Sporotrichosis: A Review of a Neglected Disease in the Last 50 Years in Brazil
Abstract
:1. Introduction
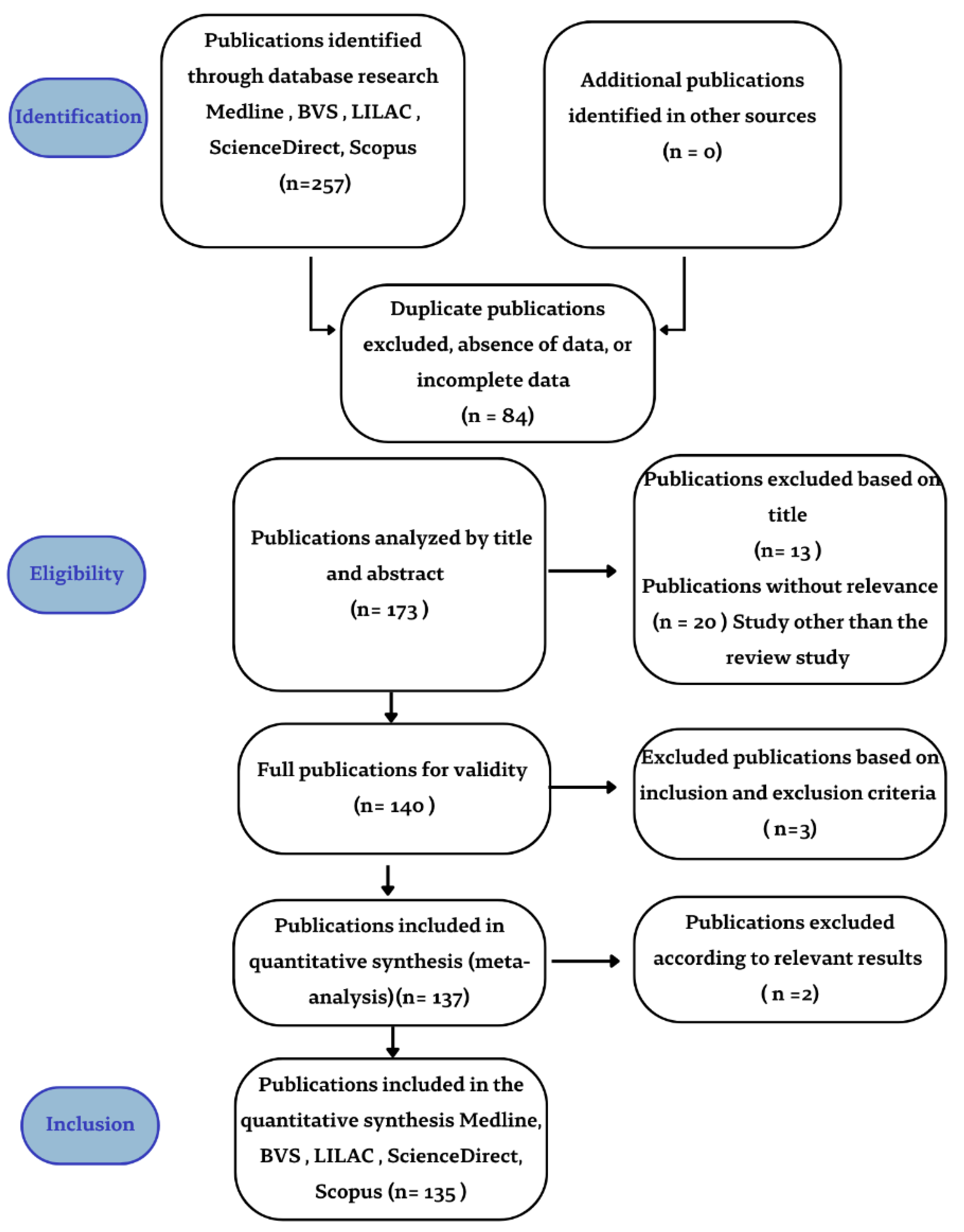
2. Materials and Methods
3. Results and Discussion
3.1. Emergence of Sporotrichosis as a Zoonotic Disease
3.2. Sporotrichosis Diagnosis
3.3. Clinical Manifestations
3.4. Sporotrichosis Treatment
3.5. Prophylaxis and Prevention
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arenas, R.; Sánchez-Cardenas, C.D.; Ramirez-Hobak, L.; Ruíz Arriaga, L.F.; Vega Memije, M.E. Sporotrichosis: From KOH to molecular biology. J. Fungi 2018, 4, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, M.B.L.; Schubach, T.P.; Coll, J.O.; Gremião, I.D.; Wanke, B.; Schubach, A. Esporotricose: A evolução e os desafios de uma epidemia. Rev. Panam. Salud Publica 2010, 27, 455–460. [Google Scholar]
- Lutz, A.; Splendore, A. Sobre uma micose observada em homens e ratos. Rev. Med. São Paulo 1907, 21, 433–450. [Google Scholar]
- De Almeida, J.R.F.; Jannuzzi, G.P.; Kaihami, G.H.; Breda, L.C.D.; Ferreira, K.S.; de Almeida, S.R. An immunoproteomic approach revealing peptides from Sporothrix brasiliensis that induce a cellular immune response in subcutaneous sporotrichosis. Sci. Rep. 2018, 8, 4192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waller, S.B.; Ripoll, M.K.; Madrid, I.M.; Acunha, T.; Cleff, M.B.; Chaves, F.C.; de Mello, J.R.B.; de Faria, R.O.; Meireles, M.C.A. Susceptibility and resistance of Sporothrix brasiliensis to branded and compounded itraconazole formulations. Braz. J. Microbiol. 2021, 52, 155–162. [Google Scholar] [CrossRef]
- Téllez, M.D.; Batista-Duharte, A.; Portuondo, D.; Quinello, C.; Bonne-Hernández, R.; Carlos, I.Z. Sporothrix schenckii complex biology: Environment and fungal pathogenicity. Microbiology 2014, 160, 2352–2365. [Google Scholar] [CrossRef]
- Cabañes, F.J. Sporotrichosis in Brazil: Animals + humans = one health. Rev. Iberoam. Micol. 2020, 37, 73–74. [Google Scholar] [CrossRef]
- De Miranda, L.H.M.; Meli, M.; Conceição-Silva, F.; Novacco, M.; Menezes, R.C.; Pereira, S.A.; Sugiarto, S.; Dos Reis, É.G.; Gremião, I.D.F.; Hofmann-Lehmann, R. Co-infection with feline retrovirus is related to changes in immunological parameters of cats with sporotrichosis. PLoS ONE 2018, 13, e0207644. [Google Scholar] [CrossRef] [Green Version]
- Madrid, H.; Cano, J.; Gené, J.; Bonifaz, A.; Toriello, C.; Guarro, J. Sporothrix globosa, a pathogenic fungus with widespread geographical distribution. Rev. Iberoam. Micol. 2009, 26, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Marimon, R.; Cano, J.; Gene, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 2007, 45, 3198–3206. [Google Scholar] [CrossRef] [Green Version]
- Marimon, R.; Gene, J.; Cano, J.; Guarro, J. Sporothrix luriei: A rare fungus from clinical origin. Med. Mycol. 2008, 46, 621–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, N.M.; Oliveira, M.M.E.; Portela, M.A.; Santos, C.; Zancope-Oliveira, R.M.; Lima, N. Sporotrichosis caused by Sporothrix mexicana, Portugal. Emerg. Infect. Dis. 2011, 17, 1975–1976. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.; Zhang, Y.; de Camargo, Z.P. Emerging sporotrichosis is driven by clonal and recombinant Sporothrix species. Emerg. Microbes Infect. 2014, 3, e32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Rodrigues, A.M.; Feng, P.; Hoog, G.S. Global ITS diversity in the Sporothrix schenckii complex. Fungal Divers. 2014, 6, 153–165. [Google Scholar] [CrossRef]
- Oliveira, M.M.E.; Almeida-Paes, R.; Gutierrez-Galhardo, M.C.; Zancope-Oliveira, R.M. Molecular identification of the Sporothrix schenckii complex. Rev. Iberoam. Micol. 2014, 31, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hagen, F.; Stielow, B.; Rodrigues, A.M.; Samerpitak, K.; Zhou, X. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14,000 human and animal case reports. Persoonia 2015, 35, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016, 12, e1005638. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2010, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Meyer, E.M.; de Beer, Z.W.; Summerbell, R.C.; Moharram, A.M.; de Hoog, G.S.; Vismer, H.F.; Wingfield, M. Taxonomy, and phylogeny of new wood-and soil-inhabiting Sporothrix species in the Ophiostoma stenoceras-porothrix schenckii complex. Mycologia 2008, 100, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Romeo, O.; Scordino, F.; Criseo, G. New insight into molecular phylogeny and epidemiology of Sporothrix schenckii species complex based on calmodulin-encoding gene analysis of Italian isolates. Mycopathologia 2011, 172, 179–186. [Google Scholar] [CrossRef]
- De Beer, Z.W.; Duong, T.A.; Wingfield, M.J. The divorce of Sporothrix and Ophiostoma: Solution to a problematic relationship. Stud. Mycol. 2016, 83, 165–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.M.; Hagen, F.; Camargo, Z.P. A Spotlight on Sporothrix and Sporotrichosis. Mycopathologia 2022, 187, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.B.; de Almeida Paes, R.; Schubach, A.O. Sporothrix schenckii and Sporotrichosis. Clin. Microbiol. Rev. 2011, 24, 633–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgado, D.S.; Castro, R.; Ribeiro-Alves, M.; Corrêa-Moreira, D.; Castro-Alves, J.; Pereira, S.A.; Menezes, R.C.; Oliveira, M.M.E. Global distribution of animal sporotrichosis: A systematic review of Sporothrix sp. identified using molecular tools. Curr. Res. Microb. Sci. 2022, 3, 100140. [Google Scholar] [CrossRef]
- Padhye, A.A.; Kaufman, L.; Durry, E.; Banerjee, C.K.; Jindal, S.K.; Talwar, P.; Chakrabarti, A. Fatal pulmonary sporotrichosis caused by Sporothrix schenckii var. luriei in India. J. Clin. Microbiol. 1992, 30, 2492–2494. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.C.; Lopes, P.G.; Spader, T.B. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J. Clin. Microbiol. 2011, 49, 3047–3049. [Google Scholar] [CrossRef] [Green Version]
- Barros, M.B.; Schubach., A.d.O.; do Valle, A.C.; Gutierrez Galhardo, M.C.; Conceicao-Silva, F.; Schubach, T.M.; Reis, R.S.; Wanke, B.; Marzochi, K.B.F.; Conceição, M.J. Cat transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: Description of a series of cases. Clin. Infect. Dis. 2004, 38, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Barros, M.B.; Schubach, A.O.; Schubach, T.M.; Wanke, B.; Lambert-Passos, S.R. An epidemic of sporotrichosis in Rio de Janeiro, Brazil: Epidemiological aspects of a series of cases. Epidemiol. Infect. 2008, 136, 1192–1196. [Google Scholar] [CrossRef]
- Silva, M.B.T.; Costa, M.M.M.; Torres, C.C.S.; Galhardo, M.C.G.; Valle, A.C.F.; Magalhães, M.A.F.M.; Sabroza, P.C.T.; Oliveira, R.M. Urban sporotrichosis: A neglected epidemic in Rio de Janeiro, Brazil Cad. Saúde Pública, 2012, 28, 1867–1879. [Google Scholar] [CrossRef] [Green Version]
- Schubach, A.; Barros, M.B.; Wanke, B. Epidemic sporotrichosis. Curr. Opin. Infect. Dis. 2008, 21, 129–133. [Google Scholar] [CrossRef]
- Bustamante, B.; Campos, P.E. Endemic sporotrichosis. Curr. Opin. Infect. Dis. 2001, 14, 45–149. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Bento, A.; de Sena Costa, A.S.; Lima, S.L.; do Monte Alves, M.; de Azevedo Melo, A.S.; Rodrigues, A.M. The spread of cat-transmitted sporotrichosis due to Sporothrix brasiliensis in Brazil towards the Northeast region. PLoS Negl. Trop. Dis. 2021, 15, e0009693. [Google Scholar] [CrossRef] [PubMed]
- Rabello, V.B.S.; Almeida, M.A.; Bernardes-Engemann, A.R.; Almeida-Paes, R.; de Macedo, P.M.; Zancopé-Oliveira, R.M. The historical burden of Sporotrichosis in Brazil: A systematic review of cases reported from 1907 to 2020. Braz. J. Microbiol. 2022, 53, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Telles, F.; Buccheri, R.; Benard, G. Sporotrichosis in immunocompromised hosts. J. Fungi (Basel) 2019, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gremião, I.D.; Miranda, L.H.; Reis, E.G.; Rodrigues, A.M.; Pereira, S.A. Zoonotic epidemic of sporotrichosis: Cat to human transmission. PLoS Pathog. 2017, 13, e1006077. [Google Scholar] [CrossRef] [Green Version]
- Gremião, I.D.F.; Oliveira, M.M.E.; Monteiro de Miranda, L.H.; Saraiva Freitas, D.F.; Pereira, S.A. Geographic expansion of sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Paes, R.; Oliveira, M.M.E.; Freitas, D.F.S.; Valle, A.C.F.; Zancopé-Oliveira, R.M.; Gutierrez-Galhardo, M.C. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl. Trop. Dis. 2014, 8, e3094. [Google Scholar] [CrossRef] [Green Version]
- Macedo, P.M.; Lopes-Bezerra, L.M.; Bernardes-Engemann, A.R.; Orofino-Costa, R. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: Study of efficacy and safety in 102 patients. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 719–724. [Google Scholar] [CrossRef]
- Lopes-Bezerra, L.M.; Schubach, A.; Costa, R.O. Sporothrix schenckii and sporotrichosis. An. Acad. Bras. Cienc. 2006, 78, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Brazil. Rio de Janeiro (State). State Department of Health. SES Resolution No. 674 of 12 July 2013. Redefines the Relationship of Diseases and Conditions of Compulsory Notification at the State Level. Published in DOE 16 July 2013. Available online: http://www.rio.rj.gov.br/dlstatic/10112/4364979/4115670/ResolucaoSESN674DE12.07.2013.pdf (accessed on 21 April 2021).
- Brazil. Minas Gerais State Health Department (SES/MG). Resolution SES/MG No. 6532, 5 December 2018. Adds Diseases to the National List of Compulsory Notification Diseases. Available online: http://vigilancia.saude.mg.gov.br/index.php/download/resolucao-ses-mg-no-6-532-de-05-de-dezembro-de-2018/?wpdmdl=5990 (accessed on 21 November 2021).
- Brazil. Paraiba State Health Department (CIB/SES-PB). Resolution No. 80/18, 7 August 2018. Approves the Institution of Compulsory Notification for Human Sporotrichosis Disease at the State Level. Available online: http://static.paraiba.pb.gov.br/2018/02/Resolucao-80-Vigilancia-Esporotricose.pdf (accessed on 21 November 2021).
- Brazil. Pernambuco State Health Department (SES/PE). Ordinance nº 390. 14 September 2016. Weekly Compulsory Notification (NCS): Compulsory Notification Carried out within 7 (Seven) Days, from the Knowledge of the Occurrence of the Disease, Injury or Public Health Event (Sporotrichosis). Available online: https://www.normasbrasil.com.br/norma/portaria-390-2016-pe_328576.html (accessed on 21 November 2021).
- Brazil. State of Bahia. Municipal Health Department. Ordinance No. 191, 27 March 2018. Institutes the Inclusion of Sporoticosis in the List of Diseases and Conditions of Compulsory Notification in the City of Salvador. Available online: https://emevz.ufba.br/sites/emevz.ufba.br/files/portaria-municipal-191-de-2018-inclusao-da-esporotricose-na-lista-de-doencas-e-agravos-de-notificacao-compulsoria.pdf (accessed on 21 November 2021).
- Rodrigues, A.M.; Della Terra, P.P.; Gremião, I.D.; Pereira, S.A.; Orofino-Costa, R.; de Camargo, Z.P. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia 2020, 185, 813–842. [Google Scholar] [CrossRef]
- Madrid, I.M.; Mattei, A.S.; Fernandes, C.G.; Nobre, M.O.; Meireles, M.C.A. Epidemiological findings and laboratory evaluation of sporotrichosis: A description of 103 cases in cats and dogs in southern Brazil. Mycopathologia 2012, 173, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Sanchotene, K.O.; Madrid, I.M.; Klafke, G.B.; Bergamashi, M.; Terra, P.P.D.; Rodrigues, A.M. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses 2015, 58, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Poester, V.R.; Mattei, A.S.; Madrid, I.M.; Pereira, J.T.B.; Klafke, G.B.; Sanchotene, K.O.; Brandolt, T.M.; Xavier, M.O. Sporotrichosis in Southern Brazil, towards an epidemic? Zoonoses Public Health 2018, 65, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Etchecopaz, A.; Toscanini, M.A.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.A.; Bendezú, K.; Nusblat, A.D.; Iachini, R.; Cuestas, M.L. Sporothrix Brasiliensis: A review of an emerging South American fungal pathogen, its related disease, presentation and spread in Argentina. J. Fungi (Basel) 2021, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Oyarce, J.A.; Garcia, C.; Alave, J.; Bustamente, B. Caracterización epidemiológica, clínica y de laboratório de esporotricosis en pacientes de un hospital de tercer nivel en Lima-Perú, entre los años 1991 y 2014. Rev. Chil. Infectol. 2016, 33, 315–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubach, A.O.; Schubach, T.M.; Barros, M.B. Epidemic cat-transmitted sporotrichosis. N. Engl. J. Med. 2005, 353, 1185–1186. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Melo Teixeira, M.; de Hoog, G.S.; Schubach, T.M.; Pereira, S.A.; Fernandes, G.F. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl. Trop. Dis. 2013, 7, e2281. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.M.; de Almeida, L.G.; Kubitschek-Barreira, P.; Alves, F.L.; Kioshima, E.S.; Abadio, A.K. Comparative genomics of the major fungal agents of human and animal Sporotrichosis: Sporothrix schenckii and Sporothrix brasiliensis. BMC Genom. 2014, 15, 943. [Google Scholar] [CrossRef] [Green Version]
- Arrillaga-Moncrieff, I.; Capilla, J.; Mayayo, E.; Marimon, R.; Mariné, M.; Gené, J. Different virulence levels of the species of Sporothrix in a murine model. Clin. Microbiol. Infect. 2009, 15, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.M.; Kubitschek-Barreira, P.H.; Fernandes, G.F.; de Almeida, S.R.; Lopes-Bezerra, L.M.; de Camargo, Z.P. Immunoproteomic analysis reveals a convergent humoral response signature in the Sporothrix schenckii complex. J. Proteom. 2015, 11, 8–22. [Google Scholar] [CrossRef]
- Kano, R.; Okubo, M.; Siew, H.H.; Kamata, H.; Hasegawa, A. Molecular typing of Sporothrix schenckii isolates from cats in Malaysia. Mycoses 2015, 58, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Siew, H.H. The current status of feline sporotrichosis in Malaysia. Med. Mycol. 2017, 58, E107–E113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.D.; Zhou, X.; Liu, T.T.; Yang, Z.B. Morphological and physiological comparison of taxa comprising the Sporothrix schenckii complex. J. Zhejiang Univ. Sci. B 2015, 16, 940–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Montes, H.M.; Dantas Ada, S.; Trujillo-Esquivel, E.; de Souza Baptista, A.R.; Lopes-Bezerra, L.M. Current progress in the biology of members of the Sporothrix schenckii complex following the genomic era. FEMS Yeast Res. 2015, 15, fov065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessica, N.; Sonia, R.L.; Rodrigo, C.; Isabella, D.F.; Tânia, M.P.; Jeferson, C.; Anna, B.F.; Sandro, A. Diagnostic accuracy assessment of cytopathological examination of feline sporotrichosis. Med. Mycol. 2015, 53, 880–884. [Google Scholar] [CrossRef] [Green Version]
- Madrid, I.M.; Mattei, A.S.; Soares, M.P.; Nobre, M.O.; Meireles, M.C.A. Ultrastructural study of the mycelial phase of clinical isolates of Sporothrix schenckii obtained from feline, canine and human cases of sporotrichosis. Braz. J. Microbiol. 2011, 42, 1147–1150. [Google Scholar] [CrossRef]
- Waller, S.B.; Dalla Lana, D.F.; Quatrin, P.M.; Ferreira, M.R.A.; Fuentefria, A.M.; Mezzari, A. Antifungal resistance on Sporothrix species: An overview. Braz. J. Microbiol. 2021, 52, 73–80. [Google Scholar] [CrossRef]
- Pereira, S.A.; Menezes, R.C.; Gremião, I.D.F.; Silva, J.N.; Honse, C.O.; Figueiredo, F.B. Sensitivity of cytopathological examination in the diagnosis of feline sporotrichosis. J. Feline Med. Surg. 2011, 13, 220–223. [Google Scholar] [CrossRef]
- Bernardes-Engemann, A.R.; Costa, R.C.; Miguens, B.R.; Penha, C.V.; Neves, E.; Pereira, B.A. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med. Mycol. 2015, 43, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Almeida-Paes, R.; Brito-Santos, F.; Galdino Figueiredo-Carvalho, M.H.; Sá Machado, A.C.; Evangelista Oliveira, M.M.; Pereira, S.A.; Gutierrez-Galhardo, M.C.; Zancopé-Oliveira, R.M. Minimal inhibitory concentration distributions and epidemiological cutoff values of five antifungal agents against Sporothrix brasiliensis. Mem. Inst. Oswaldo Cruz. 2017, 112, 376–381. [Google Scholar] [CrossRef]
- Coelho, L.M.L.; Grisolia, J.C.; Lúcia, M.; Boczar, M.; Ferreira, E.B.; Nogueira, D.A.; Chavasco, J.K.; de Camargo, Z.P.; Lopes-Bezerra, L.M.; Bezerra, L.M.L.; et al. Effects of metaperiodate and urea solutions on the serological diagnosis of human sporotrichosis using an indirect ELISA test. Braz. J. Microbiol. 2019, 50, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.; Lopes-Bezerra, L.M.; Bernardes-Engemann, A.R.; Schubach, T.M.; Dias, M.A.; Pereira, S.A. Serodiagnosis of sporotrichosis infection in cats by enzyme-linked immunosorbent assay using a specific antigen, SsCBF, and crude exoantigens. Vet. Microbiol. 2011, 147, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl. Trop. Dis. 2015, 9, e0004190. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.M.; Santos, C.; Sampaio, P.; Romeo, O.; Almeida-Paes, R.; Pais, C. Development, and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res. Microbiol. 2015, 166, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, A.M.F.; Moreira, L.M.; Barczewski, B.F.; Matos, L.X.; Oliveira, B.V.J.; Pimentel, M.I.F.; Almeida-Paes, R.; Oliveira, M.G.; Pinto, T.A.C.; Lima, N.; et al. Identification by MALDI-TOF MS of Sporothrix brasiliensis isolated from a subconjunctival infiltrative lesion in an immunocompetent patient. Microorganisms 2020, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, B.D.; Pfaller, M.A. Contemporary tools for the diagnosis and management of invasive mycoses. Clin. Infect. Dis. 2006, 43, S15–S27. [Google Scholar] [CrossRef] [Green Version]
- Luiz, R.L.F.; Menezes, R.C.; Pereira, S.A.; de Oliveira, R.V.C.; Oliveira, M.M.E. Nested PCR for the diagnosis of feline sporotrichosis from formalin-fixed and paraffin-embedded samples using different DNA extraction protocols. Front. Vet. Sci. 2022, 8, 755897. [Google Scholar] [CrossRef]
- Montenegro, H.; Rodrigues, A.M.; Dias, M.A.G.; Silva, E.A.; Bernardi, F.; Camargo, Z.P. Feline sporotrichosis due to Sporothrix brasiliensis: An emerging animal infection in São Paulo, Brazil. BMC Vet. Res. 2014, 10, 269. [Google Scholar] [CrossRef] [Green Version]
- Rossow, J.A.; Queiroz-Telles, F.; Caceres, D.H.; Beer, K.D.; Jackson, B.R.; Pereira, J.G.; Ferreira Gremião, I.D.; Pereira, S.A. A one health approach to combatting Sporothrix brasiliensis: Narrative review of an emerging zoonotic fungal pathogen in South America. J. Fungi (Basel) 2020, 26, 247. [Google Scholar] [CrossRef]
- Lavalle, P.; Mariat, F. Sporotrichosis. Bull. Inst. Pasteur 1983, 81, 295–322. [Google Scholar]
- Martínez-Herrera, E.; Arenas, R.; Hernández-Castro, R.; Frías-De-León, M.G.; Rodríguez-Cerdeira, C. Uncommon clinical presentations of sporotrichosis: A two-case report. Pathogens 2021, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Galhardo, M.C.; de Oliveira Schubach, A.; de Lima Barros, M.B.; Moita Blanco, T.C.; Cuzzi-Maya, T.; Pacheco Schubach, T.M.; dos Santos Lazéra, M.; do Valle, A.C. Erythema nodosum associated with sporotrichosis. Int. J. Dermatol. 2002, 41, 114–116. [Google Scholar] [CrossRef]
- Gutierrez-Galhardo, M.C.; Barros, M.B.; Schubach, A.O.; Cuzzi, T.; Schubach, T.M.; Lazera, M.S.; Valle, A.C. Erythema multiforme associated with sporotrichosis. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Schubach, A.; de Lima Barros, M.B.; Schubach, T.M.; Francesconi-do-Valle, A.C.; Gutierrez-Galhardo, M.C.; Sued, M.; de Matos Salgueiro, M.; Fialho-Monteiro, P.C.; Reis, R.S.; Marzochi, K.B.; et al. Primary conjunctival sporotrichosis: Two cases from a zoonotic epidemic in Rio de Janeiro, Brazil. Cornea 2005, 24, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Curi, A.L.; Felix, S.; Azevedo, K.M.; Estrela, R.; Villar, E.G.; Saraca, G. Retinal granuloma caused by Sporothrix schenckii. Am. J. Ophthalmol. 2003, 136, 205–207. [Google Scholar] [CrossRef]
- Alvarez, R.; Lopez-Villegas, A. Primary ocular sporotrichosis. Am. J. Ophthalmol. 1966, 62, 150–151. [Google Scholar] [CrossRef]
- Orofino-Costa, R.; Macedo, P.M.; Rodrigues, A.M.; Bernardes-Engemann, A.R. Sporotrichosis: An update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An. Bras Dermatol. 2017, 92, 606–620. [Google Scholar] [CrossRef] [Green Version]
- Alba-Fierro, C.A.; Pérez-Torres, A.; Toriello, C.; Romo-Lozano, Y.; López-Romero, E.; Ruiz-Baca, E. Molecular components of the Sporothrix schenckii complex that induce immune response. Curr. Microbiol. 2016, 73, 292–300. [Google Scholar] [CrossRef]
- Orofino-Costa, R.; Unterstell, N.; Carlos Gripp, A.; de Macedo, P.M.; Brota, A.; Dias, E.; de Melo Teixeira, M.; Felipe, M.S.; Bernardes-Engemann, A.R.; Lopes-Bezerra, L.M. Pulmonary cavitation and skin lesions mimicking tuberculosis in a HIV negative patient caused by Sporothrix brasiliensis. Med. Mycol. Case Rep. 2013, 2, 65–71. [Google Scholar] [CrossRef]
- Aung, A.K.; Spelman, D.W.; Thompson, P.J. Pulmonary sporotrichosis: An evolving clinical paradigm. Semin. Respir. Crit. Care Med. 2015, 36, 756–766. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected endemic mycoses. Lancet Infect. Dis. 2017, 17, 367–377. [Google Scholar] [CrossRef]
- Gremião, I.D.F.; Martins da Silva da Rocha, E.; Montenegro, H.; Carneiro, A.J.B.; Xavier, M.O.; de Farias, M.R.; Monti, F.; Mansho, W.; de Macedo Assunção Pereira, R.H.; Pereira, S.A.; et al. Guideline for the management of feline sporotrichosis caused by Sporothrix brasiliensis and literature revision. Braz. J. Microbiol. 2021, 52, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Cabo, J.F.; de las Heras Guillamon, M.; Latre Cequiel, M.W.; García de Jalón Ciércoles, J.A. Feline sporotrichosis: A case report. Mycopathologia 1989, 108, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, R.W.; Langham, R.F.; Reimann, K.A.; Wakenell, P.S. Feline sporotrichosis: A report of five cases with transmisión to humans. J. Am. Acad. Dermatol. 1986, 15, 37–45. [Google Scholar] [CrossRef]
- Crothers, S.L.; White, S.D.; Ihrke, P.J.; Affolter, V.K. Sporotrichosis: A retrospective evaluation of 23 cases seen in northern California (1987–2007). Vet. Dermatol. 2009, 20, 249–259. [Google Scholar] [CrossRef]
- Boechat, J.S.; Pereira, S.A.; de Sá Machado, A.C.; Viana, P.G.; Almeida-Paes, R.; Zancopé-Oliveira, R.M.; Gremião, I.D.F.; de Oliveira, M.M.E. Canine sporotrichosis: Polyphasic taxonomy and antifungal susceptibility profiles of Sporothrix species in an endemic area in Brazil. Braz. J. Microbiol. 2021, 52, 135–143. [Google Scholar] [CrossRef]
- Duangkaew, L.; Yurayart, C.; Limsivilai, O.; Chen, C.; Kasorndorkbua, C. Cutaneous sporotrichosis in a stray cat from Thailand. Med. Mycol. Case Rep. 2018, 23, 46–49. [Google Scholar] [CrossRef]
- Valeriano, C.A.T.; Lima-Neto, R.G.; Inacio, C.P.; Rabello, V.B.S.; Oliveira, E.P.; Zancope-Oliveira, R.M.; Almeida-Paes, R.; Neves, R.P.; de Oliveira, M.M.E. Is Sporothrix chilensis circulating outside Chile? PLoS Negl. Trop. Dis. 2020, 14, e0008151. [Google Scholar] [CrossRef] [Green Version]
- Rosser, E.J.; Dunstan, R.W. Sporotrichosis. In Infectious Diseases of the Dog and the Cat, 3rd ed.; Greene, C.E., Ed.; Saunders: St Louis, MO, USA, 2006; pp. 608–612. [Google Scholar]
- Schubach, T.M.; Schubach, A.; Okamoto, T.; Pellon, I.V.; Fialho-Monteiro, P.C.; Reis, R.S.; Barros, M.B.; Andrade-Perez, M.; Wanke, B. Haematogenous spread of Sporothrix schenckii in cats with naturally acquired sporotrichosis. J. Small Anim. Pract. 2003, 44, 395–398. [Google Scholar] [CrossRef]
- Gremião, I.D.; Menezes, R.C.; Schubach, T.M.; Figueiredo, A.B.; Cavalcanti, M.C.; Pereira, S.A. Feline sporotrichosis: Epidemiological and clinical aspects. Med. Mycol. 2015, 53, 15–21. [Google Scholar] [CrossRef]
- Schubach, T.M.; Valle, A.C.; Gutierrez-Galhardo, M.C.; Monteiro, P.C.; Reis, R.S.; Zancope-Oliveira, R.M.; Marzochi, K.B.; Schubach, A. Isolation of Sporothrix schenckii from the nails of domestic cats (Felis catus). Med. Mycol. 2001, 39, 147–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brilhante, R.S.N.; Fernandes, M.R.; Pereira, V.S.; Costa, A.D.C.; Oliveira, J.S.; de Aguiar, L.; Rodrigues, A.M.; de Camargo, Z.P.; Pereira-Neto, W.A.; Sidrim, J.J.C.; et al. Biofilm formation on cat claws by Sporothrix species: An ex vivo model. Microb. Pathog. 2021, 150, 104670. [Google Scholar] [CrossRef] [PubMed]
- Viana, P.G.; Figueiredo, A.B.F.; Gremião, I.D.F.; de Miranda, L.H.M.; da Silva Antonio, I.M.; Boechat, J.S.; de Sá Machado, A.C.; de Oliveira, M.M.E.; Pereira, S.A. Successful treatment of canine sporotrichosis with terbinafine: Case reports and literature review. Mycopathologia 2018, 183, 471–478. [Google Scholar] [CrossRef]
- Schechtman, R.C. Sporotrichosis: Part II. Skinmed. 2010, 8, 275–280. [Google Scholar] [PubMed]
- Waller, S.B.; Hoffmann, J.F.; Madrid, I.M.; Picoli, T.; Cleff, M.B.; Chaves, F.C.; Zanette, R.A.; de Mello, J.R.B.; de Faria, R.O.; Meireles, M.C.A. Polar Origanum vulgare (Lamiaceae) extracts with antifungal potential against Sporothrix brasiliensis. Med. Mycol. 2018, 56, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascarenhas, M.B.; Lopes, N.L.; Pinto, T.G.; Costa, T.S.; Peixoto, A.P.; Ramadinha, R.R.; Fernandes, J.I. Canine sporotrichosis: Report of 15 advanced cases. Pesq. Vet. Bras. 2018, 38, 477–481. [Google Scholar] [CrossRef]
- Ramos, A.C.M.O.; Oliveira, I.V.P.M.; Reis-Lima, R.K.; Paula, V.V.; Filgueira, K.D. Zoonotic transmission of canine sporotrichosis in northeastern Brazil. Acta Vet. Bras. 2017, 11, 79–84. [Google Scholar] [CrossRef]
- Lopes-Bezerra, L.M.; Mora-Montes, H.M.; Zhang, Y.; Nino-Vega, G.; Rodrigues, A.M.; de Camargo, Z.P.; de Hoog, S. Sporotrichosis between 1898 and 2017: The evolution of knowledge on a changeable disease and on emerging etiological agents. Med. Mycol. 2018, 56, 126–143. [Google Scholar] [CrossRef] [Green Version]
- Schubach, T.M.; Schubach, A.; Okamoto, T.; Barros, M.B.; Figueiredo, F.B.; Cuzzi, T.; Pereira, S.A.; Dos Santos, I.B.; Almeida Paes, R.; Paes Leme, L.R.; et al. Canine sporotrichosis in Rio de Janeiro, Brazil: Clinical presentation, laboratory diagnosis and therapeutic response in 44 cases (1998–2003). Med. Mycol. 2006, 44, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Da Rocha, R.F.D.B.; Schubach, T.M.P.; Pereira, S.A.; Dos Reis, É.G.; Carvalho, B.W.; Gremião, I.D.F. Refractory feline sporotrichosis treated with itraconazole combined with potassium iodide. J. Small Anim. Pract. 2018, 59, 720–721. [Google Scholar] [CrossRef] [Green Version]
- Fichman, V.; Freitas, D.F.S.; do Valle, A.C.F.; de Souza, R.V.; Curi, A.L.L.; Valete-Rosalino, C.M.; de Macedo, P.M.; Varon, A.G.; Figueiredo-Carvalho, M.H.G.; Almeida-Silva, F.; et al. Severe sporotrichosis treated with amphotericin B: A 20-year cohort study in an endemic area of zoonotic transmission. J. Fungi (Basel) 2022, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.G.; Gremião, I.D.; Kitada, A.A.; Rocha, R.F.; Castro, V.S.; Barros, M.B.; Menezes, R.C.; Pereira, S.A.; Schubach, T.M. Potassium iodide capsule treatment of feline sporotrichosis. J. Feline Med. Surg. 2012, 14, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, A.; Fierro, L.; Saúl, A.; Ponce, R.M. Cutaneous sporotrichosis. Intermittent treatment (pulses) with itraconazole. Eur. J. Dermatol. 2010, 18, 61–64. [Google Scholar]
- de Lima Barros, M.B.; Schubach, A.O.; de Vasconcellos Carvalhaes de Oliveira, R.; Martins, E.B.; Teixeira, J.L.; Wanke, B. Treatment of cutaneous sporotrichosis with itraconazole--study of 645 patients. Clin. Infect. Dis. 2011, 52, e200–e206. [Google Scholar] [CrossRef] [PubMed]
- Scuarcialupi, L.N.; Pereira, F.C.; Baquero, O.S. Feline sporotrichosis: Social vulnerability and prioritization of geographic areas in Guarulhos, SP, Brazil. Braz. J. Vet. Res. Anim. Sci. 2021, 58, e188291. [Google Scholar] [CrossRef]
- Reis, E.G.; Schubach, T.M.; Pereira, S.A.; Silva, J.N.; Carvalho, B.W.; Quintana, M.S.; Gremião, I.D. Association of itraconazole and potassium iodide in the treatment of feline sporotrichosis: A prospective study. Med. Mycol. 2016, 54, 684–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakasu, C.C.T.; Waller, S.B.; Ripoll, M.K.; Ferreira, M.R.A.; Conceição, F.R.; Gomes, A.D.R.; Osório, L.D.G.; de Faria, R.O.; Cleff, M.B. Feline sporotrichosis: A case series of itraconazole-resistant Sporothrix brasiliensis infection. Braz. J. Microbiol. 2021, 52, 163–171. [Google Scholar] [CrossRef]
- Orofino-Costa, R.; Bernardes-Engemann, A.R.; Azulay-Abulafia, L.; Benvenuto, F.; Neves, M.L.; Lopes-Bezerra, L.M. Sporotrichosis in pregnancy: Case reports of 5 patients in a zoonotic epidemic in Rio de Janeiro, Brazil. An. Bras. Dermatol. 2011, 86, 995–998. [Google Scholar]
- Francesconi, G.; Valle, A.C.; Passos, S.; Reis, R.; Galhardo, M.C. Terbinafine (250 mg/day): An effective and safe treatment of cutaneous sporotrichosis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1273–1276. [Google Scholar] [CrossRef]
- Kauffman C., A.; Bustamante, B.; Chapman S., W.; Pappas P., G. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Gremião, I.; Schubach, T.; Pereira, S.; Rodrigues, A.; Honse, C.; Barros, M. Treatment of refractory feline sporotrichosis with a combination of intralesional amphotericin B and oral itraconazole. Aust. Vet. J. 2011, 89, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Ottonelli Stopiglia, C.D.; Magagnin, C.M.; Castrillón, M.R.; Mendes, S.D.; Heidrich, D.; Valente, P.; Scroferneker, M.L. Antifungal susceptibilities and identification of species of the Sporothrix schenckii complex isolated in Brazil. Med. Mycol. 2014, 52, 56–64. [Google Scholar] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Approved Standard M38-A2 Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Espinel-Ingroff, A.; Abreu, D.P.B.; Almeida-Paes, R.; Brilhante, R.S.N.; Chakrabarti, A.; Chowdhary, A.; Hagen, F.; Córdoba, S.; Gonzalez, G.M.; Govender, N.P.; et al. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob. Agents Chemother. 2017, 61, e01057-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borba-Santos, L.P.; Visbal, G.; Gagini, T.; Rodrigues, A.M.; Pires De Camargo, Z.; Lopes-Bezerra, L.M.; Ishida, K.; De Souza, W.; Rozental, S. Δ24-sterol methyltransferase plays an important role in the growth and development of Sporothrix schenckii and Sporothrix brasiliensis. Front. Microbiol. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagini, T.; Colina-Vegas, L.; Villarreal, W.; Borba-Santos, L.P.; Pereira, C.S.; Batista, A.A.; Fleury, M.K.; Souza, W.; Rozental, S.; Costa, L.A.S.; et al. Metal–azole fungistatic drug complexes as anti-Sporothrix spp. agents. New J. Chem. 2018, 42, 13641–13650. [Google Scholar] [CrossRef]
- Borba-Santos, L.P.; Reis de Sá, L.F.; Ramos, J.A.; Rodrigues, A.M.; de Camargo, Z.P.; Rozental, S.; Ferreira-Pereira, A. Tacrolimus increases the effectiveness of itraconazole and fluconazole against Sporothrix spp. Front. Microbiol. 2017, 8, 1759. [Google Scholar] [CrossRef] [Green Version]
- Borba-Santos, L.P.; Gagini, T.; Ishida, K.; de Souza, W.; Rozental, R. Miltefosine is active against Sporothrix brasiliensis isolates with in vitro low susceptibility to amphotericin B or itraconazole. J. Med. Microbiol. 2015, 64, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Borba-Santos, L.P.; Ishida, K.; Calogeropoulou, T.; Souza, W.; Rozental, S. Adamantylidene-substituted alkylphosphocholine TCAN26 is more active against Sporothrix schenckii than mil;efosine. Mem. Inst. Oswaldo Cruz. 2016, 111, 523–527. [Google Scholar] [CrossRef]
- Tandon, V.K.; Yadav, D.B.; Singh, R.V.; Chaturvedi, A.K.; Shukla, P.K. Synthesis, and biological evaluation of novel (L)-alpha-amino acid methyl ester, heteroalkyl, and aryl substituted 1,4-naphthoquinone derivatives as antifungal and antibacterial agents. Bioorg. Med. Chem. Lett. 2005, 15, 5324–5328. [Google Scholar] [CrossRef]
- Tandon, V.K.; Maurya, H.K.; Tripathi, A.; ShivaKeshava, G.B.; Shukla, P.K.; Srivastava, P.; Panda, D. 2,3-Disubstituted-1,4-naphthoquinones, 12H-benzo[b]phenothiazine-6,11-diones and related compounds: Synthesis and biological evaluation as potential antiproliferative and antifungal agents. Eur. J. Med. Chem. 2009, 44, 1086–1092. [Google Scholar] [CrossRef]
- Garcia Ferreira, P.; Pereira Borba-Santos, L.; Noronha, L.L.; Deckman Nicoletti, C.; de Sá Haddad Queiroz, M.; de Carvalho da Silva, F.; Rozental, S.; Omena Futuro, D.; Francisco Ferreira, V. Synthesis, stability studies, and antifungal evaluation of substituted α- and β-2,3-dihydrofuranaphthoquinones against Sporothrix brasiliensis and Sporothrix schenckii. Molecules 2019, 24, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borba-Santos, L.P.; Vila, T.; Rozental, S. Identification of two potential inhibitors of Sporothrix brasiliensis and Sporothrix schenckii in the Pathogen Box collection. PLoS ONE 2020, 15, e0240658. [Google Scholar] [CrossRef] [PubMed]
- Honse, C.O.; Rodrigues, A.M.; Gremião, I.D.; Pereira, S.A.; Schubach, T.M. Use of local hyperthermia to treat sporotrichosis in a cat. Vet. Rec. 2010, 166, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Fichman, V.; Valle, A.C.F.D.; de Macedo, P.M.; Freitas, D.F.S.; Oliveira, M.M.E.; Almeida-Paes, R.; Gutierrez-Galhardo, M.C. Cryosurgery for the treatment of cutaneous sporotrichosis in four pregnant women. PLoS Negl. Trop. Dis. 2018, 12, e0006434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasuya, A.; Ohta, I.; Tokura, Y. Structural and immunological effects of skin cryoablation in a mouse model. PLoS ONE 2015, 10, e0123906. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.R.; Campos, M.P.; Barros, M.B.L.; Carmo, C.N.; Gremião, I.D.F.; Pereira, S.A.; Schubach, T.M. Treatment abandonment in feline sporotrichosis—Study of 147 cases. Zoonoses Public Health 2013, 60, 149–153. [Google Scholar] [CrossRef]
- Silva, M.B.T.; Costa, M.M.; Torres, C.C.; Galhardo, M.C.; Valle, A.C.; Magalhães, M.d.A.; Sabroza, P.C.; Oliveira, R.M. Esporotricose urbana: Epidemia negligenciada no Rio de Janeiro, Brasil. [Urban sporotrichosis: A neglected epidemic in Rio de Janeiro, Brazil]. Cad. Saúde Pública 2012, 28, 1867–1880. [Google Scholar] [CrossRef] [Green Version]
- Orr, E.R.; Riley, H.D., Jr. Sporotrichosis in childhood: Report of ten cases. J. Pediatr. 1971, 78, 951–957. [Google Scholar] [CrossRef]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Narrative and systematic reviews | Editorials |
| Original articles | Opinion papers |
| Cross-sectional or longitudinal studies | Thesis/Dissertation |
| Meeting Summary | |
| Studies written in English, Spanish or Portuguese | Book chapters |
| Studies from developed and developing countries |
| Specimens | Direct Exam (KOH) | Cytological Examination (Grocott/PAS/Gram/Giemsa) | Fungal Culture | Reference |
|---|---|---|---|---|
| Humans | Low diagnostic sensitivity. Difficult direct examination | Low fungal load in the lesions. Difficult direct examinations | Reference standard. Melanin production. | [45],58,59,60,61] |
| Felines | High fungal load favoring the direct examination | Cytologic examination—preliminary diagnosis. High sensitivity (79 to 85%) | Reference standard. Similar sensitivity to skin biopsy. Melanin production related to azole resistance. | [46],61,62,63] |
| Canines | Low sensitive for the diagnosis Difficult direct examination | Low fungal load in the lesions. Low sensitivity (32%) | Reference standard. Fungal load is usually low. | [61] |
| Methods | Patient Type | Species Detected | Limitation | References |
|---|---|---|---|---|
| ELISA (Enzyme Linked ImmunonoSorbent Assay) | Human, Feline | S. schenckii S. brasiliensis | Cross-reactions with other fungal diseases may occur. Addition of 6M urea reduces cross-reactivity. Decrease in serum antibody titers as the lesions heal. Combine with clinical specimen cultures. | [64,65,66,67] |
| Molecular diagnostic methods and gene sequencing | Human, Feline, Canine | S. brasiliensis, S. schenckii, S. globosa, S. mexicana, S. pallida | High cost | [9,10,11], [13,14,15], [20,21], [32], [52,53], [56], [68] |
| MALDI-TOF-MS (Matrix Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry) | Human, Feline, Canine | S. brasiliensis, S. schenckii, S. globosa, S. pallida, S. mexicana, S. luriei | High cost | [69,70] |
| Histological methods associated to PCR-based molecular diagnostic | Feline | S. chilensis, S. mexicana, S. pallida, S. globosa, S. brasiliensis, S. schenckii | Use of formalin-fixed and paraffin-embedded tissues | [71,72] |
| Antifungal | Administration Route | Patients | Dosage | Indications | Reference |
|---|---|---|---|---|---|
| Potassium iodide (KI) | oral | Humans (children/elderly) | 15 mg/kg/day | Humans in the endemic area | [74], [76], [81,82], [87], [89,90], [96] |
| cats | 2.5–20 mg/kg/day plus ITZ cat dosage | Cats presenting multiple skin and mucosal lesions, or presence of respiratory signs; Cases refractory to ITZ monotherapy | |||
| Itraconazole (ITZ) | oral | Humans (adults) | 100 to 400 mg/day | Healthy patients with limited lesions, immunosuppressed patients and in the systemic form | [74], [77,78,79], [82], [87], [90,91,92,93], [96], [105,106,107,108,109,110,111,112,113,114,115,116,117,118] |
| cats | 25 mg–100 mg/kg/day | Cats with fixed cutaneous lesions and naïve to antifungal therapy | |||
| Terbinafine (TRB) | Oral | Humans (adults) | 250–500 mg/day | Cutaneous sporotrichosis; cases which itraconazole was contraindicated | [87], [91], [99], [107], [115], [118] |
| cats | 30 mg/kg/day | ||||
| Amphotericin B (AMB) | Deoxycholate (intravenous) | Humans (adults) | 0.3–1 mg/kg/day | Disseminated forms sporotrichosis | [80], [84], [86], [87], [91], [93], [96], [107], [117,118] |
| Liposomal (intravenous) | Humans (adults) and cats (rarely) | 3–5 mg/kg/day |
| Antifungal Drug | Action Mechanism | Efficiency | Reference |
|---|---|---|---|
| 22-hydrazone-imidazolin-2-yl-chol-5-ene-3β-ol (H3) | inhibition of Δ (24)-sterol methyltransferase | Sporothrix brasiliensis; Sporothrix schenckii | [121] |
| CTZ, KTZ and their respective metal salts or metal complexes under mild conditions | interference with the cell shape | S. schenckii, S. brasiliensis, Sporothrix globosa | [122] |
| Tacrolimus and cyclosporine A alone and in combination with ITZ or FLZ | inhibiting of calcineurin (only tacrolimus showed synergism with azoles) | S. brasiliensis, S. schenckii | [123] |
| Miltefosine | changes in the lipid composition of membranes | S. brasiliensis with low susceptibility to AMB or ITZ | [124] |
| Structural analogues of miltefosine (TCAN26, TC19, and TC70) | disruption of the cell membrane and cell wall, and increased cell wall thickness | S. schenckii sensu stricto | [125] |
| 1,4-naphthoquinone derivatives | not described | S. schenckii | [126] |
| 2,3-disubstituted-1,4-naphthoquinones | not described | S. schenckii | [127] |
| Substituted α- and β-2,3-dihydrofuranaphthoquinones | arylation of the thiol groups of proteins, intercalation, induction of breaks in the DNA chain, generation of free radicals and other reactive oxygen species (ROS), and bioreductive alkylation via the formation of quinone methide | S. brasiliensis, S. schenckii | [128] |
| Pathogen Box library (400 compounds evaluated; 12 showed effectiveness) | disrupted cells and overflow of intracellular content, increase in cell size, accumulation of neutral lipids, and disruption of plasma membrane integrity | S. brasiliensis, S. schenckii | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, C.M.; Oliveira, M.M.E.; Pires, R.H. Sporotrichosis: A Review of a Neglected Disease in the Last 50 Years in Brazil. Microorganisms 2022, 10, 2152. https://doi.org/10.3390/microorganisms10112152
Alvarez CM, Oliveira MME, Pires RH. Sporotrichosis: A Review of a Neglected Disease in the Last 50 Years in Brazil. Microorganisms. 2022; 10(11):2152. https://doi.org/10.3390/microorganisms10112152
Chicago/Turabian StyleAlvarez, Carmen Magaly, Manoel Marques Evangelista Oliveira, and Regina Helena Pires. 2022. "Sporotrichosis: A Review of a Neglected Disease in the Last 50 Years in Brazil" Microorganisms 10, no. 11: 2152. https://doi.org/10.3390/microorganisms10112152
APA StyleAlvarez, C. M., Oliveira, M. M. E., & Pires, R. H. (2022). Sporotrichosis: A Review of a Neglected Disease in the Last 50 Years in Brazil. Microorganisms, 10(11), 2152. https://doi.org/10.3390/microorganisms10112152








