Prevalence of mecA, mecC and Panton-Valentine-Leukocidin Genes in Clinical Isolates of Coagulase Positive Staphylococci from Dermatological Canine Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Isolates
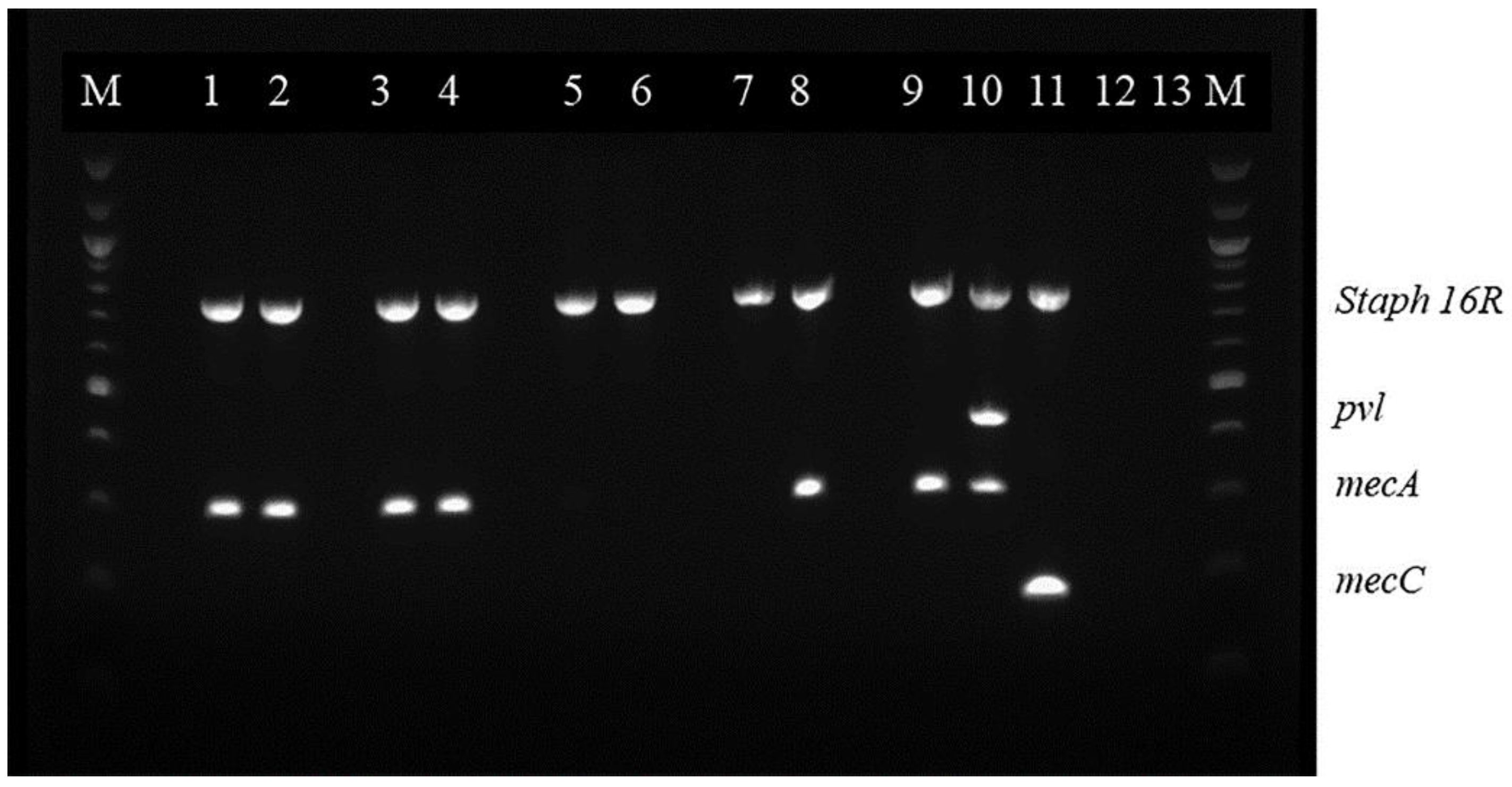
2.2. Multiplex Polymerase Chain Reaction (mPCR)
2.3. Disk Diffusion Susceptibility Tests (Kirby-Bauer Test)
2.4. Ethics Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morris, D.O.; Rook, K.A.; Shofer, F.S. Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcus schleiferi isolates obtained from small companion animals for antimicrobial resistance: A retrospective review of isolates (2003–04). Vet. Dermatol. 2006, 17, 332–337. [Google Scholar] [CrossRef]
- van Duijkeren, E.; Catry, B.; Greko, C.; Moreno, M.A.; Pomba, M.C.; Pyörälä, S.; Ruzauskas, M.; Sanders, P.; Threlfall, E.J.; Torren-Edo, J.; et al. Review on methicillin-resistant Staphylococcus pseudintermedius. J. Antimicrob. Chemother. 2011, 66, 2705–2714. [Google Scholar] [CrossRef] [Green Version]
- Han, J.-I.; Rhim, H.; Yang, C.-H.; Park, H.-M. Molecular characteristics of new clonal complexes of Staphylococcus pseudintermedius from clinically normal dogs. Vet. Q. 2018, 38, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhaiyan, M.; Wirth, J.S.; Saravanan, V.S. Phylogenomic analyses of the Staphylococcaceae family suggest the reclassification of five species within the genus Staphylococcus as heterotypic synonyms, the promotion of five subspecies to novel species, the taxonomic reassignment of five Staphylococcus species to Mammaliicoccus gen. nov., and the formal assignment of Nosocomiicoccus to the family Staphylococcaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 5926–5936. [Google Scholar] [CrossRef] [PubMed]
- Rana, E.A.; Islam, M.Z.; Das, T.; Dutta, A.; Ahad, A.; Biswas, P.K.; Barua, H. Prevalence of coagulase-positive methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in dogs in Bangladesh. Vet. Med. Sci. 2022, 8, 498–508. [Google Scholar] [CrossRef]
- Paterson, G.K. Genomic epidemiology of the opportunistic pathogen Staphylococcus coagulans from companion dogs. J. Med. Microbiol. 2021, 70, 001407. [Google Scholar] [CrossRef] [PubMed]
- Bemis, D.A.; Jones, R.D.; Hiatt, L.E.; Ofori, E.D.; Rohrbach, B.W.; Frank, L.A.; Kania, S.A. Comparison of tests to detect oxacillin resistance in Staphylococcus intermedius, Staphylococcus schleiferi, and Staphylococcus aureus isolates from canine hosts. J. Clin. Microbiol. 2006, 44, 3374–3376. [Google Scholar] [CrossRef] [Green Version]
- Pietrocola, G.; Gianotti, V.; Richards, A.; Nobile, G.; Geoghegan, J.A.; Rindi, S.; Monk, I.R.; Bordt, A.S.; Foster, T.J.; Fitzgerald, R.; et al. Fibronectin Binding Proteins SpsD and SpsL Both Support Invasion of Canine Epithelial Cells by Staphylococcus pseudintermedius. Infect. Immun. 2015, 83, 4093–4102. [Google Scholar] [CrossRef] [Green Version]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Velasco, V.; Sherwood, J.S.; Rojas-García, P.P.; Logue, C.M. Multiplex Real-Time PCR for Detection of Staphylococcus aureus, mecA and Panton-Valentine Leukocidin (PVL) Genes from Selective Enrichments from Animals and Retail Meat. PLoS ONE 2014, 9, e97617. [Google Scholar] [CrossRef]
- Zhang, K.; McClure, J.-A.; Elsayed, S.; Louie, T.; Conly, J.M. Novel Multiplex PCR Assay for Characterization and Concomitant Subtyping of Staphylococcal Cassette Chromosome mec Types I to V in Methicillin-Resistant Staphylococcus aureus. J. Clin. Microbiol. 2005, 43, 5026–5033. [Google Scholar] [CrossRef] [Green Version]
- Petersen, A.; Stegger, M.; Heltberg, O.; Christensen, J.; Zeuthen, A.; Knudsen, L.; Urth, T.; Sorum, M.; Schouls, L.; Larsen, J.; et al. Epidemiology of methicillin-resistant Staphylococcus aureus carrying the novel mecC gene in Denmark corroborates a zoonotic reservoir with transmission to humans. Clin. Microbiol. Infect. 2013, 19, E16–E22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardiau, M.; Yamazaki, K.; Ote, I.; Misawa, N.; Mainil, J.G. Characterization of methicillin- resistant Staphylococcus pseudintermedius isolated from dogs and cats. Microbiol. Immunol. 2013, 57, 496–501. [Google Scholar]
- Loncaric, I.; Kübber-Heiss, A.; Posautz, A.; Ruppitsch, W.; Lepuschitz, S.; Schauer, B.; Feßler, A.T.; Krametter-Frötscher, R.; Harrison, E.M.; Holmes, M.A.; et al. Characterization of mecC gene-carrying coagulase-negative Staphylococcus spp. isolated from various animals. Vet. Microbiol. 2019, 230, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Paterson, G.K.; Harrison, E.M.; Holmes, M.A. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014, 22, 42–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, G.K.; Morgan, F.J.E.; Harrison, E.; Cartwright, E.J.P.; Torok, E.; Zadoks, R.N.; Parkhill, J.; Peacock, S.J.; Holmes, M. Prevalence and characterization of human mecC methicillin-resistant Staphylococcus aureus isolates in England. J. Antimicrob. Chemother. 2014, 69, 907–910. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.F.J. Detection of methicillin/oxacillin resistance in staphylococci. J. Antimicrob. Chemother. 2001, 48, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, A.; Jana, D.; Dutta, K.; Dua, P.; Ghosh, C. Prevalence of Panton-Valentine Leukocidin Gene among Community Acquired Staphylococcus aureus: A Real-Time PCR Study. J. Pathog. 2018, 2018, 4518541. [Google Scholar] [CrossRef] [Green Version]
- Johnsson, D.; Mölling, P.; Strålin, K.; Söderquist, B. Detection of Panton–Valentine leukocidin gene in Staphylococcus aureus by LightCycler PCR: Clinical and epidemiological aspects. Clin. Microbiol. Infect. 2004, 10, 884–889. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.T.; Burnham, C.-A.D.; Westblade, L.F.; Bard, J.D.; Lawhon, S.D.; Wallace, M.A.; Stanley, T.; Burd, E.; Hindler, J.; Humphries, R.M. Evaluation of Oxacillin and Cefoxitin Disk and MIC Breakpoints for Prediction of Methicillin Resistance in Human and Veterinary Isolates of Staphylococcus intermedius Group. J. Clin. Microbiol. 2016, 54, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.; Larsen, A.R.; Skov, R.L.; Paterson, G.K.; Holmes, M.A.; Sabat, A.J.; Friedrich, A.W.; Köck, R.; Peters, G.; Kriegeskorte, A. Evaluation of a Modular Multiplex-PCR Methicillin-Resistant Staphylococcus aureus Detection Assay Adapted for mecC Detection. J. Clin. Microbiol. 2013, 51, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Surveillance for Methicillin-Resistant Staphylococcus aureus: Principles, Practices, and Challenges, A Report.; CLSI Docu ment X07-R; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolates form Animals, 3rd ed.; CLSI Supplement VET01S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; 25th Informational Supplement. M100-S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Sasaki, T.; Kikuchi, K.; Tanaka, Y.; Takahashi, N.; Kamata, S.; Hiramatsu, K. Methicillin-Resistant Staphylococcus pseudintermedius in a Veterinary Teaching Hospital. J. Clin. Microbiol. 2007, 45, 1118–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GENEWIZ, USA. Available online: https://www.genewiz.com/en/Public/Services/Next-Generation-Sequencing/Standalone-NGS-Solutions (accessed on 2 February 2022).
- QIAGEN Multiplex PCR Kit Handbook. Available online: https://www.qiagen.com/us/resources/download.aspx?id=a541a49c-cd06-40ca-b1d2-563d0324ad6c&lang=en (accessed on 2 February 2022).
- Kawakami, T.; Shibata, S.; Murayama, N.; Nagata, M.; Nishifuji, K.; Iwasaki, T.; Fukata, T. Antimicrobial Susceptibility and Methicillin Resistance in Staphylococcus pseudintermedius and Staphylococcus schleiferi subsp. coagulans Isolated from Dogs with Pyoderma in Japan. J. Vet. Med. Sci. 2010, 72, 1615–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, Z.; Stoakes, L.; Garrow, S.; Longo, S.; Fitzgerald, V.; Lannigan, R. Rapid detection of mecA-positive and mecA-negative Staphylococci by an anti-penicillin binding protein 2a slide latex agglutination test. J. Clin. Microbiol. 2000, 38, 2051–2054. [Google Scholar] [CrossRef]
- Los, F.C.O.; Randis, T.; Aroian, R.V.; Ratner, A. Role of Pore-Forming Toxins in Bacterial Infectious Diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef] [Green Version]
- Savini, V.; Di Giuseppe, N.; Fazii, P.; D’Amario, C.; D’Antonio, D.; Carretto, E. Staphylococcus pseudintermedius heterogeneously expresses the mecA gene. Vet. Microbiol. 2013, 165, 489–490. [Google Scholar] [CrossRef]
- Seidel, C.; Peters, S.; Eschbach, E.; Feßler, A.T.; Oberheitmann, B.; Schwarz, S. Development of a nucleic acid lateral flow immunoassay (NALFIA) for reliable, simple and rapid detection of the methicillin resistance genes mecA and mecC. Vet. Microbiol. 2017, 200, 101–106. [Google Scholar] [CrossRef]
- González-Domínguez, M.S.; Carvajal, H.D.; Calle-Echeverri, D.A.; Chinchilla-Cárdenas, D. Molecular Detection and Characterization of the mecA and nuc Genes From Staphylococcus Species (S. aureus, S. pseudintermedius, and S. schleiferi) Isolated From Dogs Suffering Superficial Pyoderma and Their Antimicrobial Resistance Profiles. Front. Vet. Sci. 2020, 7, 376. [Google Scholar] [CrossRef]

| Primer | 5′–3″ | Target Gene | Amplicon Length (bp) | Reference |
|---|---|---|---|---|
| mecA-for | TGAAAAATGATTATGGCTCAGGTACT | mecA | 173 | [16] |
| mecA-rev | CTGGAACTTGTTGAGCAGWGGTTCT | mecA | ||
| mecC-for | ATCTCGCCTTGGCCATATCCTGAA | mecC | 171 | [16] |
| mecC-rev | TGCCCGCATTGCATTAGCATTAGG | mecC | ||
| pvl-for | ACA CAC TAT GGC AAT AGT TAT TT | Pvl | 868 | [26] |
| pvl-rev | AAA GCA ATG CAA TTG ATG TA | Pvl | ||
| Staph756F | AACTCTGTTATTAGGGAAGAACA | 16S | 756 | [22] |
| Staph 750R | CCACCTTCCTCCGGTTTGTCACC | 16S |
| MecA Positive Isolates | Staphylococcus pseudintermedius | Staphylococcus coagulans | Staphylococcus aureus |
|---|---|---|---|
| Oxacillin resistant/cefoxitin sensitive | 54 | 26 | 0 |
| Oxacillin sensitive/cefoxitin sensitive | 31 | 3 | 2 |
| Oxacillin resistant/cefoxitin resistant | 30 | 1 | 1 |
| Oxacillin sensitive/cefoxitin resistant | 1 | 0 | 0 |
| Total | 116 | 30 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platenik, M.O.; Archer, L.; Kher, L.; Santoro, D. Prevalence of mecA, mecC and Panton-Valentine-Leukocidin Genes in Clinical Isolates of Coagulase Positive Staphylococci from Dermatological Canine Patients. Microorganisms 2022, 10, 2239. https://doi.org/10.3390/microorganisms10112239
Platenik MO, Archer L, Kher L, Santoro D. Prevalence of mecA, mecC and Panton-Valentine-Leukocidin Genes in Clinical Isolates of Coagulase Positive Staphylococci from Dermatological Canine Patients. Microorganisms. 2022; 10(11):2239. https://doi.org/10.3390/microorganisms10112239
Chicago/Turabian StylePlatenik, Marcela O., Linda Archer, Lopamudra Kher, and Domenico Santoro. 2022. "Prevalence of mecA, mecC and Panton-Valentine-Leukocidin Genes in Clinical Isolates of Coagulase Positive Staphylococci from Dermatological Canine Patients" Microorganisms 10, no. 11: 2239. https://doi.org/10.3390/microorganisms10112239






