Antibacterial Properties and Potential Mechanism of Serum from Chinese Alligator
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent Preparation
2.2. Bacterial Strains
2.3. Bacterial Cultures
2.4. Antibacterial Properties in Relation to CAS Concentration
2.5. Antibacterial Ability of the CAS
2.6. Proteome Analysis of the CAS
2.6.1. Protein Preparation
2.6.2. TMT Protein Labeling and Mass Spectrometry Analysis
2.6.3. Bioinformatics Analysis
2.7. Analysis of the Complement Component 3 Found in the CAS
2.8. Sequence Alignment and Phylogenetic Analysis of Complement Component 3d
3. Results
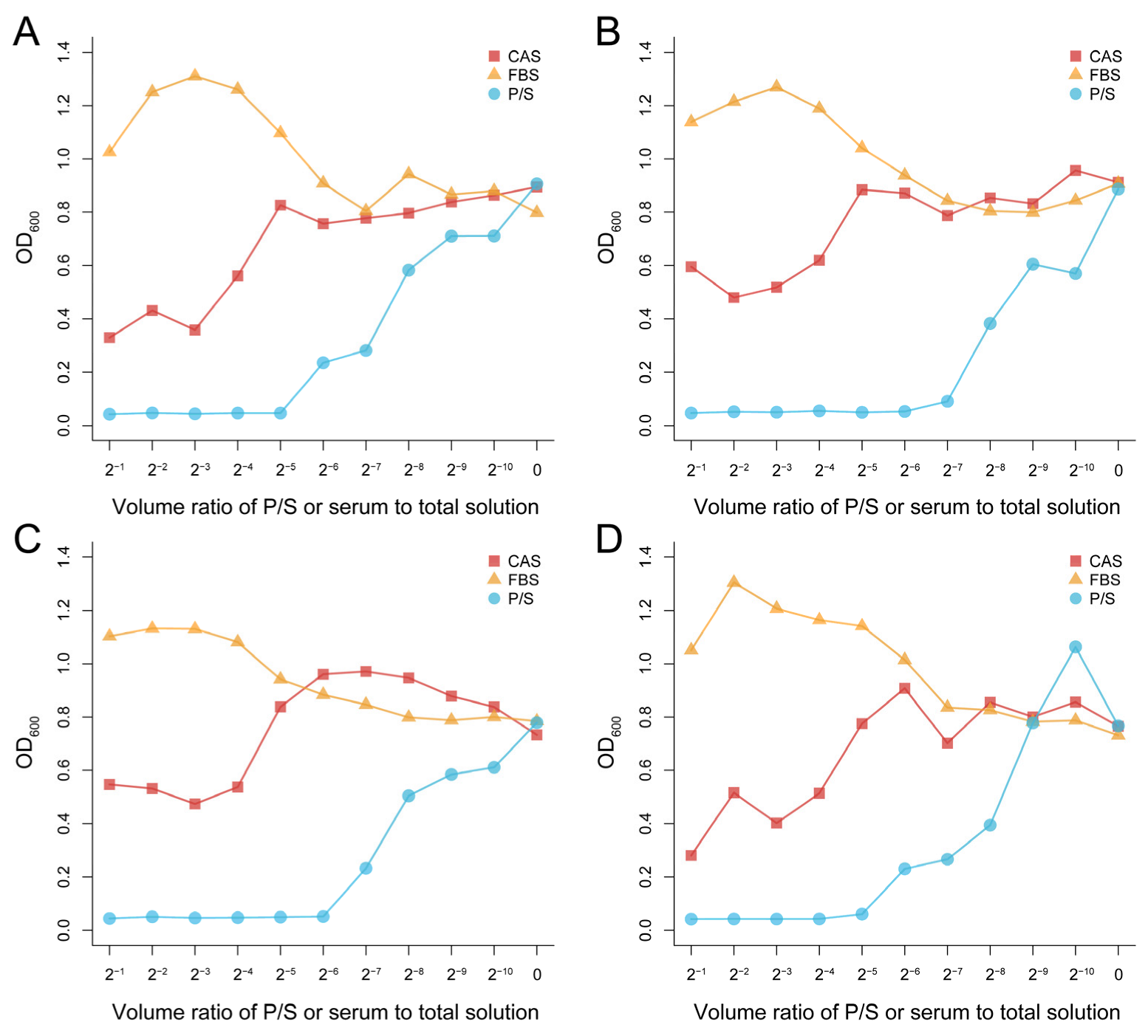
3.1. Effect of Concentration of CAS on the Antibacterial Activity
3.2. Antibacterial Effect of the CAS on Different Strains at a Given Concentration
3.3. Antibacterial Effect of the CAS with Protease K
3.4. Proteomic Analysis of the CAS
3.5. Analysis of Complement C3 Protein in Chinese Alligator
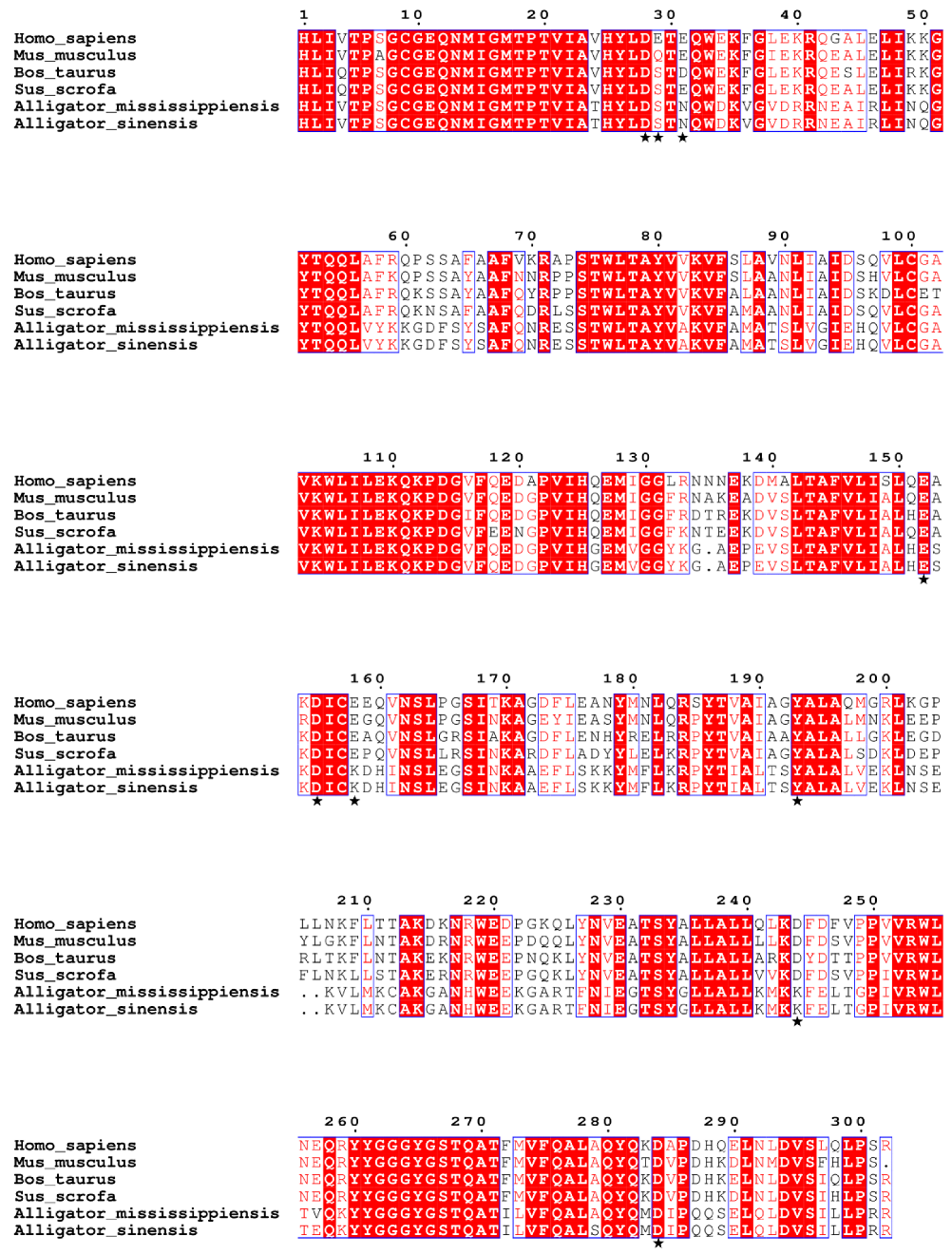
3.6. Sequence Analysis and Phylogenetic Analysis of Complement Component 3d
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. R. 2010, 74, 417. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J. The bactericidal power of normal serum. J. Pathol. Bacteriol. 1933, 37, 367–380. [Google Scholar] [CrossRef]
- Barksdale, S.M.; Hrifko, E.J.; van Hoek, M.L. Cathelicidin antimicrobial peptide from Alligator mississippiensis has antibacterial activity against multi-drug resistant Acinetobacter baumanii and Klebsiella pneumoniae. Dev. Comp. Immunol. 2017, 70, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Hitt, S.J.; Bishop, B.M.; van Hoek, M.L. Komodo-dragon cathelicidin-inspired peptides are antibacterial against carbapenem-resistant Klebsiella pneumoniae. J. Med. Microbiol. 2020, 69, 1262–1272. [Google Scholar] [CrossRef]
- Gong, Y.; Li, H.; Wu, F.; Zhang, X.; Zhou, Y.; Zhang, S. A short peptide derived from zebrafish AP-2 complex subunit mu-A AP2M1A(354)(-382) has antimicrobial activity against multi-drug resistant bacteria. Pept. Sci. 2022, 114, e24258. [Google Scholar] [CrossRef]
- Merchant, M.E.; Roche, C.M.; Thibodeaux, D.; Elsey, R.M. Identification of alternative pathway serum complement activity in the blood of the American alligator (Alligator mississippiensis). Comp. Biochem. Phys. B 2005, 141, 281–288. [Google Scholar] [CrossRef]
- Wan, Q.; Pan, S.; Hu, L.; Zhu, Y.; Xu, P.; Xia, J.; Chen, H.; He, G.; He, J.; Ni, X.; et al. Genome analysis and signature discovery for diving and sensory properties of the endangered Chinese alligator. Cell Res. 2013, 23, 1091–1105. [Google Scholar] [CrossRef]
- Rost, B.; Yachdav, G.; Liu, J.F. The PredictProtein server. Nucleic Acids Res. 2004, 322, W321–W326. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; Mcwilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Korpela, J.; Salonen, E.; Kuusela, P.; Sarvas, M.; Vaheri, A. Binding of avidin to bacteria and to the outer membrane porin of Escherichia coli. FEMS Microbiol. Lett. 1984, 22, 3–10. [Google Scholar] [CrossRef]
- Chen, X.J.; Niyonsaba, F.; Ushio, H.; Okuda, D.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J. Dermatol. Sci. 2005, 40, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Bishop, B.M.; van Hoek, M.L. Susceptibility of Pseudomonas aeruginosa biofilm to alpha-helical peptides: D-enantiomer of LL-37. Front. Microbiol. 2011, 2, 128. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Bishop, B.M.; van Hoek, M.L. Natural and synthetic cathelicidin peptides with anti-microbial and anti-biofilm activity against Staphylococcus aureus. BMC Microbiol. 2011, 11, 114. [Google Scholar] [CrossRef]
- Kovach, M.A.; Ballinger, M.N.; Newstead, M.W.; Zeng, X.; Bhan, U.; Yu, F.; Moore, B.B.; Gallo, R.L.; Standiford, T.J. Cathelicidin-Related Antimicrobial Peptide Is Required for Effective Lung Mucosal Immunity in Gram-Negative Bacterial Pneumonia. J. Immunol. 2012, 189, 304–311. [Google Scholar] [CrossRef]
- Mccormack, R.; de Armas, L.R.; Shiratsuchi, M.; Ramos, J.E.; Podack, E.R. Inhibition of Intracellular Bacterial Replication in Fibroblasts Is Dependent on the Perforin-Like Protein (Perforin-2) Encoded by Macrophage-Expressed Gene 1. J. Innate Immun. 2013, 5, 185–194. [Google Scholar] [CrossRef]
- Mccormack, R.; Podack, E.R. Perforin-2/Mpeg1 and other pore-forming proteins throughout evolution. J. Leukocyte Biol. 2015, 98, 761–768. [Google Scholar] [CrossRef]
- Sorensen, C.A.; Rosbjerg, A.; Jensen, B.H.; Krogfelt, K.A.; Garred, P. The Lectin Complement Pathway Is Involved in Protection Against Enteroaggregative Escherichia coli Infection. Front. Immunol. 2018, 9, 1153. [Google Scholar] [CrossRef]
- Doorduijn, D.J.; Rooijakkers, S.H.M.; van Schaik, W.; Bardoel, B.W. Complement resistance mechanisms of Klebsiella pneumoniae. Immunobiology 2016, 221, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, H.I.; Ali, Y.M.; Rajakumar, K.; Lynch, N.J.; Kadioglu, A.; Stover, C.M.; Schwaeble, W.J. Absence of the lectin activation pathway of complement does not increase susceptibility to Pseudomonas aeruginosa infections. Immunobiology 2012, 217, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Han, D.; Liu, C.; Gao, Y.; Li, D.; Liu, Y.; Yang, G. Staphylococcus aureus VraX specifically inhibits the classical pathway of complement by binding to C1q. Mol. Immunol. 2017, 88, 38–44. [Google Scholar] [CrossRef]
- Laarman, A.J.; Ruyken, M.; Malone, C.L.; van Strijp, J.A.G.; Horswill, A.R.; Rooijakkers, S.H.M. Staphylococcus aureus Metalloprotease Aureolysin Cleaves Complement C3 To Mediate Immune Evasion. J. Immunol. 2011, 186, 6445–6453. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef]
- Goldberg, B.S.; Ackerman, M.E. Antibody-mediated complement activation in pathology and protection. Immunol. Cell Biol. 2020, 98, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, L.; Al-Rammahi, N.; Haffner, S.M.; Stromstedt, A.A.; Browning, K.L.; Malmsten, M. Avidin-Biotin Cross-Linked Microgel Multilayers as Carriers for Antimicrobial Peptides. Biomacromolecules 2018, 19, 4691–4702. [Google Scholar] [CrossRef]
- Janssen, B.; Huizinga, E.G.; Raaijmakers, H.; Roos, A.; Daha, M.R.; Nilsson-Ekdahl, K.; Nilsson, B.; Gros, P. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature 2005, 437, 505–511. [Google Scholar] [CrossRef]
- Dempsey, P.W.; Allison, M.; Akkaraju, S.; Goodnow, C.C.; Fearon, D.T. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science 1996, 271, 348–350. [Google Scholar] [CrossRef]
- Nagar, B.; Jones, R.G.; Diefenbach, R.J.; Isenman, D.E.; Rini, J.M. X-ray crystal structure of C3d: A C3 fragment and ligand for complement receptor 2. Science 1998, 280, 1277–1281. [Google Scholar] [CrossRef]
- Isenman, D.E.; Leung, E.; Mackay, J.D.; Bagby, S.; van den Elsen, J.M.H. Mutational Analyses Reveal that the Staphylococcal Immune Evasion Molecule Sbi and Complement Receptor 2 (CR2) Share Overlapping Contact Residues on C3d: Implications for the Controversy Regarding the CR2/C3d Cocrystal Structure. J. Immunol. 2010, 184, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.; Britton, A. Characterization of serum complement activity of saltwater (Crocodylus porosus) and freshwater (Crocodylus johnstoni) crocodiles. Comp. Biochem. Phys. A 2006, 143, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Kommanee, J.; Preecharram, S.; Daduang, S.; Temsiripong, Y.; Dhiravisit, A.; Yamada, Y.; Thammasirirak, S. Antibacterial activity of plasma from crocodile (Crocodylus siamensis) against pathogenic bacteria. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, D.A.; Hanington, P.C.; Walsh, J.G.; Wilson, E.C.; Belosevic, M. Comparison of select innate immune mechanisms of fish and mammals. Xenotransplantation 2005, 12, 266–277. [Google Scholar] [CrossRef]
- Merchant, M.E.; Trahan, C.; Moran, C.; White, M.E. Two Different Complement C3 Genes in Crocodilians. Copeia 2016, 104, 756–762. [Google Scholar] [CrossRef]
- Linscott, W.D.; Triglia, R.P. The bovine complement system. Oxyg. Transp. Tissue XXXIII 1981, 137, 413–430. [Google Scholar]
- Campbell, L.M.; Michaels, G.; Klein, R.D.; Roth, I.L. Isolation of Klebsiella pneumoniae from lake water. Can. J. Microbiol. 1976, 22, 1762–1767. [Google Scholar] [CrossRef]
- Fogarty, L.R.; Haack, S.K.; Johnson, H.E.; Brennan, A.K.; Isaacs, N.M.; Spencer, C. Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) at ambient freshwater beaches. J. Water Health 2015, 13, 680–692. [Google Scholar] [CrossRef]
- Pellett, S.; Bigley, D.V.; Grimes, D.J. Distribution of Pseudomonas aeruginosa in a riverine ecosystem. Appl. Environ. Microbiol. 1983, 45, 328–332. [Google Scholar] [CrossRef]
- Ishii, S.; Sadowsky, M.J. Escherichia coli in the environment: Implications for water quality and human health. Microbes Environ. 2008, 23, 101–108. [Google Scholar] [CrossRef]
- Merchant, M.E.; Mills, K.; Leger, N.; Jenkins, E.; Vliet, K.A.; Mcdaniel, N. Comparisons of innate immune activity of all known living crocodylian species. Comp. Biochem. Phys. B 2006, 143, 133–137. [Google Scholar] [CrossRef]



| Cathelicidin | Perforin | Complement | Avidin | |
|---|---|---|---|---|
| K. pneumoniae | Inhibition | Uncertain | Inhibition | Binding (inhibition effect is negligible) |
| S. aureus | Inhibition | Inhibition | Not inhibition | |
| E. coli | Inhibition | Uncertain | Inhibition | |
| P. aeruginosa | Inhibition | Uncertain | Inhibition |
| Human | Alligators |
|---|---|
| Asp28 | Asp |
| Glu29 | Ser |
| Glu31 | Asn |
| Glu152 | Glu |
| Asp155 | Asp |
| Glu158 | Lys |
| Tyr193 | Tyr |
| Asp244 | Lys |
| Asp284 | Asp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.-Y.; Chen, Y.-W.; Chai, Z.-F.; Wang, Y.-Z.; Lin, J.-Q.; Fang, S.-G. Antibacterial Properties and Potential Mechanism of Serum from Chinese Alligator. Microorganisms 2022, 10, 2210. https://doi.org/10.3390/microorganisms10112210
Hu M-Y, Chen Y-W, Chai Z-F, Wang Y-Z, Lin J-Q, Fang S-G. Antibacterial Properties and Potential Mechanism of Serum from Chinese Alligator. Microorganisms. 2022; 10(11):2210. https://doi.org/10.3390/microorganisms10112210
Chicago/Turabian StyleHu, Meng-Yuan, Yi-Wen Chen, Zhi-Fan Chai, Yin-Zhi Wang, Jian-Qing Lin, and Sheng-Guo Fang. 2022. "Antibacterial Properties and Potential Mechanism of Serum from Chinese Alligator" Microorganisms 10, no. 11: 2210. https://doi.org/10.3390/microorganisms10112210
APA StyleHu, M.-Y., Chen, Y.-W., Chai, Z.-F., Wang, Y.-Z., Lin, J.-Q., & Fang, S.-G. (2022). Antibacterial Properties and Potential Mechanism of Serum from Chinese Alligator. Microorganisms, 10(11), 2210. https://doi.org/10.3390/microorganisms10112210







