Effects of Below-Ground Microbial Biostimulant Trichoderma harzianum on Diseases, Insect Community, and Plant Performance in Cucurbita pepo L. under Open Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crop Cultivation
2.2. Meteorological Data
2.3. Experimental Design
2.4. Fungal Inoculation
2.5. Arthropod Sampling
2.5.1. Arthropod Sampling on Zucchini Leaves
2.5.2. Arthropod Sampling with Coloured Pan Traps
2.6. Evaluation of Diseases in Zucchini Plants
2.6.1. Evaluation of Zucchini Viruses in the Field
2.6.2. ELISA Assay
2.6.3. Powdery Mildew Evaluation Assay
2.7. Evaluation of Plant Growth and Productivity
2.8. Statistical Analysis
3. Results
3.1. Trichoderma harzianum T22 Inoculation
3.2. Arthropods Sampling
3.2.1. Arthropod Sampling on Zucchini Leaves
3.2.2. Arthropod Sampling with Coloured Pan Traps
3.3. Plant Diseases
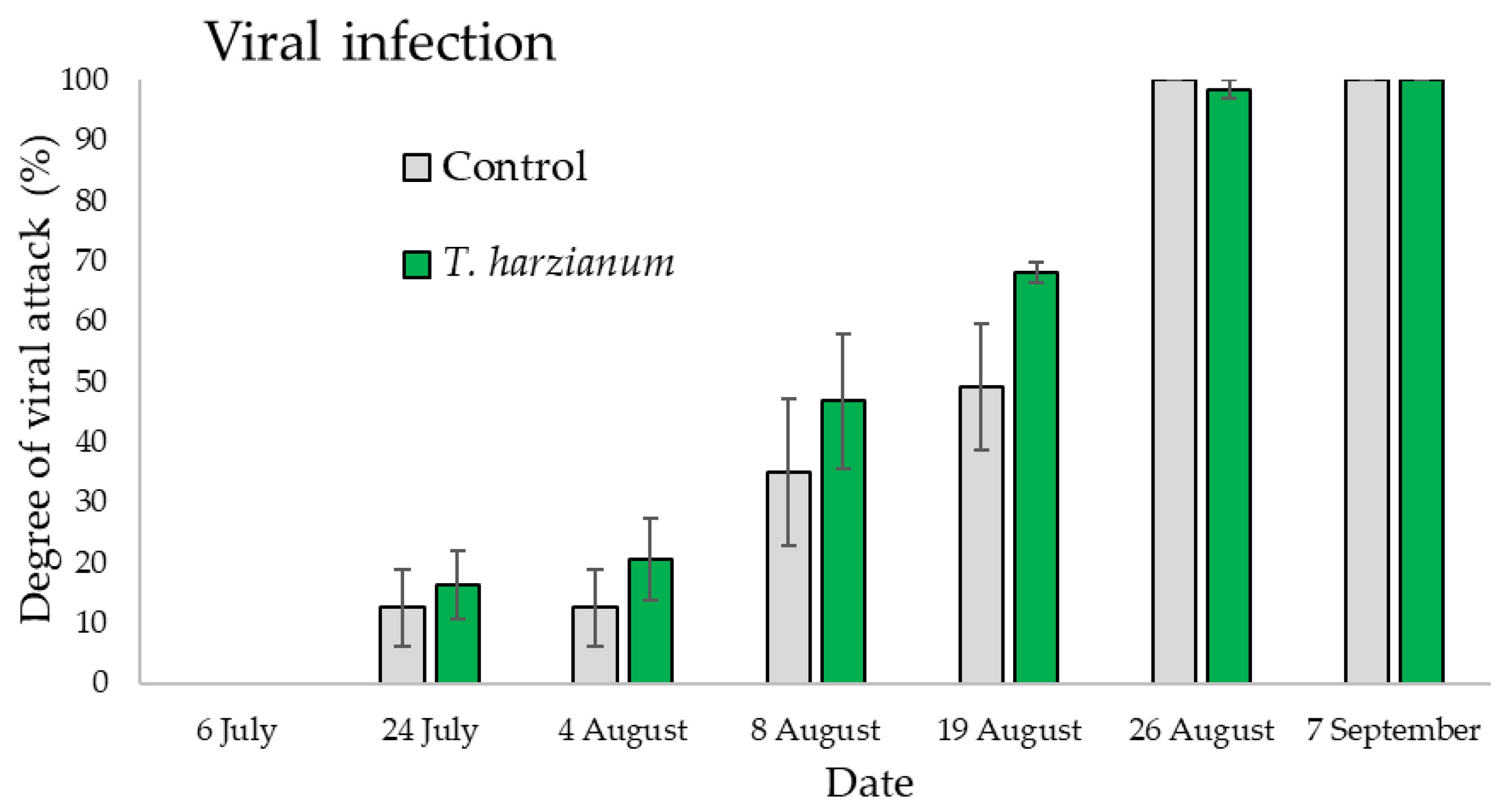
3.3.1. Field Evaluation of Zucchini Viral Diseases
3.3.2. ELISA Test for Viruses in Zucchini Plants
3.3.3. Powdery Mildew
3.4. Crop Sampling
3.4.1. Plant Length
3.4.2. Plant Productivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a Cultivated Planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and Function of the Soil Microbial Community in a Long-Term Fertilizer Experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Khanna, R.; Gupta, S. Agrochemicals as a Potential Cause of Ground Water Pollution: A Review. Int. J. Chem. Stud. 2018, 6, 985–990. [Google Scholar]
- Bass, C.; Jones, C.M. Editorial Overview: Pests and Resistance: Resistance to Pesticides in Arthropod Crop Pests and Disease Vectors: Mechanisms, Models and Tools. Curr. Opin. Insect Sci. 2018, 27, 4–7. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The Evolutionary Origins of Pesticide Resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef] [Green Version]
- Carletto, J.; Martin, T.; Vanlerberghe-Masutti, F.; Brévault, T. Insecticide Resistance Traits Differ among and within Host Races in Aphis gossypii. Pest Manag. Sci. 2010, 66, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Pozo de la Hoz, J.; Rivero, J.; Azcón-Aguilar, C.; Urrestarazu, M.; Pozo, M.J. Mycorrhiza-Induced Resistance against Foliar Pathogens Is Uncoupled of Nutritional Effects under Different Light Intensities. J. Fungi 2021, 7, 402. [Google Scholar] [CrossRef]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef]
- Batista, B.D.; Singh, B.K. Realities and Hopes in the Application of Microbial Tools in Agriculture. Microb. Biotechnol. 2021, 14, 1258–1268. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Contreras-Cornejo, H.A.; Adame-Garnica, S.G.; Del-Val, E.; Larsen, J. The Interactions of Trichoderma at Multiple Trophic Levels: Inter-Kingdom Communication. Microbiol. Res. 2020, 240, 126552. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, D.; Bossi, S.; Cascone, P.; Digilio, M.C.; Prieto, J.D.; Fanti, P.; Guerrieri, E.; Iodice, L.; Lingua, G.; Lorito, M.; et al. Tomato below Ground-above Ground Interactions: Trichoderma longibrachiatum Affects the Performance of Macrosiphum euphorbiae and Its Natural Antagonists. Mol. Plant. Microbe. Interact. 2013, 26, 1249–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppola, M.; Cascone, P.; Di Lelio, I.; Woo, S.L.; Lorito, M.; Rao, R.; Pennacchio, F.; Guerrieri, E.; Digilio, M.C. Trichoderma atroviride P1 Colonization of Tomato Plants Enhances Both Direct and Indirect Defense Barriers against Insects. Front. Physiol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alınç, T.; Cusumano, A.; Peri, E.; Torta, L.; Colazza, S. Trichoderma harzianum Strain T22 Modulates Direct Defense of Tomato Plants in Response to Nezara viridula Feeding Activity. J. Chem. Ecol. 2021, 47, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Caccavo, V.; Forlano, P.; Mang, S.M.; Fanti, P.; Nuzzaci, M.; Battaglia, D.; Trotta, V. Effects of Trichoderma harzianum Strain T22 on the Arthropod Community Associated with Tomato Plants and on the Crop Performance in an Experimental Field. Insects 2022, 13, 418. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernández, I.; Sánchez-Guzmán, M.J.; Jung, S.C.; Pascual, J.A.; Pozo, M.J. Deciphering the Hormonal Signaling Network behind the Systemic Resistance Induced by Trichoderma harzianum in Tomato. Front. Plant Sci. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jogaiah, S.; Abdelrahman, M.; Tran, L.S.P.; Ito, S.I. Different Mechanisms of Trichoderma virens-Mediated Resistance in Tomato against Fusarium wilt Involve the Jasmonic and Salicylic Acid Pathways. Mol. Plant Pathol. 2018, 19, 870–882. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Téllez, V.I.; Cruz-Olmedo, A.K.; Plasencia, J.; Gavilanes-Ruíz, M.; Arce-Cervantes, O.; Hernández-León, S.; Saucedo-García, M. The Protective Effect of Trichoderma asperellum on Tomato Plants against Fusarium oxysporum and Botrytis cinerea Diseases Involves Inhibition of Reactive Oxygen Species Production. Int. J. Mol. Sci. 2019, 20, 2007. [Google Scholar] [CrossRef] [Green Version]
- Heflish, A.A.; Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Protective and Curative Effects of Trichoderma asperelloides Ta41 on Tomato Root Rot Caused by Rhizoctonia solani Rs33. Agronomy 2021, 11, 1162. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species–Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studholme, D.J.; Harris, B.; Le Cocq, K.; Winsbury, R.; Perera, V.; Ryder, L.; Ward, J.L.; Beale, M.H.; Thornton, C.R.; Grant, M. Investigating the Beneficial Traits of Trichoderma hamatum GD12 for Sustainable Agriculture-Insights from Genomics. Front. Plant Sci. 2013, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological Functions of Trichoderma Spp. and Their Secondary Metabolites in the Rhizosphere: Interactions with Plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andolfo, G.; Di Donato, A.; Darrudi, R.; Errico, A.; Cigliano, R.A.; Ercolano, M.R. Draft of Zucchini (Cucurbita pepo L.) Proteome: A Resource for Genetic and Genomic Studies. Front. Genet. 2017, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Paris, H.S. A Proposed Subspecific Classification for Cucurbita Pepo. Phytologia 1986, 61, 133–138. [Google Scholar]
- Gilardi, G.; Pugliese, M.; Gullino, M.L.; Garibaldi, A. Effect of Biocontrol Agents and Potassium Phosphite against Phytophthora crown Rot, Caused by Phytophthora capsici, on Zucchini in a Closed Soilless System. Sci. Hortic. 2020, 265, 109207. [Google Scholar] [CrossRef]
- Formisano, L.; Miras-Moreno, B.; Ciriello, M.; El-Nakhel, C.; Corrado, G.; Lucini, L.; Colla, G.; Rouphael, Y. Trichoderma and Phosphite Elicited Distinctive Secondary Metabolite Signatures in Zucchini Squash Plants. Agronomy 2021, 11, 1205. [Google Scholar] [CrossRef]
- Shen, J.; Li, X.; Wang, J.; Zhang, C.; Li, L.; Zhang, L. Dynamic Distribution of Aphis gossypii Glover (Homoptera: Aphididae) and Incidence of Viral Disease in Different Zucchini (Cucurbita Pepo L.) Cultivars. Biochem. Syst. Ecol. 2018, 77, 31–36. [Google Scholar] [CrossRef]
- Hinds, J.; Hooks, C.R.R. Population Dynamics of Arthropods in a Sunn-Hemp Zucchini Interplanting System. Crop Prot. 2013, 53, 6–12. [Google Scholar] [CrossRef]
- Koné, K.; Tuo, Y.; Yapo, M.L.; Soro, F.; Traoré, D.; Koua, K.H. Main Insect Pests of Zucchini (Cucurbita pepo L), in the Dry Season and Impact on Production in Northern Côte d ’ Ivoire. J. Entomol. Zool. Stud. 2019, 7, 523–527. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Herbaceous Plants and Shrubs. Volume 1: Host Lists and Keys. Volume 2: The Aphids; John Wiley & Sons, Ltd.: Chichester, UK, 2006; ISBN 9780471489733. [Google Scholar]
- Ng, J.C.K.; Perry, K.L. Transmission of Plant Viruses by Aphid Vectors. Mol. Plant Pathol. 2004, 5, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Manco, E.; Vitiello, A.; Di Lelio, I.; Giorgini, M.; Rao, R.; Pennacchio, F.; Digilio, M.C. Plant Response to Feeding Aphids Promotes Aphid Dispersal. Entomol. Exp. Appl. 2018, 166, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Ebert, T.A.; Cartwright, B.O. Biology and Ecology of Aphis gossypii Glover (Homoptera: Aphididae). Southwest. Entomol. 1997, 22, 116–153. [Google Scholar]
- Al-Shahwan, I.M.; Abdalla, O.A.; Al-Saleh, M.A. Response of Greenhouse-Grown Cucumber Cultivars to an Isolate of Zucchini Yellow Mosaic Virus (ZYMV). Plant Dis. 1995, 79, 898–901. [Google Scholar] [CrossRef]
- Nováková, S.; Flores-Ramírez, G.; Glasa, M.; Danchenko, M.; Fiala, R.; Skultety, L. Partially Resistant Cucurbita pepo Showed Late Onset of the Zucchini Yellow Mosaic Virus Infection Due to Rapid Activation of Defense Mechanisms as Compared to Susceptible Cultivar. Front. Plant Sci. 2015, 6, 263. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Xu, B.; Zhou, J. Differential Responses of Cucurbita pepo to Podosphaera xanthii Reveal the Mechanism of Powdery Mildew Disease Resistance in Pumpkin. Front. Plant Sci. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Cao, C.W.; Zhang, J.; Gao, X.W.; Liang, P.; Guo, H.L. Overexpression of Carboxylesterase Gene Associated with Organophosphorous Insecticide Resistance in Cotton Aphids, Aphis gossypii (Glover). Pestic. Biochem. Physiol. 2008, 90, 175–180. [Google Scholar] [CrossRef]
- Herron, G.A.; Wilson, L.J. Can Resistance Management Strategies Recover Insecticide Susceptibility in Pests?: A Case Study with Cotton Aphid Aphis gossypii (Aphididae: Hemiptera) in Australian Cotton. Austral Entomol. 2017, 56, 1–13. [Google Scholar] [CrossRef]
- Desbiez, C.; Lecoq, H. Zucchini Yellow Mosaic Virus. Plant Pathol. 1997, 46, 809–829. [Google Scholar] [CrossRef]
- Yuan, C.; Ullman, D.E. Comparison of Efficiency and Propensity as Measures of Vector Importance in Zucchini Yellow Mosaic Potyvirus Transmission by Aphis gossypii and A. craccivora. Phytopathology 1996, 86, 698–703. [Google Scholar] [CrossRef]
- Herron, G.; Powis, K.; Rophail, J. Baseline Studies and Preliminary Resistance Survey of Australian Populations of Cotton Aphid Aphis gossypii Glover (Hemiptera: Aphididae). Aust. J. Entomol. 2000, 39, 33–38. [Google Scholar] [CrossRef]
- Herron, G.A.; Powis, K.; Rophail, J. Insecticide Resistance in Aphis gossypii Glover (Hemiptera: Aphididae), a Serious Threat to Australian Cotton. Aust. J. Entomol. 2001, 40, 85–91. [Google Scholar] [CrossRef]
- Ahmad, M.; Iqbal Arif, M. Susceptibility of Pakistani Populations of Cotton Aphid Aphis gossypii (Homoptera: Aphididae) to Endosulfan, Organophosphorus and Carbamate Insecticides. Crop Prot. 2008, 27, 523–531. [Google Scholar] [CrossRef]
- Pocurull, M.; Fullana, A.M.; Ferro, M.; Valero, P.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Commercial Formulates of Trichoderma Induce Systemic Plant Resistance to Meloidogyne incognita in Tomato and the Effect Is Additive to That of the Mi-1.2 Resistance Gene. Front. Microbiol. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell Science: Oxford, UK, 2000; Volume 278. [Google Scholar]
- Leong, J.M.; Thorp, R.W. Colour-Coded Sampling: The Pan Trap Colour Preferences of Oligolectic and Nonoligolectic Bees Associated with a Vernal Pool Plant. Ecol. Entomol. 1999, 24, 329–335. [Google Scholar] [CrossRef]
- Clark, M.F.; Adams, A.N. Characteristics of the Microplate Method of Enzyme-Linked Immunosorbent Assay for the Detection of Plant Viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Miazzi, M.; Laguardia, C.; Faretra, F. Variation in Podosphaera xanthii on Cucurbits in Southern Italy. J. Phytopathol. 2011, 159, 538–545. [Google Scholar] [CrossRef]
- Braun, U.; Cook, R.T.A. Taxonomic Manual of the Erysiphales (Powdery Mildews); CBS Biodiv.; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2012; ISBN 978-90-70351-89-2. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: Https://Www.R-Project.Org/ (accessed on 15 December 2021).
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Shoresh, M.; Harman, G.E. The Molecular Basis of Shoot Responses of Maize Seedlings to Trichoderma harzianum T22 Inoculation of the Root: A Proteomic Approach. Plant Physiol. 2008, 147, 2147–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The Beneficial Effect of Trichoderma Spp. on Tomato Is Modulated by the Plant Genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef]
- Ponzio, C.; Gols, R.; Pieterse, C.M.J.; Dicke, M. Ecological and Phytohormonal Aspects of Plant Volatile Emission in Response to Single and Dual Infestations with Herbivores and Phytopathogens. Funct. Ecol. 2013, 27, 587–598. [Google Scholar] [CrossRef]
- Walling, L.L. The Myriad Plant Responses to Herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Salas-Marina, M.A.; Silva-Flores, M.A.; Uresti-Rivera, E.E.; Castro-Longoria, E.; Herrera-Estrella, A.; Casas-Flores, S. Colonization of Arabidopsis Roots by Trichoderma atroviride Promotes Growth and Enhances Systemic Disease Resistance through Jasmonic Acid/Ethylene and Salicylic Acid Pathways. Eur. J. Plant Pathol. 2011, 131, 15–26. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-Induced Plant Immunity Likely Involves Both Hormonal- and Camalexin-Dependent Mechanisms in Arabidopsis thaliana and Confers Resistance against Necrotrophic Fungus Botrytis cinerea. Plant Signal. Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef] [Green Version]
- Bari, R.; Jones, J.D.G. Role of Plant Hormones in Plant Defence Responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dagostin, S.; Ferrari, A.; Elad, Y.; Pertot, I. Induction of Systemic Resistance against Plasmopara viticola in Grapevine by Trichoderma harzianum T39 and Benzothiadiazole. Biol. Control 2008, 47, 228–234. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as Biocontrol Agent against Pests: New Uses for a Mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Coppola, M.; Diretto, G.; Digilio, M.C.; Woo, S.L.; Giuliano, G.; Molisso, D.; Pennacchio, F.; Lorito, M.; Rao, R. Transcriptome and Metabolome Reprogramming in Tomato Plants by Trichoderma harzianum Strain T22 Primes and Enhances Defense Responses against Aphids. Front. Physiol. 2019, 10, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Cornejo, H.A.; Viveros-Bremauntz, F.; Del-Val, E.; Macías-Rodríguez, L.; López-Carmona, D.A.; Alarcón, A.; González-Esquivel, C.E.; Larsen, J. Alterations of Foliar Arthropod Communities in a Maize Agroecosystem Induced by the Root-Associated Fungus Trichoderma harzianum. J. Pest Sci. 2020, 94, 363–374. [Google Scholar] [CrossRef]
- Afechtal, M.; Aarabe, A.; Chebli, B.; Mounir, M. The Occurrence of Major Viruses Infecting Zucchini Squash (Cucurbita pepo L.) in Morocco. Eur. Sci. J. 2019, 15, 188–196. [Google Scholar] [CrossRef]
- El-Sharkawy, E.E.S.; Abdelrazik, E. Biocontrol of Fusarium Root Rot in Squash Using Mycorrhizal Fungi and Antagonistic Microorganisms. Egypt. J. Biol. Pest Control 2022, 32, 13. [Google Scholar] [CrossRef]
- Li, M.; Ma, G.; Lian, H.; Su, X.; Tian, Y.; Huang, W.; Mei, J.; Jiang, X. The Effects of Trichoderma on Preventing Cucumber Fusarium wilt and Regulating Cucumber Physiology. J. Integr. Agric. 2019, 18, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.B.; Adriaanse, I.C.T.; Belshaw, R.; Godfray, H.C.J. The Structure of an Aphid-Parasitoid Community. J. Anim. Ecol. 1999, 68, 346–370. [Google Scholar] [CrossRef]
- Weisser, W.W. Metapopulation Dynamics in an Aphid-Parasitoid System. Entomol. Exp. Appl. 2000, 97, 83–92. [Google Scholar] [CrossRef]
- Karley, A.J.; Parker, W.E.; Pitchford, J.W.; Douglas, A.E. The Mid-Season Crash in Aphid Populations: Why and How Does It Occur? Ecol. Entomol. 2004, 29, 383–388. [Google Scholar] [CrossRef]
- Reimer-Michalski, E.-M.; Conrath, U. Innate Immune Memory in Plants. Semin. Immunol. 2016, 28, 319–327. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Chiusano, M.L.; Colantuono, C.; Lorito, M.; Pennacchio, F.; Rao, R.; Woo, S.L.; Guerrieri, E.; Digilio, M.C. Trichoderma harzianum Enhances Tomato Indirect Defense against Aphids. Insect Sci. 2017, 24, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Boege, K.; Dirzo, R.; Siemens, D.; Brown, P. Ontogenetic Switches from Plant Resistance to Tolerance: Minimizing Costs with Age? Ecol. Lett. 2007, 10, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Boege, K.; Marquis, R.J. Facing Herbivory as You Grow up: The Ontogeny of Resistance in Plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef]
- Trotta, V.; Toma, I.; Forlano, P.; Fanti, P.; Prieto, J.D.; Battaglia, D. The Age of Tomato Plants Affects the Development of Macrosiphum euphorbiae (Thomas, 1878) (Hemiptera) Colonies. Agron. Colomb. 2021, 39, 3–7. [Google Scholar] [CrossRef]
- Vitti, A.; Pellegrini, E.; Nali, C.; Lovelli, S.; Sofo, A.; Valerio, M.; Scopa, A.; Nuzzaci, M. Trichoderma harzianum T-22 Induces Systemic Resistance in Tomato Infected by Cucumber Mosaic Virus. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hafez, Y.M.; El-Nagar, A.S.; Elzaawely, A.A.; Kamel, S.; Maswada, H.F. Biological Control of Podosphaera xanthii the Causal Agent of Squash Powdery Mildew Disease by Upregulation of Defense-Related Enzymes. Egypt. J. Biol. Pest Control 2018, 28. [Google Scholar] [CrossRef] [Green Version]
- Elsisi, A.A. Evaluation of Biological Control Agents for Managing Squash Powdery Mildew under Greenhouse Conditions. Egypt. J. Biol. Pest Control 2019, 29, 89. [Google Scholar] [CrossRef] [Green Version]
- El-Sharkawy, H.H.A.; Abbas, M.S.; Soliman, A.S.; Ibrahim, S.A.; El-Nady, I.A.I. Synergistic Effect of Growth-Promoting Microorganisms on Bio-Control of Fusarium oxysporum f. Sp. Pisi, Growth, Yield, Physiological and Anatomical Characteristics of Pea Plants. Pestic. Biochem. Physiol. 2021, 178, 104939. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forlano, P.; Mang, S.M.; Caccavo, V.; Fanti, P.; Camele, I.; Battaglia, D.; Trotta, V. Effects of Below-Ground Microbial Biostimulant Trichoderma harzianum on Diseases, Insect Community, and Plant Performance in Cucurbita pepo L. under Open Field Conditions. Microorganisms 2022, 10, 2242. https://doi.org/10.3390/microorganisms10112242
Forlano P, Mang SM, Caccavo V, Fanti P, Camele I, Battaglia D, Trotta V. Effects of Below-Ground Microbial Biostimulant Trichoderma harzianum on Diseases, Insect Community, and Plant Performance in Cucurbita pepo L. under Open Field Conditions. Microorganisms. 2022; 10(11):2242. https://doi.org/10.3390/microorganisms10112242
Chicago/Turabian StyleForlano, Pierluigi, Stefania Mirela Mang, Vittoria Caccavo, Paolo Fanti, Ippolito Camele, Donatella Battaglia, and Vincenzo Trotta. 2022. "Effects of Below-Ground Microbial Biostimulant Trichoderma harzianum on Diseases, Insect Community, and Plant Performance in Cucurbita pepo L. under Open Field Conditions" Microorganisms 10, no. 11: 2242. https://doi.org/10.3390/microorganisms10112242
APA StyleForlano, P., Mang, S. M., Caccavo, V., Fanti, P., Camele, I., Battaglia, D., & Trotta, V. (2022). Effects of Below-Ground Microbial Biostimulant Trichoderma harzianum on Diseases, Insect Community, and Plant Performance in Cucurbita pepo L. under Open Field Conditions. Microorganisms, 10(11), 2242. https://doi.org/10.3390/microorganisms10112242









