Visceral Leishmaniasis Urbanization in the Brazilian Amazon Is Supported by Significantly Higher Infection Transmission Rates Than in Rural Area
Abstract
:1. Introduction
2. Materials and Methods
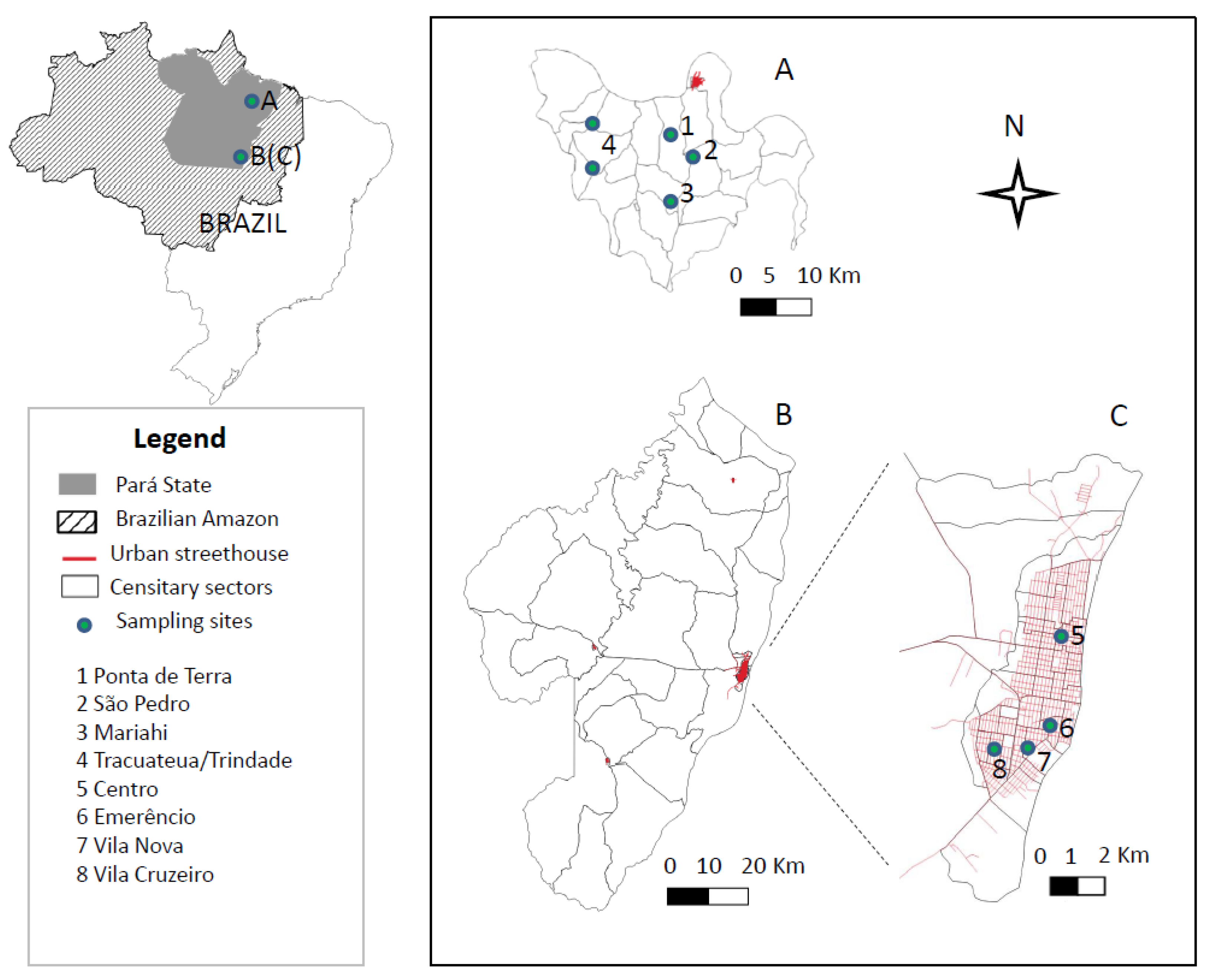
2.1. Study Area
2.2. Study Population
2.3. Study Design
2.4. Clinical Evaluation of Infected Individuals in Distinct Urban and Rural Scenarios
2.5. Serological Diagnostic Survey of Canine L. (L.) Infantum Chagasi-Infection in Distinct Urban and Rural Scenarios
2.6. Spatial Distribution of Human L. (L.) Infantum Chagasi-Infection in Distinct Urban and Rural Scenarios
2.7. Data Analysis
2.8. Ethical Approval
3. Results
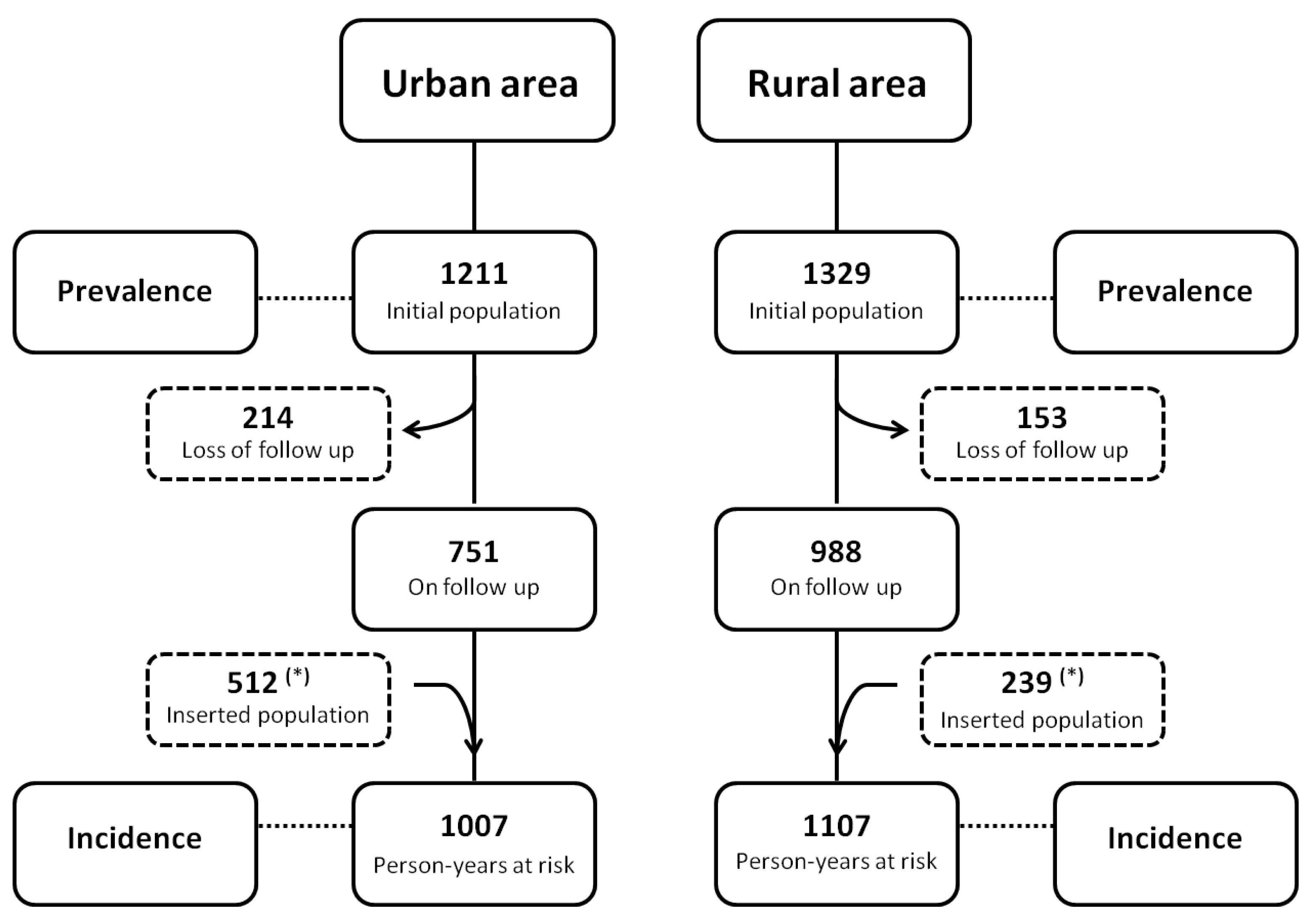
3.1. Prevalence of Human L. (L.) Infantum Chagasi-Infection in Distinct Urban and Rural Scenarios
3.2. Incidence of Human L. (L.) Infantum Chagasi-Infections in Distinct Urban and Rural Scenarios
3.3. Frequency of Human L. (L.) Infantum Chagasi-Infections According to Age and Gender in Distinct Urban and Rural Scenarios
3.4. Prevalence and Incidence of the Clinical-Immunological Profiles of Human L. (L.) Infantum Chagasi-Infections in Distinct Urban and Rural Scenarios
3.5. Evolution of III Profile Cases of Human L. (L.) Infantum Chagasi-Infections in Distinct Urban and Rural Scenarios
3.6. Serological Diagnostic Survey of Canine L. (L.) Infantum Chagasi-Infections in Distinct Urban and Rural Scenarios
3.7. Spatial Distribution of Human L. (L.) Infantum Chagasi-Infections in Distinct Urban and Rural Scenarios
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Leishmaniasis: World Health Organization. 2 March 2020. Available online: http://www.who.int/news-room/factsheets/detail/leishmaniasis (accessed on 1 August 2022).
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; Who Leishmaniasis Control the WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R.; Shaw, J.J. New World Leishmaniasis. In Topley & Wilson’s Microbiology and Microbial Infections, 10th ed.; Collier, L., Balows, A., Sussman, M., Eds.; Parasitology, Arnold: London, UK, 2010; Volume 5, pp. 313–349. [Google Scholar]
- Silveira, F.T.; Lainson, R.; de Souza, A.A.A.; Crescente, J.A.B.; Corbett, C.E.P. Leishmaniose visceral americana. In Medicina Tropical e Infectologia na Amazônia, 1st ed.; Leão, R., Ed.; Samauma: Belém, Brazil, 2013; Volume 2, pp. 1245–1274. [Google Scholar]
- Lainson, R.; Shaw, J.J. Leishmaniasis in the New World. In Topley & Wilson’s Microbiol and Microbial Infections, 10th ed.; Collier, L., Balows, A., Sussman, M., Eds.; Parasitology, Arnold: London, UK, 2005; Volume 5, pp. 313–349. [Google Scholar]
- Lainson, R. The Neotropical Leishmania species: A brief historical review of their discovery, ecology and taxonomy. Rev. Pan-Amaz. Saude 2010, 1, 13–32. [Google Scholar] [CrossRef]
- Silveira, F.T.; Corbett, C.E.P. Leishmania chagasi Cunha & Chagas, 1937: Indigenous or introduced? A brief review. Rev. Pan-Amaz. Saúde 2010, 1, 143–147. [Google Scholar] [CrossRef]
- Marcili, A.; Sperança, M.A.; da Costa, A.P.; Madeira, M.D.F.; Soares, H.S.; Sanches, C.D.O.; Acosta, I.D.C.; Girotto, A.; Minervino, A.H.; Horta, M.C.; et al. Phylogenetic relationships of Leishmania species based on trypanosomatid barcode (SSU rDNA) and gGAPDH genes: Taxonomic revision of Leishmania (L.) infantum chagasi in South America. Infect. Genet. Evol. 2014, 25, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveira, F.T.; Junior, E.C.S.; Silvestre, R.V.; Costa-Martins, A.G.; Pinheiro, K.D.C.; Ochoa, W.S.; dos Santos, T.V.; Ramos, P.K.; Casseb, S.; da Silva, S.P.; et al. Whole-Genome Sequencing of Leishmania infantum chagasi Isolates from Honduras and Brazil. Microbiol. Resour. Announc. 2021, 10, e00471-21. [Google Scholar] [CrossRef]
- Lainson, R.; Rangel, E.F. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil—A review. Mem. Inst. Oswaldo Cruz 2005, 100, 811–827. [Google Scholar] [CrossRef]
- Badaro, R.; Jones, T.C.; Lorenco, R.; Cerf, B.J.; Sampaio, D.; Carvalho, E.M.; Rocha, H.; Teixeira, R.; Johnson, W.D. A Prospective Study of Visceral Leishmaniasis in an Endemic Area of Brazil. J. Infect. Dis. 1986, 154, 639–649. [Google Scholar] [CrossRef]
- Badaro, R.; Jones, T.C.; Carvalho, E.M.; Sampaio, D.; Reed, S.G.; Barral, A.; Teixeira, R.; Johnson, W.D. New Perspectives on a Subclinical Form of Visceral Leishmaniasis. J. Infect. Dis. 1986, 154, 1003–1011. [Google Scholar] [CrossRef]
- Pearson, R.D.; Sousa, A.D.Q. Clinical Spectrum of Leishmaniasis. Clin. Infect. Dis. 1996, 22, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Crescente, J.B.; Silveira, F.T.; Lainson, R.; Gomes, C.M.; Laurenti, M.D.; Corbett, C.E. A cross-sectional study on the clinical and immunological spectrum of human Leishmania (L.) infantum chagasi infection in the Brazilian Amazon region. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1250–1256. [Google Scholar] [CrossRef]
- Silveira, F.T.; Lainson, R.; Crescente, J.Â.; De Souza, A.A.; Campos, M.B.; Gomes, C.M.; Laurenti, M.D.; Corbett, C.E. A prospective study on the dynamics of the clinical and immunological evolution of human Leishmania (L.) infantum chagasi infection in the Brazilian Amazon region. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 529–535. [Google Scholar] [CrossRef]
- Silveira, F.T.; Lainson, R.; De Souza, A.A.A.; Campos, M.B.; Carneiro, L.A.; Lima, L.V.R.; Ramos, P.K.S.; Gomes, C.M.D.C.; Laurenti, M.D.; Corbett, C.E.P. Further evidences on a new diagnostic approach for monitoring human Leishmania (L.) infantum chagasi infection in Amazonian Brazil. Parasitol. Res. 2010, 106, 377–386. [Google Scholar] [CrossRef]
- da Matta, V.L.; Gonçalves, A.N.A.; Gomes, C.M.C.; Furtado, R.R.; Campos, M.B.; Lima, L.V. The clinical-immunological spectrum of human Leishmania (L.) infantum chagasi infection in the Brazilian Amazon: A transcriptomic approach. In Proceedings of the 7th World Congress on Leishmaniasis, Cartagena, Colombia, 1–6 August 2022; p. 1363. [Google Scholar]
- Moreno, E.C.; Melo, M.N.; Lambertucci, J.R.; Serufo, J.C.; Andrade, A.S.; Antunes, C.M.; Genaro, O.; Carneiro, M. Diagnosing human asymptomatic visceral leishmaniasis in an urban area of the State of Minas Gerais, using serological and molecular biology techniques. Rev. Soc. Bras. Med. Trop. 2006, 39, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Silveira, F.T.; Lainson, R.; Pereira, E.A.; De Souza, A.A.A.; Campos, M.B.; Chagas, E.J.; Gomes, C.M.C.; Laurenti, M.D.; Corbett, C.E.P. A longitudinal study on the transmission dynamics of human Leishmania (Leishmania) infantum chagasi infection in Amazonian Brazil, with special reference to its prevalence and incidence. Parasitol. Res. 2009, 104, 559–567. [Google Scholar] [CrossRef]
- Silva, L.A.; Romero, H.D.; Fagundes, A.; Nehme, N.; Fernandes, O.; Rodrigues, V.; Costa, R.T.; Prata, A. Use of the polymerase chain reaction for the diagnosis of asymptomatic Leishmania infection in a visceral leishmaniasis-endemic area. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 101–104. [Google Scholar] [CrossRef] [Green Version]
- de Araujo, V.E.; Pinheiro, L.C.; Almeida, M.C.; de Menezes, F.C.; Morais, M.H.; Reis, I.A.; Assunção, R.M.; Carneiro, M. Relative risk of visceral leishmaniasis in Brazil: A spatial analysis in urban area. PLoS Negl. Trop. Dis. 2013, 7, e2540. [Google Scholar] [CrossRef] [Green Version]
- Brasil, Ministério da Saúde, Secretaria de Vigilância da Saúde. Sistema de Informação de Agravos de Notificação—Sinan/Net 2019. Available online: https://datasus.saude.gov.br/ (accessed on 1 August 2022).
- IBGE—Instituto Brasileiro de Geografia e Estatística. Estimativas da População Residente para os Municípios e para as Unidades da Federação Brasileira com Data de Referência em 1º de Julho. 2017:11p. Available online: https://www.ibge.gov.br/ (accessed on 1 August 2022).
- Jeronimo, S.; Holst, A.K.B.; Jamieson, S.E.; Francis, R.; Martins, D.R.A.; Bezerra, F.L.; Ettinger, N.A.; Nascimento, E.T.; Monteiro, G.R.; Lacerda, H.G.; et al. Genes at human chromosome 5q31.1 regulate delayed-type hypersensitivity responses associated with Leishmania chagasi infection. Genes Immun. 2007, 8, 539–551. [Google Scholar] [CrossRef]
- Gama, M.E.A.; Costa, J.M.L.; Gomes, C.M.C.; Corbett, C.E.P. Subclinical form of the American visceral leishmaniasis. Mem. Inst. Oswaldo Cruz 2004, 99, 889–893. [Google Scholar] [CrossRef] [Green Version]
- Silveira, F.T.; Lima, L.V.R.; Vasconcelos dos Santos, T.; Ramos, P.K.S.; Campos, M.C. Reviewing the trajectory of American visceral leishmaniasis in the Amazon, Brazil: From Evandro Chagas to the present days. Rev. Pan-Amaz. Saúde 2016, 7, 15–22. [Google Scholar] [CrossRef]
- Manual de Vigilância e Controle da Leishmaniose Visceral, 1st ed.; Ministério da Saúde. Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica: Brasília, Brazil, 2006.
- Lima, L.V.; Ramos, P.K.; Campos, M.B.; Santos, T.V.; Gomes, C.M.; Laurenti, M.D.; Corbett, C.E.; Silveira, F.T. Preclinical diagnosis of American visceral leishmaniasis during early onset of human Leishmania (L.) infantum chagasi-infection. Pathog. Glob. Health 2014, 108, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Vasconcelos-Dos-Santos, T.; Campos, M.; Ramos, P.K.; Gomes, C.; Laurenti, M.; da Matta, V.; Corbett, C.; Silveira, F. New record of preclinical diagnosis of American visceral leishmaniasis in Amazonian Brazil encourages optimizing disease control. Parasite Epidemiol. Control. 2020, 10, e00154. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.T.; Han, A.; Bittencourt, L.F.; Ramos, P.C.; Campos, M.B.; Gomes, C.M.; Laurenti, M.D.; Corbett, C.E. IgM antibody response in symptomatic (Visceral Leishmaniasis) and asymptomatic (Indeterminate Initial Infection) human Leishmania (L.) infantum chagasi-infection in Amazonian Brazil. Eur. J. Trop. Med. Int. Health 2011, 16, 64. [Google Scholar]
- Silveira, F.T.; Carneiro, L.A.; Ramos, P.K.S.; Chagas, E.J.; Lima, L.V.; Campos, M.B.; Laurenti, M.D.; Gomes, C.M.; Corbett, C.E. A cross-sectional study on canine Leishmania (L.) infantum chagasi infection in Amazonian Brazil ratifies a higher prevalence of specific IgG-antibody response than delayed-type hypersensitivity in symptomatic and asymptomatic dogs. Parasitol. Res. 2012, 111, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.; Ayres, M., Jr.; Ayres, D.; Santos, A.S. Bioestat 5.0: Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas; Soc Civ Mamirauá-Brasília: Belém, Brazil, 2008. [Google Scholar]
- Dantas-Torres, F.; Miró, G.; Baneth, G.; Bourdeau, P.; Breitschwerdt, E.; Capelli, G.; Cardoso, L.; Day, M.J.; Dobler, G.; Ferrer, L.; et al. Canine Leishmaniasis Control in the Context of One Health. Emerg. Infect. Dis. 2019, 25, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, L.A.; Dos Santos, T.V.; do Rêgo Lima, L.V.; Ramos, P.K.; Campos, M.B.; Silveira, F.T. First report on feline leishmaniasis caused by Leishmania (L.) amazonensis in Amazonian Brazil. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100360. [Google Scholar] [CrossRef]
- Caldas, A.J.M.; Silva, D.R.C.; Pereira, C.C.R.; Nunes, P.M.S.; Silva, B.P.; Silva, A.A.M.; Barral, A.; Costa, J.M. Infecção por Leishmania (Leishmania) chagasi em crianças de uma área endêmica de leishmaniose visceral americana na ilha de São Luis-MA, Brasil. Rev. Soc. Bras. Med. Trop. 2001, 34, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Caldas, A.; Costa, J.; Silva, A.; Vinhas, V.; Barral, A. Risk factors associated with asymptomatic infection by Leishmania chagasi in north-east Brazil. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 21–28. [Google Scholar] [CrossRef]
- Jeronimo, S.M.B.; Teixeira, M.J.; Sousa, A.D.Q.; Thielking, P.; Pearson, R.D.; Evans, T.G. Natural History of Leishmania (Leishmania) chagasi infection in Northeastern Brazil: Long-Term Follow-Up. Clin. Infect. Dis. 2000, 30, 608–609. [Google Scholar] [CrossRef] [Green Version]
- Lima, I.D.; Queiroz, J.W.; Lacerda, H.G.; Queiroz, P.V.S.; Pontes, N.N.; Barbosa, J.D.A.; Martins, D.R.; Weirather, J.L.; Pearson, R.D.; Wilson, M.; et al. Leishmania infantum chagasi in Northeastern Brazil: Asymptomatic Infection at the Urban Perimeter. Am. J. Trop. Med. Hyg. 2012, 86, 99–107. [Google Scholar] [CrossRef]
- Nascimento, M.D.S.B.; Souza, E.C.; da Silva, L.M.; da Cunha Leal, P.; de Lima Cantanhede, K.; de Barros Bezerra, G.F.; de Castro Viana, G.M. Prevalência de infecção por Leishmania chagasi utilizando os métodos de ELISA (rK39 e CRUDE) e intradermorreação de Montenegro em área endêmica do Maranhão, Brasil. Cad Saúde Pública Rio Jan. 2005, 21, 1801–1807. [Google Scholar] [CrossRef] [Green Version]
- Da Rocha, I.C.M.; Dos Santos, L.H.M.; Coura-Vital, W.; Da Cunha, G.M.R.; Magalhães, F.D.C.; Da Silva, T.A.M.; Morais, M.H.F.; Oliveira, E.; Reis, I.A.; Carneiro, M. Effectiveness of the Brazilian Visceral Leishmaniasis Surveillance and Control Programme in reducing the prevalence and incidence of Leishmania infantum infection. Parasites Vectors 2018, 11, 586. [Google Scholar] [CrossRef]
- Araujo, A.D.C.; Gonçalves, N.N.V.M.; Torres, F.D.; Ferreira, F.; Horta, M.C. Visceral Leishmaniasis in Petrolina, State of Pernambuco, Brazil, 2007–2013. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 29. [Google Scholar] [CrossRef] [Green Version]
- Diniz, L.F.B.; de Souza, C.D.F.; Carmo, R.F.D. Epidemiology of human visceral leishmaniasis in the urban centers of the lower-middle São Francisco Valley, Brazilian semiarid region. Rev. Soc. Bras. Med. Trop. 2018, 51, 461–466. [Google Scholar] [CrossRef]
- Reis, L.L.; Balieiro, A.A.; Fonseca, F.R.; Gonçalves, M.J. Changes in the epidemiology of visceral leishmaniasis in Brazil from 2001 to 2014. Rev. Soc. Bras. Med. Trop. 2017, 50, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, W.H.S.; Zúniga, C.; Chaves, L.F.; Flores, G.V.A.; Pacheco, C.M.S.; Da Matta, V.L.R.; Corbett, C.E.P.; Silveira, F.T.; Laurenti, M.D. Clinical and Immunological Features of Human Leishmania (L.) infantum-Infection, Novel Insights Honduras, Central America. Pathogens 2020, 9, 554. [Google Scholar] [CrossRef]
- Gama, M.E.A.; Gomes, C.M.D.C.; Silveira, F.T.; Laurenti, M.D.; Goncalves, E.D.G.; Da Silva, A.R.; Corbett, C.E.P. Severe visceral leishmaniasis in children: The relationship between cytokine patterns and clinical features. Rev. Soc. Bras. Med. Trop. 2013, 46, 741–745. [Google Scholar] [CrossRef] [Green Version]
- Ramos, P.K.; Carvalho, K.I.; Rosa, D.S.; Rodrigues, A.P.; Lima, L.V.; Campos, M.B.; Gomes, C.M.C.; Laurenti, M.D.; Corbett, C.E.; Silveira, F.T. Serum Cytokine Responses over the Entire Clinical-Immunological Spectrum of Human Leishmania (L.) infantum chagasi Infection. BioMed Res. Int. 2016, 2016, 6937980. [Google Scholar] [CrossRef]


| Clinical- Immunological Profiles | Urban Scenario n = 1211 (%) n = 246 (%) | 95% CI | Rural Scenario n = 1329 (%) n = 188 (%) | 95% CI |
|---|---|---|---|---|
| AI * | 13.4 (162) | 11.5–15.3 | 8.3 (110) | 6.8–9.8 |
| SRI | 3.4 (41) | 2.4–4.4 | 2.6 (34) | 1.7–3.4 |
| III | 3.0 (36) | 2.0–3.9 | 2.3 (30) | 1.5–3.1 |
| SOI | 0.4 (5) | n/a | 0.5 (6) | 0.1–0.8 |
| SI | 0.2 (2) | n/a | 0.6 (8) | 0.2–1.0 |
| Clinical- Immunological Profiles | Urban Scenario n = 1007 (100-py) n = 137 (%) | 95% CI | Rural Scenario n = 1107 (100-py) n = 75 (%) | 95% CI |
|---|---|---|---|---|
| AI * | 9.6 (97) | 7.8–11.5 | 4.3 (48) | 3.1–5.5 |
| SRI | 2.2 (22) | 1.3–3.1 | 1.1 (12) | 0.5–1.7 |
| III | 1.6 (16) | 0.8–2.4 | 1.1 (12) | 0.5–1.7 |
| SOI | 0.2 (2) | n/a | 0.1 (1) | n/a |
| SI | 0.0 (0) | n/a | 0.2 (2) | n/a |
| Gender | Age Range *** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Urban (n = 1211) | Rural (n = 1329) | Urban (n = 1211) | Rural (n = 1329) | |||||||
| Male (%) | Female (%) | Male (%) | Female (%) | 1–10 (%) | 11–20 (%) | ≥21 (%) | 1–10 (%) | 11–20 (%) | ≥21 (%) | |
| AI | 6.8 (82) | 6.6 (80) | 5.0 * (66) | 3.3(44) | 2.1 (25) | 2.6 (31) | 8.8 * (106) | 0.7 (9) | 2.4 * (32) | 5.2 * (69) |
| SRI | 1.6 (19) | 1.8 (22) | 1.6 (21) | 1.0 (13) | 0.3 (4) | 1.2 (14) | 1.9 * (23) | 0.3 (4) | 0.5 (7) | 1.7 * (23) |
| III | 1.2 (15) | 1.7 (21) | 1.1 (15) | 1.1 (15) | 0.5 (6) | 0.5 (6) | 2.0 * (24) | 0.3 (4) | 0.7 (9) | 1.3 (17) |
| SOI | 0.2 (2) | 0.2 (3) | 0.2 (2) | 0.3 (4) | 0.0 (0) | 0.1 (1) | 0.3 (4) | 0.1 (2) | 0.1 (1) | 0.2 (3) |
| SI | 0.0 (0) | 0.2 (2) | 0.4 (5) | 0.2 (3) | 0.1 (1) | 0.0 (0) | 0.1 (1) | 0.4 (5) | 0.1 (1) | 0.2 (3) |
| Gender | Age Range *** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Urban (n = 1007) | Rural (n = 1107) | Urban (n = 1007) | Rural (n = 1107) | |||||||
| Male (/100-py) | Female (100-py) | Male (100-py) | Female (100-py) | 1–10 (100-py) | 11–20 (100-py) | ≥21 (100-py) | 1–10 (100-py) | 11–20 (100-py) | ≥21 (100-py) | |
| AI | 4.2 (42) | 5.5 (55) | 2.4 (27) | 1.9 (21) | 0.7 (7) | 1.5 (15) | 7.4 * (75) | 0.2 (2) | 1.3 (14) | 2.9 * (32) |
| SRI | 1.2 (12) | 1.0 (10) | 0.7 (8) | 0.4 (4) | 0.3 (3) | 0.6 (6) | 1.3 (13) | 0.3 (3) | 0.1 (1) | 0.7 (8) |
| III | 1.0 (10) | 0.6 (6) | 0.6 (7) | 0.5 (5) | 0.3 (3) | 0.4 (4) | 0.9 (9) | 0.3 (3) | 0.3 (3) | 0.5 (6) |
| SOI | 0.0 (0) | 0.2 (2) | 0.1 (1) | 0.0 (0) | 0.0 (0) | 0.1 (1) | 0.1 (1) | 0.0 (0) | 0.0 (0) | 0.1 (1) |
| SI | 0.0 (0) | 0.0 (0) | 0.2 (2) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.1 (1) | 0.1 (1) | 0.0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtado, R.R.; Alves, A.C.; Lima, L.V.R.; Vasconcelos dos Santos, T.; Campos, M.B.; Ramos, P.K.S.; Gomes, C.M.C.; Laurenti, M.D.; da Matta, V.L.; Corbett, C.E.; et al. Visceral Leishmaniasis Urbanization in the Brazilian Amazon Is Supported by Significantly Higher Infection Transmission Rates Than in Rural Area. Microorganisms 2022, 10, 2188. https://doi.org/10.3390/microorganisms10112188
Furtado RR, Alves AC, Lima LVR, Vasconcelos dos Santos T, Campos MB, Ramos PKS, Gomes CMC, Laurenti MD, da Matta VL, Corbett CE, et al. Visceral Leishmaniasis Urbanization in the Brazilian Amazon Is Supported by Significantly Higher Infection Transmission Rates Than in Rural Area. Microorganisms. 2022; 10(11):2188. https://doi.org/10.3390/microorganisms10112188
Chicago/Turabian StyleFurtado, Rodrigo R., Ana Camila Alves, Luciana V. R. Lima, Thiago Vasconcelos dos Santos, Marliane B. Campos, Patrícia Karla S. Ramos, Claudia Maria C. Gomes, Márcia D. Laurenti, Vânia Lucia da Matta, Carlos Eduardo Corbett, and et al. 2022. "Visceral Leishmaniasis Urbanization in the Brazilian Amazon Is Supported by Significantly Higher Infection Transmission Rates Than in Rural Area" Microorganisms 10, no. 11: 2188. https://doi.org/10.3390/microorganisms10112188







