobacillus rhamnosus), Lactobacillus helveticus, Streptococcus salivarius subsp. thermophilus, Enterococcus faecium, Weissella spp., and Leuconostoc spp.; several strains of Bacillus subtilis, Bacillus licheniformis, and Bacillus amyloliquefaciens were also employed. Moreover, some probiotic cultures were also added to the novel formulation, including Bifidobacterium lactis, Bifidobacterium animalis, Bifidobacterium spp., and strains of Lb. acidophilus and Lpb. plantarum. Interestingly, two papers dealt with the use of a natural mixed starter culture, such as milk kefir grains and water kefir grains, while two studies developed novel products by fermentation with only pure yeast species, namely, Debaryomyces hansenii, Kluyveromyces marxianus, Saccharomyces cerevisiae, and Hansenula polymorpha. The scientific articles were then clustered according to topic: (i) 22 articles on the development and characterization of new fermented food products supplemented with microalgae, (ii) 13 articles on microalgae as the sole substrate for fermentation to obtain innovative functional foods or ingredients, and (iii) 6 articles on the effect of the addition of microalgae on the growth and viability of LAB and probiotics. These articles are listed in Table 2.

Table 2.
Studies on microalgal fermentation for food applications.
Table 2.
Studies on microalgal fermentation for food applications.
| Application | Microalga Species | Formulation | Concentration | Microbial Inoculum/Starter Cultures | Fermentation Conditions | Storage Conditions | Food Product | Reference |
|---|---|---|---|---|---|---|---|---|
| Microalgae as microbial growth promoter | Arthrospira platensis | Filtrate | 1:1 filtrate added to synthetic media | Lactococcus lactis subsp. lactis C2, Lactococcus casei YK3, Lactobacillus delbruekii subsp. bulgaricus YL1, Streptococcus salivarius subsp. thermophilus TH4, Lactobacillus spp. JL2 | 37 °C, 24 h | n.d. | n.s. | [50] |
| Arthrospira platensis | Extract | 3 mg/mL in fermented milk | Streptococcus thermophilus TH4, Lactococcus lactis subsp. lactis C2, Lactobacillus delbruekii subsp. bulgaricus YL1, Lactobacillus acidophilus LO1 | 37 °C, 20 h | n.d. | Fermented milk | [51] | |
| Arthrospira platensis | Powder | 3g/L in milk | Lactobacillus acidophilus, Bifidobacterium spp., Streptococcus thermophilus | 40 °C, 6 h | 4 °C, 42 days, and 15 °C, 18 days | Fermented ABT milk | [52] | |
| Arthrospira platensis; Chlorella vulgaris | Spray-dried biomass | 3 g/L in milk | Lactiplantibacillus plantarum, Enterococcus faecium | 30 °C or 37 °C | n.d. | Fermented milk | [53] | |
| Arthrospira platensis | Powder | 1, 5, 10 mg/mL | Lactobacillus acidophilus MTCC447, Streptococcus thermophilus MTCC1938, Lacticaseibacillus casei MTCC1423 | 37 °C, 10 h | n.d. | n.s. | [54] | |
| Arthrospira platensis | Powder | 0.5, 1.0% (w/v) in milk | Bifidobacterium animalis subsp. lactis Bb12, Lactobacillus acidophilus La-5 | 42 °C, 6 h | 5 ± 1 °C, 15 days | Fermented milks | [55] | |
| Microalgae supplementation to fermented foods | Arthrospira platensis | Powder | 0.5, 1.0% (w/v) in milk | Streptococcus thermophylus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus acidophilus | 40 °C and 42 °C, until the pH 4.7 was reached | 4 °C, 30 days | Yogurt and acidophilus milk | [56] |
| Arthrospira platensis; Chlorella vulgaris | Powder | 0.25, 0.5, 1.0% (w/v) in milk | Lactobacillus acidophilus LA-5, Bifidobacterium lactis BB-12, Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus | 40 °C until the pH 4.7 was reached | 5 °C, 28 days | Probiotic yogurt | [57] | |
| Arthrospira platensis | Powder | 0.3, 0.5, 0.8% in milk | Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. cremoris, Lactobacillus acidophilus LA-5 | 30 °C, 48 h | 4 °C, 45 days | Probiotic feta cheese | [58] | |
| Arthrospira platensis | Powder | 0.25, 0.5, 0.75, 1.0% (w/v) in milk | Lactobacillus bulgaricus, Streptococcus thermophilus | 42 °C, 4 h | 7, 14, 21, 28 days of refrigerated storage | Yogurt | [59] | |
| Arthrospira platensis | Grains | 0.5, 1.0, 1.5% | Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus | n.r. | n.d. | Kareish cheese | [60] | |
| Arthrospira platensis | Dry biomass | 1.0% (w/v) in soy milk | Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus | 37 °C, 24 h | 4 °C | Soy yogurt | [61] | |
| Arthrospira platensis | Dry biomass | 0.5, 1.0, 1.5% in soy milk | Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus | 37 °C, 16 h | 4 °C | Soy yogurt | [62] | |
| Arthrospira platensis | Powder | 5 mg/mL in milk | Streptococcus salivarius subsp. thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, Bifidobacterium spp. | 37 °C, 48 h | n.d. | Labneh | [63] | |
| Arthrospira platensis | Fresh vs oven-dried | 0.1, 0.3, 0.5% (w/v) in milk | Streptococcus thermophilus, Lactobacillus bulgaricus | 42 °C, 4 h | 4 °C, 24 h | Yogurt | [64] | |
| Arthrospira platensis | Powder | 0.5, 1.0, 1.5% (w/w) | Lacticaseibacillus casei, Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. cremoris | 40 °C until the pH 5.2 was reached | 4 °C, 60 days | Feta-type cheese | [65] | |
| Arthrospira platensis SP6/CFTRI | Fresh wet biomass (spirulina milk emulsion) | 10% (w/w) | Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus | 42 °C until the pH 4.6–4.7 was reached | 6–8 °C | Probiotic yogurt | [66] | |
| Arthrospira platensis | Powder | 0.25, 0.50, 1.0% (w/v) in milk | Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus acidophilus, Bifidobacterium lactis | 40 °C until the pH 4.4 was reached (about 4 h) | 4 ± 1 °C, 21 days | Ayran | [67,68] | |
| Arthrospira platensis | Powder | 1.0% (w/w) | Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus | 42 °C until the pH 4.6 was reached | 4 °C | Low-fat yogurt | [69] | |
| Arthrospira platensis F&M-C256 | Lyophilized biomass | 10% (w/v) in vegetal soybean drink or water | Lactiplantibacillus plantarum ATCC 8014 | 37 °C, 72 h, 100 rpm stirring | n.d. | Vegetal soybean drink | [70] | |
| Arthrospira platensis | Dried biomass | n.s. | Lacticaseibacillus paracasei | 40 °C until the pH 4.7 was reached | 4 °C | Probiotic yogurt | [71] | |
| Arthrospira platensis | Algae biomass in water (5% w/v) | 0.25, 0.50% (w/v) in skim milk powder (SSM) and in commercial soy-based beverages (SBB) | Lactococcus lactis subsp. lactis, Lactococcus lactis subsp. cremoris, Lactococcus casei, Lactobacillus delbruekii subsp. bulgaricus, Streptococcus thermophilus, Lacticaseibacillus rhamnosus, Lactobacillus helveticus, Lactobacillus delbruekii subsp. lactis, Weissella spp., Leuconostoc spp. | 37 °C, 48 h | n.d. | Milk and soy fermented beverages | [72] | |
| Arthrospira spp. | Dry biomass | 1.0, 2.0% (w/v) in milk | Milk kefir grains | 25–37 °C, 24 h | n.d. | Milk kefir | [73] | |
| Arthrospira platensis | Powder | 0.25, 0.50% in almond milk and soy milk | Lactobacilli and lactococci plant-based kefir culture | 42 °C until a pH 4.5 ± 0.02 was reached | 4 °C, 21 days | Vegan kefir (soy milk kefir and almond milk kefir) | [74] | |
| Arthrospira platensis | Powder | 0.25, 0.5, 1.0 g/kg | Yogurt culture | 20 °C, 24 h and then 12 °C, 48 h | n.d. | Greek soft cheese | [75] | |
| Chlorella vulgaris | Powder | 1.5% (w/v) in soya drink | Levilactobacillus brevis ŁOCK 0944 | 30 °C, 4 h and then matured at 18 °C, 20 h | n.d. | Soya drink | [76] | |
| Arthrospira spp. | Lyophilized biomass | 1.6 g, 2.4 g in 100 mL of distilled water | Water kefir grains | 25 °C, 48 h | n.d. | Water kefir | [77] | |
| Microalgae as the sole substrate for fermentation | Pavlova lutheri | Powder | 1:15 (w/v) | Hansenula polymorpha | 37 °C, 12 days | n.d. | n.s. | [78] |
| Arthrospira platensis | Powder | 2.0% (w/v) | Lactiplantibacillus plantarum B7, Lactiplantibacillus plantarum C8-1, Lactiplantibacillus plantarum 121, Lactobacillus acidophilus NCFM, Bacillus subtilis 168 | 37 °C, 24 h | n.d. | n.s. | [79] | |
| Arthrospira maxima | Powder | 10% (w/v) | Lactiplantibacillus plantarum HY-08 | 37 °C, 4 days | n.d. | n.s. | [80] | |
| Arthrospira maxima | Powder | 10% (w/v) | Lactiplantibacillus plantarum HY-08 | 37 °C | n.d. | n.s. | [81] | |
| Arthrospira platensis | Wet biomass | 5 g in 30 mL of distilled water | Lactiplantibacillus plantarum | 37 °C, 72 h in a shaker | n.d. | Fermented nutraceutical product | [47] | |
| Arthrospira platensis F&M-C256 | Lyophilized biomass | 10% (w/v) | Lactiplantibacillus plantarum ATCC 8014 | 37 °C, 72 h, 100 rpm stirring | n.d. | Probiotic-based products | [82] | |
| Chlorella vulgaris | Powder | 0.1, 1.5% (w/v) | Levilactobacillus brevis ŁOCK 0944, Levilactobacillus brevis ŁOCK 0980, Levilactobacillus brevis ŁOCK 0992, Levilactobacillus brevis MG451814 | 30 °C, 24 h | n.d. | n.s. | [83] | |
| Arthrospira platensis | Powder | 2.0% (w/v) | Lactiplantibacillus plantarum DY-1, Lacticaseibacillus casei KDB-LC, Lactobacillus acidophilus KDB-03, Lactobacillus acidophilus KDB-08, Bacillus subtilis ND, Bacillus spp., Bacillus amyloliquefaciens LXZ | 37 °C, 72 h | n.d. | n.s. | [84] | |
| Arthrospira platensis | Dehydrated biomass | n.s. | Lacticaseibacillus casei 2240, Lacticaseibacillus rhamnosus GG | 37 °C, 48 h | n.d. | New fermented food supplements | [85] | |
| Nostochopsis lobatus, Nostoc commune, Nostoc flagelliforme, Nostoc verrucosum, Arthrospira platensis, Dunaliella tertiolecta, Chlorogonium spp., Porphyridium purpureum, Pleurochrysis carterae, Euglena spp. | Powder | 10% (w/v) | Lactococcus lactis subsp. lactis, Lactiplantibacillus plantarum | 37 °C, 72 h | n.d. | n.s. | [86] | |
| Arthrospira platensis | Fresh | 10 g with 10 mL of physiological solution | Lactiplantibacillus plantarum | 30°C, 72 h | n.d. | n.s. | [87] | |
| Arthrospira platensis | Powder | 4.0% (w/v) | Debaryomyces hansenii, Kluyveromyces marxianus, Saccharomyces cerevisiae | 28 °C, 48 h, 130 rpm stirring | n.d. | n.s. | [88] | |
| Arthrospira platensis | Dried powder | 4.0% (w/v) | Lactobacillus helveticus B-4526, Lactiplantibacillus plantarum B531, Lacticaseibacillus rhamnosus B-442, Lacticaseibacillus casei B-1922, Bacillus subtilis B-3384, Bacillus subtilis B-3387, Bacillus licheniformis NRS-1264 | LAB strains at 37°C, 48 h, 100 rpm stirring; Bacillus strains at 28°C, 48 h, 130 rpm stirring | n.d. | n.s. | [89] |
n.d., not determined; n.s., not specified; w/v, weight/volume; w/w, weight/weight; rpm, revolutions per minute.
3.2. Effect of the Addition of Microalgae on Growth and Viability of LAB and Probiotics
The study of Parada et al. [50] was the first to explore the feasibility of the fermentation of A. platensis filtrate using several LAB species added to a synthetic medium, and a stimulatory effect on LAB growth was observed (Table 2). These authors hypothesized the presence of extracellular products released by A. platensis within the medium that promoted LAB growth in vitro. Subsequently, the studies of de Caire et al. [51], Varga et al. [52], Gyenis et al. [53], and Mocanu et al. [55] tested the potential of A. platensis and C. vulgaris as bacterial growth promoters in milk and confirmed that the algae boosted the growth and survival of several LAB and probiotic strains: Str. thermophilus, Lc. lactis subsp. lactis, Lb. delbruekii subsp. bulgaricus, Lb. acidophilus, Bifidobacterium spp., Lpb. plantarum, E. faecium, and Bifidobacterium animalis subsp. lactis. Their results paved the way for research on the development of innovative microalga-based dairy products. In addition to stimulating LAB growth, A. platensis biomass was also able to inhibit some human pathogenic bacteria, such as Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris, Staphylococcus aureus, B. subtilis, and Bacillus pumulis, in an in vitro agar well diffusion assay [54]. It has been hypothesized that the antimicrobial activity shown by A. platensis was due to some intracellular or extracellular biologically active substances produced as secondary metabolites [30,54].
Overall, these studies confirmed the untapped potential of A. platensis and C. vulgaris in acting as prebiotic factors in fermented foods.
3.3. Development and Characterization of New Fermented Food Products Supplemented with Microalgae
Most of the existing scientific studies on algal fermentation have used macroalgae or seaweeds [46,90], but microalgal fermentation may expand the applications of algae in the food industry. The enrichment of fermented dairy products with microalgae as suppliers of bioactive compounds is considered an interesting route towards the development of sustainable and healthier dairy products that are also sources of beneficial microorganisms [91,92]. To the best of the authors’ knowledge, Arthrospira spp. and C. vulgaris are the two main species applied for fermented food fortification (Table 2). The analyzed studies were mainly focused on the production of novel dairy products, such as milk-based fermented beverages (Ayran, Labneh, fermented milks, acidophilus milks, milk kefir, yogurt, and probiotic yogurt) and different cheeses (probiotic Feta-type cheese, Kareish cheese, and Greek soft cheese), as well as lactose-free beverages, including soy yogurt and soy fermented beverages, vegan kefir, and water kefir (Table 2). In detail, the studies on ayran [67,68] confirmed the positive influence of A. platensis incorporation on the growth and survival of Str. thermophilus, Lb. delbrueckii spp. bulgaricus, Lb. acidophilus, and Bifidobacterium lactis due to the high amounts of nitrogenous materials (such as proteins, free amino acids, peptone, and peptides), minerals, B vitamins, EPS, adenine, hypoxanthine, and all the organic and inorganic nutrients that acted as LAB growth promoters and were obtained from Spirulina. However, as also demonstrated for probiotic yogurt, while Spirulina promotes LAB growth, it may impair the rheological properties of the final product. For example, in Ayran, as well as in a probiotic yogurt, a decrease in viscosity with an increasing amount of Spirulina was observed, probably due to the high LAB load and titratable acidity level, which enhanced casein proteolysis [67,68,71]. Studies on Ayran enriched with Spirulina also showed that an algal concentration higher than 0.25% decreased taste and thickness scores [67,68]. Abbas et al. [63] reported similar behavior regarding the viability of probiotic bacteria, but demonstrated that the addition of 5 mg/mL of A. platensis powder in milk is the recommended formulation for preparing an acceptable product, in terms of taste and color, called Labneh (a probiotic concentrated-yogurt from the Middle East). By contrast, the studies of Guldas and Irkin [56], Beheshtipour et al. [57], and Barkallah et al. [59] demonstrated that the addition of 0.25–0.5% of Spirulina powder to milk for yogurt production was sufficient to promote the fermentation process and to develop a product with acceptable sensory and rheological properties, as well as nutraceutical properties. Spirulina is a good source of molecules with antioxidant activity, including carotenoids, phycocyanin, chlorophylls, and natural flavoring and coloring agents. Moreover, Spirulina serves as a carrier of proteins and dietary fibers that play an important role in maintaining the product’s texture, acting as a physical stabilizer [59]. Additionally, the study of Patel et al. [66] aimed at developing a probiotic yogurt enriched with carotenoids by adding fresh Spirulina biomass. As underlined by the study, upon homogenization for complete biomass dispersion, up to 7% of the algal biomass was efficiently incorporated into the milk, avoiding the non-uniform distribution and unpleasant sensory attributes that may occur when Spirulina powder is used. Accordingly, Bchir et al. [64] reported that the addition of 0.3% of fresh Spirulina resulted in a yogurt with enhanced nutritional value and an apparent viscosity with higher organoleptic acceptability with respect to that obtained with dried Spirulina biomass.
To the authors’ knowledge, the study of Laela et al. [73] represents one of the very few studies that has employed mixed natural starter cultures, such as kefir grains, instead of pure cultures to produce Spirulina-based fermented products (Table 2). Kefir grains are composed of a mixture of LAB, yeasts, and sometimes acetic acid bacteria that coexist in a symbiotic relationship embedded within a matrix of EPS and proteins of microbial origin [93,94]. Due to this complex and unique microbial community, kefir is a popular drink rich in bioactive compounds such as peptides, amino acids, bacteriocins, folic acid, calcium, and vitamins, and several health benefits are linked to its daily consumption [93,94]. Milk supplemented with Spirulina fermented in the presence of kefir grains resulted in a functional kefir–Spirulina beverage with increased nutritional value and the capacity to control glycemic status; it enhanced the antioxidant status in a diabetic rat model, thus showing its potential for use in the human diet to manage diabetes [73].
Concerning cheeses, the two studies on Feta cheese demonstrated a positive effect of Spirulina in stimulating bacterial growth and viability, even during prolonged storage, as well as beneficial results in terms of the iron and protein amounts and the texture of the final product [58,65]. The Greek soft cheese produced in the presence of Spirulina was characterized by an increased protein content, a good microbiological profile, and an acceptable sensory profile, which was preferred by panelists when Spirulina was added at 0.25–0.5% w/w, due to a reduction in the typical flavor of the algae [75]. Intriguingly, Kareish cheese was enriched with small grains instead of Spirulina powder, and this solution was preferred by the panelists, since the product mimicked Roquefort cheese [60]. Fortification with Spirulina increased the nutritional value (in terms of protein, fat, ash, acidity, iron, total phenols, total flavonoids, and carotenoid contents), improved the antioxidant activity, and enhanced the texture profile of the cheese [60].
Besides fermented dairy products, a few studies have explored the development of vegetal alternatives to milk-based products fortified with microalgae. In detail, soy yogurt [61,62], soy milk or soya drink [70,72,74,76], almond milk [74], and plain water [70,77] were supplemented with A. platensis or C. vulgaris. The selected studies confirmed that the products based on Spirulina or C. vulgaris ingredients had added value and further supported the hypothesis that microalgae boost LAB metabolic activity (i.e., by increasing lactic acid production) and viability [70,72,74,76]. In detail, it has been observed that the total phenolic content, as well as the promoting effect on LAB growth during cold storage, increased in vegan kefir with an increase in the concentration of A. platensis [74]. Similarly, Niccolai et al. [70] showed an increase in the total phenolic content and antioxidant activity of a soybean drink upon its enrichment with A. platensis once it was fermented by the probiotic bacterium Lpb. plantarum ATCC 8014. The strong intracellular antioxidant activity that was observed in this drink has been attributed to the antioxidant components (e.g., phenols and phycocyanin) released by the cyanobacterial biomass during fermentation, acting in a synergistic manner with the bacterial culture. The in vitro digestibility of the soybean drink and water with added Spirulina was also tested. A significant improvement in digestibility was observed for water supplemented with Spirulina after 72 h of fermentation, while no effect of fermentation was found for the enriched soybean drink [70]. These data are in agreement with the study of Niccolai et al. [82], who observed a slight increase in digestibility in a fermented Spirulina extract. The authors hypothesized that the high number of bacterial cells at the end of the fermentation could have lowered the final digestibility of the products [70,82]. To the authors’ knowledge, only a few studies have focused on the in vitro digestibility of fermented microalgae, thus suggesting that this issue warrants further investigation.
Moreover, by using a gastrointestinal tract simulator, Scieszka et al. [76] demonstrated the effectiveness of a soya drink supplemented with C. vulgaris in increasing the survival rate of Lev. brevis ŁOCK 0944. They proposed the development of a new functional, plant-based, lactose-free fermented probiotic product. Accordingly, Sengupta et al. [61] proposed a soy yogurt fortified with S. platensis as a functional food due to its in vivo therapeutic effect on hypercholesteremic cardiovascular disease mediated by lowering cholesterol, improving serum HDL-C, and increasing hepatic antioxidant enzymes, with positive effects on liver function. Finally, Spirulina biomass was also used in water kefir fermentation to replace sugar [77]. As expected, the addition of Spirulina increased the nutritional profile of the water kefir in terms of the protein content, but, intriguingly, the fermentation process carried out by water kefir grains further improved the protein content with respect to the content in the unfermented samples. Moreover, a range of various saturated, unsaturated, and polyunsaturated fatty acids that are beneficial for health were detected after fermentation, thus confirming the significant contribution of kefir grains’ microbiota to the overall quality of the final product [77]. Notably, Martelli et al. [72] showed that Spirulina’s boosting effect on LAB growth and fermentation is variable depending on the LAB cultures, substrate, and Spirulina concentration; therefore, further research is required for the technological optimization of a specific fermentation process. The maximum boosting effect on LAB growth was obtained with the use of 0.25% Spirulina wet biomass, while a higher Spirulina concentration exhibited fluctuating effects on LAB growth.
3.4. Fermented Microalgae as Innovative Functional Foods or Ingredients
Some studies specifically focused on the evaluation of using microalgal biomass or extract as the sole substrate for LAB or yeast fermentation in order to obtain new functional foods or supplement formulations (Table 2). Overall, these studies aimed to test how the microbial fermentation changed the nutritional composition, sensory attributes, and functional properties of microalgae. LAB cultures were able to hydrolyze the cell wall polymers of microalgae into simpler compounds via their metabolic activities, producing molecules with nutraceutical properties [46,47]. Moreover, Spirulina wet biomass was fermented by Lpb. plantarum, resulting in a higher content of total phenolic compounds, C-phycocyanin, and free methionine, as well as improved DPPH-radical-scavenging capacity, ferric reducing antioxidant power, oxygen radical absorbance capacity, and protein fragmentation, with the release of bioactive peptides, compared to the results obtained for the unfermented sample, following 72 h of fermentation [47]. Niccolai et al. [82] reported that A. platensis was a suitable growth substrate for the probiotic bacterium Lpb. plantarum ATCC 8014, and the fermentation product showed a marked increase in antioxidant activity and total phenolic content (79% and 320%, respectively) with respect to the values in the control samples. Accordingly, the study of Jamnik et al. [87] confirmed a higher cellular antioxidant capacity and total phenolic content for biomass of A. platensis fermented by Lpb. plantarum compared to those observed in the unfermented control. Furthermore, a maximum LAB count, higher lactic acid concentration, and a drop in pH were recorded in the first 24 h of fermentation. The low pH and the absence of pathogenic bacteria also suggested that fermented A. platensis may be included in new food formulations to promote microbial stability. Additionally, the non-protein nitrogen level increased, indicating higher protein degradation and bioavailability and fat content reduction in comparison with the unfermented control. Intriguingly, the microalga P. lutheri also showed high antioxidant activity after fermentation with the yeasts H. polymorpha, and it has been suggested as a potential source of natural antioxidants [78]. Furthermore, 12 strains from 10 species of microalgae and cyanobacteria—N. lobatus, N. commune, N. flagelliforme, N. verrucosum, A. platensis, D. tertiolecta, Chlorogonium spp., P. purpureum, P. carterae, and Euglena spp.—have been assayed under fermentation with Lc. lactis subsp. lactis and Lpb. plantarum [86]. Not all the species were able to support the LAB fermentation, and only the fermented aqueous extracts of wild N. commune and sake lees cultured Euglena spp. fermented with Lc. lactis were found to be promising in terms of O2—radical-scavenging capacity and anti-glycation activity.
Other studies aimed to demonstrate the neuroprotective effect and the memory-enhancing activity of A. maxima biomass fermented by a Lpb. plantarum strain isolated from fermented vegetables [80,81]. The fermentation process was combined with the ultrasonic extraction of β-carotene at 40 kHz for 4 h [80]. The high antioxidant capacity of this extract was attributed to the high β-carotene content obtained and to other biologically active substances produced by LAB fermentation, which were able to strongly enhance the brain-derived neurotrophic factor (BDNF)/p-CREB signaling pathways that prevent dementia in mice induced by oxidative stress.
Recently, the study of Ścieszka and Klewicka [83] examined the effect of C. vulgaris on the growth kinetics, acidifying activity, proportion of lactic acid isomers, and enzymatic profiles of four strains of Lev. brevis. Notably, these LAB strains accelerated their growth by shortening the logarithmic phase when cultured in synthetic media containing C. vulgaris, and the growth rate is a crucial parameter from a technological point of view. In parallel, the LAB acidifying activity increased, thus reflecting a potential improvement in the LAB antagonistic activity against the growth of pathogenic bacteria. Moreover, the amount of L-lactic acid increased relative to that of D-lactic acid, another advantageous feature for food exploitation. Finally, the β-glucosidase and leucine arylamidase activities were enhanced in all the strains, and high activities of valine arylamidase, α-galactosidase, and α-glucosidase were also detected in one LAB strain. The cited enzymatic activities play an important role in food production, since they convert complex molecules into antioxidants and flavor compounds.
Finally, several studies aimed to investigate the influence of different microbial species and treatments on the sensory properties of fermented Spirulina. Despite the well-known nutritional properties of algae, their use in food is limited due to their unique aroma, taste, flavor, and color attributes [88]. The study of Sahin et al. [88] successfully showed a reduction in the typical aromas of Spirulina, such as “seaweed,” “umami,” “cardboard,” “earthy/muddy,” and “cereal,” by testing the fermentation performance of three pure cultures of yeasts: D. hansenii, K. marxianus, and S. cerevisiae. The three yeasts were able to grow in the sole presence of spirulina, and the K. marxianus-fermented spirulina was characterized by “fermented” and “rose” attributes. K. marxianus also exhibited the strongest ability regarding protein hydrolysis; the amounts of glutamic acid, methionine, lysine, isoleucine, and valine in the final product were all increased. Martelli et al. [85] evaluated the LAB fermentation of sterilized Spirulina biomass based on the volatile organic compound (VOC) profile. The sterilization of the matrix before the fermentation process was the best treatment for improving the final aroma of the product, reducing off-flavors and ensuring that it was safe for consumers. Bao et al. [79] applied different LAB strains and a B. subtilis culture to Spirulina biomass to improve the volatile component profile, to reduce off-flavors through deodorization, and to hydrolyze the protein. The mixed fermentation of spirulina by Lpb. plantarum and B. subtilis efficiently produced a pleasing aroma in the final product, since most of the undesirable aromatic notes associated with spirulina were removed, while other volatile molecules, such as acetoin (responsible for a creamy flavor), ethyl L(-)-lactate, lactic acid, and (R,R)-2,3-butanediol were produced via the fermentation. Furthermore, high protein bioavailability was observed: more than 30% of the protein was converted into polypeptides and amino acids. Similarly, Yu et al. [84] tested several LAB and Bacillus strains, including in combination, to investigate the effects on the components and bioactivity of Spirulina. The study confirmed the efficacy of all of the strains in enhancing the protein content, the content of amino acids, and the ratio of essential amino acids to total amino acids, and the LAB and Bacillus strain mixture showed the best effect. The fermentation also affected the VOC profile, thus mitigating the unpleasant odor of spirulina. Finally, the antioxidant and antibacterial activity of the fermented Spirulina product was also confirmed. Very recently, Kurt et al. [89] confirmed the positive influence of four different LAB and three different Bacillus strains on spirulina biomass transformation in terms of the VOCs and sensory properties, as well as total and free amino acids and protein hydrolysis. LAB strains were responsible for the highest proteolytic activity and highest sensory attribute score, while the Bacillus strains increased the total amino acids and total essential amino acids.
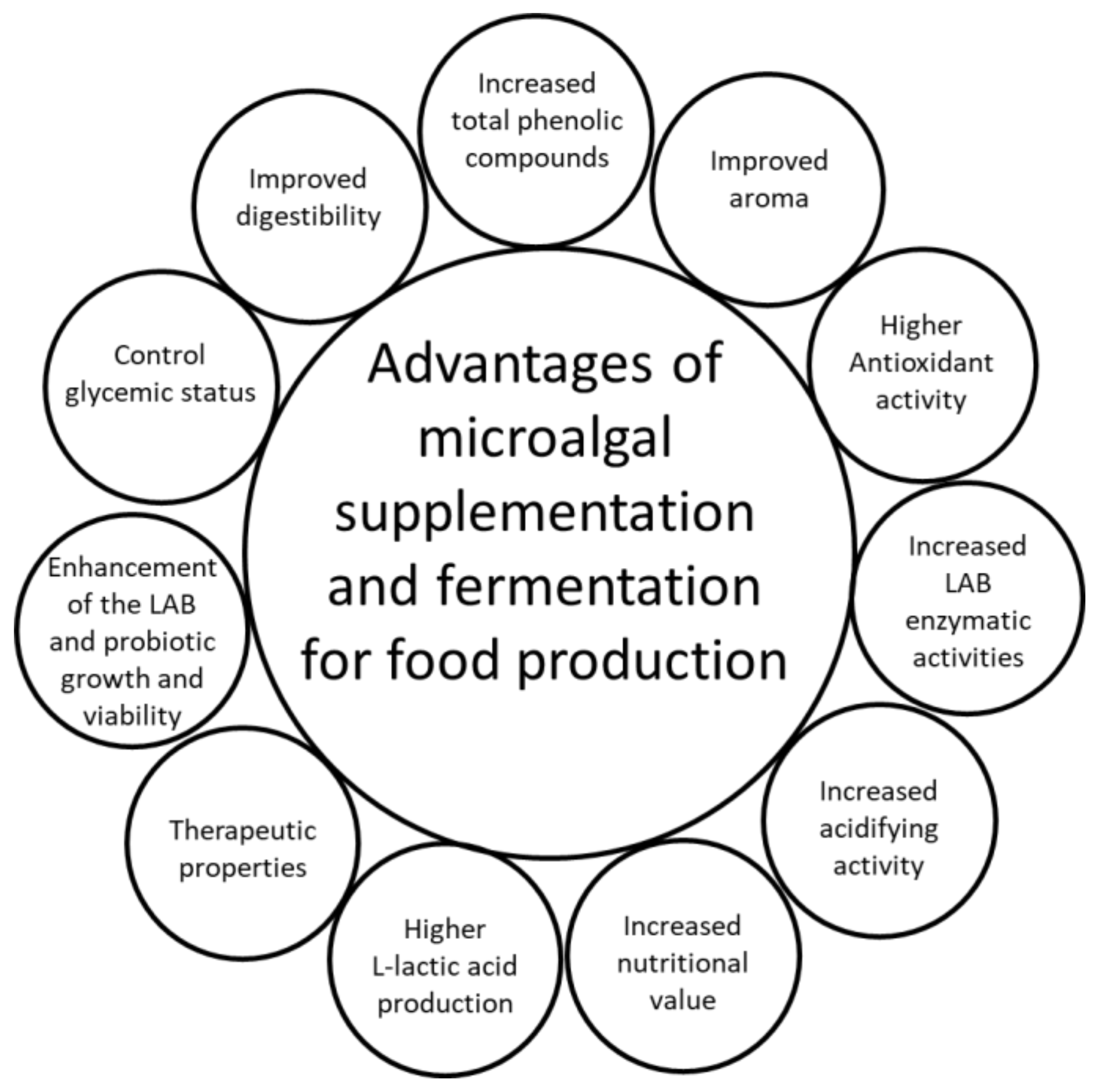
The beneficial effects of microalgal supplementation and fermentation for potential use in innovative food production are summarized in Figure 3.
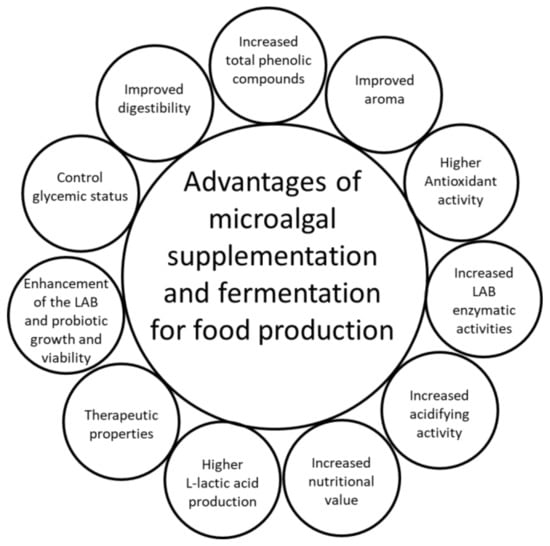
Figure 3.
Advantages of microalgal supplementation and fermentation for potential use in innovative food production; LAB, lactic acid bacteria.
4. Conclusions and Future Perspectives
The increasing growth of the population, in conjunction with reduced food resources, has resulted in increasing global demand for sustainable protein sources and functional foods. Microalga-based products are recognized as a promising solution for reducing global hunger and mitigating climate change [95]. According to the present review, microalgal fermentation driven by microorganisms may represent a safe and cheap technology for increasing the nutritional value, digestibility, and acceptability of microalgae. Indeed, based on the literature, microalgae are able to support and enhance pure microbial cultures, mixed cultures, and probiotic growth without the supplementation of other carbon sources, as well as through the enrichment of synthetic media, milk, or vegetal-based substrates with macro- and micronutrients. Fermentation has been proved to increase the bioactive profiles of microalgae by (i) reducing lipids, (ii) increasing the protein content, (iii) increasing the total phenolic content, (iv) increasing the pigment content (i.e., phycocyanin), (v) promoting the antioxidant activity, (vi) releasing free amino acids and bioactive peptides, (vii) increasing protein bioavailability, (viii) affecting the fatty acid composition, and (ix) producing functional metabolites.
Among the few microalgae tested in research trials to date, A. platensis has the potential to be exploited as a suitable substrate for microbial growth and fermentation to produce valuable fermented foods and beverages from nutritional, nutraceutical, and economic points of view. The fermented microalga field is in its infancy and has been only partially explored; the results obtained for spirulina are encouraging and pave the way for further studies on other microalgal species. Being able to manipulate the composition of microalgae may also be crucial for optimizing the fermentation process. Further studies to identify and purify the specific bioactive compounds obtained from microalgal fermentation that play key roles in many biological activities are needed.
Besides the potential health benefits, an overall reduction in the sensory acceptability of fermented products is often reported, especially when microalgae are added at high percentages. Future studies to improve the sensory attributes of such products are needed in order to meet the consumer demand for food that is both nutritious and pleasant. Moreover, the variability of LAB growth within fermentation mixtures due to several factors (e.g., the microbial strains and natural starter cultures, substrates, and microalga concentrations) is another area that needs to be researched in greater depth in order to guarantee the optimization and reproducibility of the fermentation process as required by the food industry.
Author Contributions
Conceptualization, writing—review and editing, supervision, C.G.; writing—review and editing, A.N., L.M., A.O. and L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rockström, J.; Edenhofer, O.; Gaertner, J.; DeClerck, F. Planet-Proofing the Global Food System. Nat. Food 2020, 1, 3–5. [Google Scholar] [CrossRef]
- UN DESA. World Population Prospects 2022: Summary of Results 2022; UN DESA: New York, NY, USA, 2022. [Google Scholar]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Progress and Recent Trends in Biodiesel Fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.P.; Probert, I.; Michaud, P. What Is in Store for EPS Microalgae in the Next Decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef]
- Keeling, P.J. Diversity and Evolutionary History of Plastids and Their Hosts. Am. J. Bot. 2004, 91, 1481–1493. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic Cultures of Microalgae: Metabolism and Potential Products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe; Publications Office of the European Union: Luxembourg, 2014; ISBN 978-92-79-34037-6. [Google Scholar]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic Micro-Algae and Their Potential Contribution in Biotechnology. Bioresour. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic Diversity in the Macromolecular Composition of Microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A Novel Ingredient in Nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of Interest as Food Source: Biochemical Composition and Digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef]
- Procházková, G.; Brányiková, I.; Zachleder, V.; Brányik, T. Effect of Nutrient Supply Status on Biomass Composition of Eukaryotic Green Microalgae. J. Appl. Phycol. 2014, 26, 1359–1377. [Google Scholar] [CrossRef]
- Giordano, M.; Olivieri, C.; Ratti, S.; Norici, A.; Raven, J.A.; Knoll, A.H. A tale of two eras: Phytoplankton composition influenced by oceanic paleochemistry. Geobiology 2018, 16, 498–506. [Google Scholar] [CrossRef]
- Giordano, M.; Palmucci, M.; Norici, A. Taxonomy and growth conditions concur to determine the energetic suitability of fatty acid complements in algae. J. Appl. Phycol. 2015, 27, 1401–1413. [Google Scholar] [CrossRef]
- Ratti, S.; Knoll, A.H.; Giordano, M. Grazers and Phytoplankton Growth in the Oceans: An Experimental and Evolutionary Perspective. PLoS ONE 2013, 8, e77349. [Google Scholar] [CrossRef]
- Petrucciani, A.; Chaerle, P.; Norici, A. Diatoms Versus Copepods: Could Frustule Traits Have a Role in Avoiding Predation? Front. Mar. Sci. 2022, 8, 804960. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Monteiro, C.M.; Malcata, F.X. Simultaneous Effect of Irradiance and Temperature on Biochemical Composition of the Microalga Pavlova Lutheri. J. Appl. Phycol. 2009, 21, 543–552. [Google Scholar] [CrossRef]
- Klin, M.; Pniewski, F.; Latała, A. Growth Phase-Dependent Biochemical Composition of Green Microalgae: Theoretical Considerations for Biogas Production. Bioresour. Technol. 2020, 303, 122875. [Google Scholar] [CrossRef] [PubMed]
- Hockin, N.L.; Mock, T.; Mulholland, F.; Kopriva, S.; Malin, G. The Response of Diatom Central Carbon Metabolism to Nitrogen Starvation Is Different from That of Green Algae and Higher Plants. Plant Physiol. 2012, 158, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q. Environmental Effects on Cell Composition. In Handbook of Microalgal Culture; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 114–122. ISBN 978-1-118-56716-6. [Google Scholar]
- Borowitzka, M.A. Microalgae in Medicine and Human Health. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 195–210. ISBN 978-0-12-811405-6. [Google Scholar]
- Barsanti, L.; Birindelli, L.; Gualtieri, P. Paramylon and Other Bioactive Molecules in Micro and Macroalgae. Int. J. Mol. Sci. 2022, 23, 8301. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.; Peteiro, C. Algae as Food and Food Supplements in Europe; EUR 30779 EN; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-40548-1. [Google Scholar]
- Grosshagauer, S.; Kraemer, K.; Somoza, V. The True Value of Spirulina. J. Agric. Food Chem. 2020, 68, 4109–4115. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Edible Seaweeds and Spirulina Extracts for Food Application: In Vitro and In Situ Evaluation of Antimicrobial Activity towards Foodborne Pathogenic Bacteria. Foods 2020, 9, 1442. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef]
- Gilroy, D.J.; Kauffman, K.W.; Hall, R.A.; Huang, X.; ChU, F.S. Assessing Potential Health Risks from Microcystin Toxins in Blue-Green Algae Dietary Supplements. Environ. Health Perspect. 2000, 1081, 5. [Google Scholar] [CrossRef]
- Ovando, C.A.; de Carvalho, J.C.; Vinícius de Melo Pereira, G.; Jacques, P.; Soccol, V.T.; Soccol, C.R. Functional Properties and Health Benefits of Bioactive Peptides Derived from Spirulina: A Review. Food Rev. Int. 2018, 34, 34–51. [Google Scholar] [CrossRef]
- Capelli, B.; Cysewski, G.R. Potential Health Benefits of Spirulina Microalgae: A Review of the Existing Literature. Nutrafoods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- Kuddus, M.; Singh, P.; Thomas, G.; Al-Hazimi, A. Recent Developments in Production and Biotechnological Applications of C-Phycocyanin. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Lyon-Colbert, A.; Su, S.; Cude, C. A Systematic Literature Review for Evidence of Aphanizomenon Flos-Aquae Toxigenicity in Recreational Waters and Toxicity of Dietary Supplements: 2000–2017. Toxins 2018, 10, 254. [Google Scholar] [CrossRef]
- Rani, K.; Sandal, N.; Sahoo, P. A Comprehensive Review on Chlorella-Its Composition, Health Benefits, Market and Regulatory Scenario. Pharma Innov. 2018, 7, 584–589. [Google Scholar]
- Murthy, K.N.C.; Vanitha, A.; Rajesha, J.; Swamy, M.M.; Sowmya, P.R.; Ravishankar, G.A. In Vivo Antioxidant Activity of Carotenoids from Dunaliella Salina—A Green Microalga. Life Sci. 2005, 76, 1381–1390. [Google Scholar] [CrossRef]
- Abalde, J.; Fabregas, J. β-Carotene, Vitamin C and Vitamin E Content of the Marine Microalga Dunaliella Tertiolecta Cultured with Different Nitrogen Sources. Bioresour. Technol. 1991, 38, 121–125. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and Other Nutrients from Haematococcus Pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive Compounds in Microalgae and Their Potential Health Benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Fan, C.; Wu, W.; Zhang, W.; Wang, Y. Health Benefits, Food Applications, and Sustainability of Microalgae-Derived N-3 PUFA. Foods 2022, 11, 1883. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Iskandarov, U.; Cohen, Z. LC-PUFA from Photosynthetic Microalgae: Occurrence, Biosynthesis, and Prospects in Biotechnology. Appl. Microbiol. Biotechnol. 2011, 91, 905–915. [Google Scholar] [CrossRef]
- Shanab, S.M.M.; Hafez, R.M.; Fouad, A.S. A Review on Algae and Plants as Potential Source of Arachidonic Acid. J. Adv. Res. 2018, 11, 3–13. [Google Scholar] [CrossRef]
- Eilers, U.; Bikoulis, A.; Breitenbach, J.; Büchel, C.; Sandmann, G. Limitations in the Biosynthesis of Fucoxanthin as Targets for Genetic Engineering in Phaeodactylum Tricornutum. J. Appl. Phycol. 2016, 28, 123–129. [Google Scholar] [CrossRef]
- Pérez-Alva, A.; MacIntosh, A.J.; Baigts-Allende, D.K.; García-Torres, R.; Ramírez-Rodrigues, M.M. Fermentation of Algae to Enhance Their Bioactive Activity: A Review. Algal. Res. 2022, 64, 102684. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of Fermentation on Enhancing the Nutraceutical Properties of Arthrospira platensis (spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Peng, K.; Koubaa, M.; Bals, O.; Vorobiev, E. Recent Insights in the Impact of Emerging Technologies on Lactic Acid Bacteria: A Review. Food Res. Int. 2020, 137, 109544. [Google Scholar] [CrossRef]
- Parada, J. Lactic Acid Bacteria Growth Promoters from Spirulina platensis. Int. J. Food Microbiol. 1998, 45, 225–228. [Google Scholar] [CrossRef]
- De Caire, G.Z.; Parada, J.L.; Zaccaro, M.C.; de Cano, M.M.S. Effect of Spirulina platensis Biomass on the Growth of Lactic Acid Bacteria in Milk. World J. Microbiol. Biotechnol. 2000, 16, 563–565. [Google Scholar] [CrossRef]
- Varga, L.; Szigeti, J.; Kovács, R.; Földes, T.; Buti, S. Influence of a Spirulina platensis Biomass on the Microflora of Fermented ABT Milks During Storage (R1). J. Dairy Sci. 2002, 85, 1031–1038. [Google Scholar] [CrossRef]
- Gyenis, B.; Szigeti, J.; Molnár, N.; Varga, L. Use of Dried Microalgal Biomasses to Stimulate Acid Production and Growth of Lactobacillus plantarum and Enterococcus faecium in Milk. Acta Agrar. Kaposváriensis 2005, 9, 53–59. [Google Scholar]
- Bhowmik, D.; Dubey, J.; Mehra, S. Probiotic Efficiency of Spirulina platensis–Stimulating Growth of Lactic Acid Bacteria. World J. Dairy Food Sci. 2009, 4, 160–163. [Google Scholar]
- Mocanu, G.D.; Botez, E.; Nistor, O.V.; Georgeta, D.; Vlăsceanu, G. Influence of Spirulina platensis Biomass over Some Starter Culture of Lactic Bacteria. J. Agroaliment. Process. Technol. 2013, 19, 474–479. [Google Scholar]
- Guldas, M.; Irkin, R. Influence of Spirulina platensis Powder on the Microflora of Yoghurt and Acidophilus Milk. Mljekarstvo 2010, 60, 237–243. [Google Scholar]
- Beheshtipour, H.; Mortazavian, A.M.; Mohammadi, R.; Sohrabvandi, S.; Khosravi-Darani, K. Supplementation of Spirulina platensis and Chlorella vulgaris Algae into Probiotic Fermented Milks: Algae Addition into Probiotic Fermented Milks. Compr. Rev. Food Sci. Food Saf. 2013, 12, 144–154. [Google Scholar] [CrossRef]
- Mazinani, S.; Fadaei, V.; Khosravi-Darani, K. Impact of Spirulina platensis on Physicochemical Properties and Viability of Lactobacillus acidophilus of Probiotic UF Feta Cheese: Microalgal Incorporation Probiotic UF Feta Cheese. J. Food Process. Preserv. 2016, 40, 1318–1324. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis Fortification on Physicochemical, Textural, Antioxidant and Sensory Properties of Yogurt during Fermentation and Storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Darwish, A.M.I. Physicochemical Properties, Bioactive Compounds and Antioxidant Activity of Kareish Cheese Fortified with Spirulina platensis. World J. Dairy Food Sci. 2017, 12, 71–78. [Google Scholar]
- Sengupta, S.; Bhowal, J. Optimization of Ingredient and Processing Parameter for the Production of Spirulina platensis Incorporated Soy Yogurt Using Response Surface Methodology. J. Microb. Biotechnol. Food Sci. 2017, 6, 1081–1085. [Google Scholar] [CrossRef]
- Sengupta, S.; Koley, H.; Dutta, S.; Bhowal, J. Hypocholesterolemic Effect of Spirulina platensis (SP) Fortified Functional Soy Yogurts on Diet-Induced Hypercholesterolemia. J. Funct. Foods 2018, 48, 54–64. [Google Scholar] [CrossRef]
- Abbas, M.H.; Farahat, E.S.A.K.; Zaky, W.M.; Mohamed, A.G. Applicability of Using Edible Algae (Spirulina platensis) to Prepare High Protein Quality Labenah. J. Biol. Sci. 2019, 19, 143–147. [Google Scholar] [CrossRef]
- Bchir, B.; Felfoul, I.; Bouaziz, M.A.; Gharred, T.; Yaich, H.; Noumi, E.; Snoussi, M.; Bejaoui, H.; Kenzali, Y.; Blecker, C.; et al. Investigation of Physicochemical, Nutritional, Textural, and Sensory Properties of Yoghurt Fortified with Fresh and Dried spirulina (Arthrospira platensis). Int. Food Res. J. 2019, 26, 1565–1576. [Google Scholar]
- Golmakani, M.T.; Soleimanian-Zad, S.; Alavi, N.; Nazari, E.; Eskandari, M.H. Effect of Spirulina (Arthrospira platensis) Powder on Probiotic Bacteriologically Acidified Feta-Type Cheese. J. Appl. Phycol. 2019, 31, 1085–1094. [Google Scholar] [CrossRef]
- Patel, P.; Jethani, H.; Radha, C.; Vijayendra, S.V.N.; Mudliar, S.N.; Sarada, R.; Chauhan, V.S. Development of a Carotenoid Enriched Probiotic Yogurt from Fresh Biomass of Spirulina and Its Characterization. J. Food Sci. Technol. 2019, 56, 3721–3731. [Google Scholar] [CrossRef]
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. üseyin. Influence of Incorporated Spirulina platensis on the Growth of Microflora and Physicochemical Properties of Ayran as a Functional Food. Algal Res. 2019, 44, 101710. [Google Scholar] [CrossRef]
- Çelekli, A.; Alslibi, Z.A.; Bozkurt, H. Boosting Effects of Spirulina platensis, Whey Protein, and Probiotics on the Growth of Microflora and the Nutritional Value of Ayran. Eng. Rep. 2020, 2, e12235. [Google Scholar] [CrossRef]
- Atallah, A.A.; Morsy, O.M.; Gemiel, D.G. Characterization of Functional Low-Fat Yogurt Enriched with Whey Protein Concentrate, Ca-Caseinate and Spirulina. Int. J. Food Prop. 2020, 23, 1678–1691. [Google Scholar] [CrossRef]
- Niccolai, A.; Bažec, K.; Rodolfi, L.; Biondi, N.; Zlatić, E.; Jamnik, P.; Tredici, M.R. Lactic Acid Fermentation of Arthrospira platensis (spirulina) in a Vegetal Soybean Drink for Developing New Functional Lactose-Free Beverages. Front. Microbiol. 2020, 11, 560684. [Google Scholar] [CrossRef]
- Alizadeh Khaledabad, M.; Ghasempour, Z.; Moghaddas Kia, E.; Rezazad Bari, M.; Zarrin, R. Probiotic Yoghurt Functionalised with Microalgae and Zedo Gum: Chemical, Microbiological, Rheological and Sensory Characteristics. Int. J. Dairy Technol. 2020, 73, 67–75. [Google Scholar] [CrossRef]
- Martelli, F.; Alinovi, M.; Bernini, V.; Gatti, M.; Bancalari, E. Arthrospira platensis as Natural Fermentation Booster for Milk and Soy Fermented Beverages. Foods 2020, 9, 350. [Google Scholar] [CrossRef]
- Laela, N.; Legowo, A.M.; Fulyani, F. The Effect of Kefir-Spirulina on Glycemic Status and Antioxidant Activity in Hyperglycemia Rats. Potr. S. J. F. Sci. 2021, 15, 101–110. [Google Scholar] [CrossRef]
- Sözeri Atik, D.; Gürbüz, B.; Bölük, E.; Palabıyık, İ. Development of Vegan Kefir Fortified with Spirulina platensis. Food Biosci. 2021, 42, 101050. [Google Scholar] [CrossRef]
- Bosnea, L.; Terpou, A.; Pappa, E.; Kondyli, E.; Mataragas, M.; Markou, G.; Katsaros, G. Incorporation of Spirulina platensis on Traditional Greek Soft Cheese with Respect to Its Nutritional and Sensory Perspectives. Proceedings 2021, 70, 99. [Google Scholar] [CrossRef]
- Ścieszka, S.; Gorzkiewicz, M.; Klewicka, E. Innovative Fermented Soya Drink with the Microalgae Chlorella vulgaris and the Probiotic Strain Levilactobacillus brevis ŁOCK 0944. LWT 2021, 151, 112131. [Google Scholar] [CrossRef]
- Nascimento, R.Q.; Deamici, K.M.; Tavares, P.P.L.G.; de Andrade, R.B.; Guimarães, L.C.; Costa, J.A.V.; Magalhães-Guedes, K.T.; Druzian, J.I.; de Souza, C.O. Improving Water Kefir Nutritional Quality via Addition of Viable Spirulina Biomass. Bioresour. Technol. Rep. 2022, 17, 100914. [Google Scholar] [CrossRef]
- Qian, Z.J.; Jung, W.K.; Kang, K.H.; Ryu, B.; Kim, S.K.; Je, J.Y.; Heo, S.J.; Oh, C.; Kang, D.H.; Park, W.S.; et al. In Vitro Antioxidant Activities of The Fermented Marine Microalga Pavlova lutheri (Haptophyta) With the Yeast Hansenula polymorpha. J. Phycol. 2012, 48, 475–482. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Zheng, J.H.; Ren, D.F.; Lu, J. Mixed Fermentation of Spirulina platensis with Lactobacillus plantarum and Bacillus subtilis by Random-Centroid Optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef]
- Choi, W.; Kang, D.; Heo, S.J.; Lee, H. Enhancement of the Neuroprotective Effect of Fermented Spirulina maxima Associated with Antioxidant Activities by Ultrasonic Extraction. Appl. Sci. 2018, 8, 2469. [Google Scholar] [CrossRef]
- Choi, W.Y.; Kang, D.H.; Lee, H.Y. Effect of Fermented Spirulina maxima Extract on Cognitive-Enhancing Activities in Mice with Scopolamine-Induced Dementia. Evid. Based Complement. Altern. Med. 2018, 7218504. [Google Scholar] [CrossRef]
- Niccolai, A.; Shannon, E.; Abu-Ghannam, N.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Lactic acid fermentation of Arthrospira platensis (Spirulina) biomass for probiotic-based products. J. Appl. Phycol. 2019, 31, 1077–1083. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Influence of the Microalga Chlorella vulgaris on the Growth and Metabolic Activity of Lactobacillus spp. Bacteria. Foods 2020, 9, 959. [Google Scholar] [CrossRef]
- Yu, J.; Ma, D.; Qu, S.; Liu, Y.; Xia, H.; Bian, F.; Zhang, Y.; Huang, C.; Wu, R.; Wu, J.; et al. Effects of Different Probiotic Combinations on the Components and Bioactivity of Spirulina. J. Basic Microbiol. 2020, 60, 543–557. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-State Fermentation of Arthrospira platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction. Foods 2020, 10, 67. [Google Scholar] [CrossRef]
- Kaga, Y.; Kuda, T.; Taniguchi, M.; Yamaguchi, Y.; Takenaka, H.; Takahashi, H.; Kimura, B. The Effects of Fermentation with Lactic Acid Bacteria on the Antioxidant and Anti-Glycation Properties of Edible Cyanobacteria and Microalgae. LWT 2021, 135, 110029. [Google Scholar] [CrossRef]
- Jamnik, P.; Mahnič, N.; Mrak, A.; Pogačnik, L.; Jeršek, B.; Niccolai, A.; Masten Rutar, J.; Ogrinc, N.; Dušak, L.; Ferjančič, B.; et al. Fermented Biomass of Arthrospira platensis as a Potential Food Ingredient. Antioxidants 2022, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Sahin, B.; Hosoglu, M.I.; Guneser, O.; Karagul-Yuceer, Y. Fermented Spirulina Products with Saccharomyces and Non- Saccharomyces Yeasts: Special Reference to Their Microbial, Physico-Chemical and Sensory Characterizations. Food Biosci. 2022, 47, 101691. [Google Scholar] [CrossRef]
- Kurt, H.; Hosoglu, M.I.; Guneser, O.; Karagul-Yuceer, Y. Influence of Different Bacteria Species in Chemical Composition and Sensory Properties of Fermented Spirulina. Food Chem. 2023, 400, 133994. [Google Scholar] [CrossRef]
- Uchida, M.; Miyoshi, T. Algal Fermentation—The Seed for a New Fermentation Industry of Foods and Related Products. Jpn. Agric. Res. Q. 2013, 47, 53–63. [Google Scholar] [CrossRef]
- Kavimandan, A. Incorporation of Spirulina platensis into Probiotic Fermented Dairy Products. Int. J. Dairy Sci. 2014, 10, 1–11. [Google Scholar] [CrossRef]
- Hernández, H.; Nunes, M.C.; Prista, C.; Raymundo, A. Innovative and Healthier Dairy Products through the Addition of Microalgae: A Review. Foods 2022, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Osimani, A.; Milanović, V.; Aquilanti, L.; De Filippis, F.; Stellato, G.; Di Mauro, S.; Turchetti, B.; Buzzini, P.; Ercolini, D.; et al. Bacteria and Yeast Microbiota in Milk Kefir Grains from Different Italian Regions. Food Microbiol. 2015, 49, 123–133. [Google Scholar] [CrossRef]
- Garofalo, C.; Ferrocino, I.; Reale, A.; Sabbatini, R.; Milanović, V.; Alkić-Subašić, M.; Boscaino, F.; Aquilanti, L.; Pasquini, M.; Trombetta, M.F.; et al. Study of Kefir Drinks Produced by Backslopping Method Using Kefir Grains from Bosnia and Herzegovina: Microbial Dynamics and Volatilome Profile. Food Res. Int. 2020, 137, 109369. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).