Manganese Pollution and Its Remediation: A Review of Biological Removal and Promising Combination Strategies
Abstract
1. Introduction
2. Mn in the Environment
2.1. Mn Characteristics and Essentiality
2.2. Source and Geochemical Cycle of Mn
2.3. Mn Toxicity
2.4. Transporters of Mn in Living Organisms
3. Abiotic Treatment of Mn
4. Biological Treatment of Mn
4.1. Biosorption
4.1.1. Mechanisms
4.1.2. Influencing Factors
4.1.3. Advantages and Disadvantages
4.2. Bioaccumulation
4.2.1. Mechanisms
4.2.2. Influencing Factors
4.3. Bio-Oxidation
4.3.1. Mechanisms
4.3.2. Influencing Factors
4.3.3. Advantages and Limitations
4.4. Microbially Induced-Carbonate Precipitation (MICP)
4.4.1. Mechanisms
4.4.2. Influencing Factors
4.4.3. Advantages and Limitations
5. Combination Methods to Treat Mn
5.1. MnOB and Microalgae
5.2. MnOB and BioMnOx
5.3. MnOB and Biochar
5.4. Microorganisms and Phytoremediation Plants
6. Conclusions and Future Perspectives
- In terms of biosorption research, more low-cost adsorbents derived from plants or agricultural by-products, functioning as economic substitutes for expensive traditional removal methods of heavy metal have yet to be produced.
- As far as biological oxidation, more microorganisms with strong ability to oxidize Mn remain to be explored and discovered and the activity of available microorganisms needs efforts to be improved, by optimization of environmental conditions or other methods.
- Although MICP has exhibited excellent feasibility in treating Mn pollution by converting Mn into Mn carbonates and then filtrating them, some important parameters of this method, such as influencing factors, characteristics of products, component analysis and application of products has not been clearly clarified. By referring to the more mature applications of MICP in other heavy metals removal, Mn remediation via MICP should transit gradually from laboratory level to few fields’ application in actual contaminated sites.
- Furthermore, more synergistic treatments of Mn involving various physical, chemical, biological mechanisms in a gesture to acquire higher efficiency and lower costs and overcome the intrinsic restrictions of single method could be explored extensively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Chakraborty, S.; Mukhopadhyay, S.; Lee, E.; Paoliello, M.M.B.; Bowman, A.B.; Aschner, M. Mn homeostasis in the nervous system. J. Neurochem. 2015, 134, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Mn is essential for neuronal health. In Annual Review of Nutrition, 2nd ed.; Bowman, B.A., Stover, P.J., Eds.; Annual Reviews: Palo Alto, CA, USA, 2015; Volume 35, pp. 71–108. [Google Scholar]
- Kwakye, G.F.; Paoliello, M.M.B.; Mukhopadhyay, S.; Bowman, A.B.; Aschner, M. Mn-induced parkinsonism and Parkinson’s disease: Shared and distinguishable features. Int. J. Environ. Res. Public Health 2015, 12, 7519–7540. [Google Scholar] [CrossRef] [PubMed]
- Bricker, T.M.; Roose, J.L.; Fagerlund, R.D.; Frankel, L.K.; Eaton-Rye, J.J. The extrinsic proteins of Photosystem II. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 121–142. [Google Scholar] [CrossRef]
- Requena, L.; Bornemann, S. Barley (Hordeum vulgare) oxalate oxidase is a Mn-containing enzyme. Biochem. J. 1999, 343, 185–190. [Google Scholar] [CrossRef]
- Lane, B.G. Oxalate, germins, and higher-plant pathogens. Iubmb Life 2002, 53, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Bosma, E.F.; Rau, M.H.; van Gijtenbeek, L.A.; Siedler, S. Regulation and distinct physiological roles of manganese in bacteria. FEMS Microbiol. Rev. 2021, 45. [Google Scholar] [CrossRef] [PubMed]
- Sihaib, Z.; Puleo, F.; Garcia-Vargas, J.M.; Retailleau, L.; Descorme, C.; Liotta, L.F.; Valverde, J.L.; Gil, S.; Giroir-Fendler, A. Mn oxide-based catalysts for toluene oxidation. Appl. Catal. B-Environ. 2017, 209, 689–700. [Google Scholar] [CrossRef]
- Peng, X.H.; Yu, H.B.; Wang, P.; Liu, Y.; Yang, L.; Dong, H.; Ren, Y.X.; Wang, H.W. Production assessment in the electrolytic Mn metal industry in China. Rev. Metall. 2011, 108, 437–442. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Lyu, C.; Yang, X.; Liu, W.; Su, X. A cost-effective catalytically adsorbent for in situ remediation of Mn contaminated groundwater. Water Sci. Technol.-Water Supply 2018, 18, 504–514. [Google Scholar] [CrossRef]
- Amorim, S.S.; Dias Ruas, F.A.; Barboza, N.R.; de Oliveira Neves, V.G.; Leao, V.A.; Guerra-Sa, R. Mn (Mn2+) tolerance and biosorption by Meyerozyma guilliermondii and Meyerozyma caribbica strains. J. Environ. Chem. Eng. 2018, 6, 4538–4545. [Google Scholar] [CrossRef]
- Miah, M.R.; Ijomone, O.M.; Okoh, C.O.A.; Ijomone, O.K.; Akingbade, G.T.; Ke, T.; Krum, B.; Martins, A.D.; Akinyemi, A.; Aranoff, N.; et al. The effects of Mn overexposure on brain health. Neurochem. Int. 2020, 135, 104688. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Wu, H.; Chen, M.; Wei, L.; Wang, B.; Li, B.; Liu, R.; Liu, Z. Simultaneous optimizing removal of Mn and ammonia nitrogen from electrolytic metal Mn residue leachate using chemical equilibrium model. Ecotoxicol. Environ. Saf. 2019, 172, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Shu, J.C.; Chen, M.J.; Wang, J.Y.; Wang, Y.; Luo, Z.G.; Wang, R.; Yang, F.H.; Xiu, F.R.; Sun, Z. Mn and ammonia nitrogen recovery from electrolytic Mn residue by electric field enhanced leaching. J. Clean. Prod. 2019, 236, 8. [Google Scholar] [CrossRef]
- Roccaro, P.; Barone, C.; Mancini, G.; Vagliasindi, F.G.A. Removal of Mn from water supplies intended for human consumption: A case study. Desalination 2007, 210, 205–214. [Google Scholar] [CrossRef]
- Therdkiattikul, N.; Ratpukdi, T.; Kidkhunthod, P.; Chanlek, N.; Siripattanakul-Ratpukdi, S. Mn-contaminated groundwater treatment by novel bacterial isolates: Kinetic study and mechanism analysis using synchrotron-based techniques. Sci. Rep. 2020, 10, 12. [Google Scholar] [CrossRef]
- Erikson, K.M.; Aschner, M. Mn: Its role in disease and health. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic, 2nd ed.; Carver, P.L., Ed.; Walter De Gruyter Gmbh: Berlin, Germany, 2019; Volume 19, pp. 253–266. [Google Scholar]
- Lv, M.Z.; Chen, M.X.; Zhang, R.; Zhang, W.; Wang, C.G.; Zhang, Y.; Wei, X.M.; Guan, Y.K.; Liu, J.J.; Feng, K.C.; et al. Mn is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef]
- Sun, T.; Cui, Y.L.; Berg, B.; Zhang, Q.Q.; Dong, L.L.; Wu, Z.J.; Zhang, L.L. A test of Mn effects on decomposition in forest and cropland sites. Soil Biol. Biochem. 2019, 129, 178–183. [Google Scholar] [CrossRef]
- Wong, D.W.S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef]
- Tang, X.K.; Zhao, J.R.; Li, K.Q.; Chen, Z.; Sun, Y.D.; Gao, J. Streptomyces cyaneochromogenes sp. nov., a blue pigment-producing actinomycete from Mn-contaminated soil. Int. J. Syst. Evol. Microbiol. 2019, 69, 2202–2207. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kowalczuk-Vasilev, E.; Kwiatkowska, K.; Kwiecien, M.; Baranowska-Wojcik, E.; Kiczorowska, B.; Klebaniuk, R.; Samolinska, W. Dietary intake and content of Cu, Mn, Fe, and Zn in selected cereal products marketed in Poland. Biol. Trace Elem. Res. 2019, 187, 568–578. [Google Scholar] [CrossRef]
- Bae, Y.J.; Choi, M.K. The estimated daily manganese intake of Koran children aged 11–12. Nutr. Res. Pract. 2011, 5, 548–552. [Google Scholar] [CrossRef]
- Filippini, T.; CilIoni, S.; Malavolti, M.; Violi, F.; Malagoli, C.; Tesauro, M.; Bottecchi, I.; Ferrari, A.; Vescovi, L.; Vinceti, M. Dietary intake of cadmium, chromium, copper, Mn, selenium and zinc in a Northern Italy community. J. Trace Elem. Med. Biol. 2018, 50, 508–517. [Google Scholar] [CrossRef]
- Lan, J.R.; Sun, Y.; Guo, L.; Du, Y.G.; Du, D.Y.; Zhang, T.C.; Li, J.; Ye, H.P. Highly efficient removal of As(V) with modified electrolytic Mn residues (M-EMRs) as a novel adsorbent. J. Alloys Compd. 2019, 811, 10. [Google Scholar] [CrossRef]
- Devi, P.; Kothari, P.; Dalai, A.K. Stabilization and solidification of arsenic and iron contaminated canola meal biochar using chemically modified phosphate binders. J. Hazard. Mater. 2020, 385, 11. [Google Scholar] [CrossRef]
- Lan, J.R.; Sun, Y.; Huang, P.; Du, Y.G.; Zhan, W.; Zhang, T.C.; Du, D.Y. Using electrolytic Mn residue to prepare novel nanocomposite catalysts for efficient degradation of Azo Dyes in Fenton-like processes. Chemosphere 2020, 252, 12. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yang, L.; Liu, N.; Jiang, W.J.; Jiang, X.; Li, J.J.; Yang, Z.Y.; Song, Z.P. Sulfur resource recovery based on electrolytic Mn residue calcination and Mn oxide ore desulfurization for the clean production of electrolytic Mn. Chin. J. Chem. Eng. 2020, 28, 864–870. [Google Scholar] [CrossRef]
- Tang, B.W.; Gao, S.; Wang, Y.G.; Liu, X.M.; Zhang, N. Pore structure analysis of electrolytic Mn residue based permeable brick by using industrial CT. Constr. Build. Mater. 2019, 208, 697–709. [Google Scholar] [CrossRef]
- Wang, Y.G.; Gao, S.; Liu, X.M.; Tang, B.; Mukiza, E.M.L.; Zhang, N. Preparation of non-sintered permeable bricks using electrolytic Mn residue: Environmental and NH3-N recovery benefits. J. Hazard. Mater. 2019, 378, 11. [Google Scholar] [CrossRef]
- Zheng, F.; Zhu, H.; Luo, T.; Wang, H.J.; Hou, H.B. Pure water leaching soluble Mn from electrolytic Mn residue: Leaching kinetics model analysis and characterization. J. Environ. Chem. Eng. 2020, 8, 7. [Google Scholar] [CrossRef]
- Wang, X.; Ren, B.Z.; Zhou, Y.Y.; Shi, X.Y. Study on the mechanism and kinetics of Mn release from waste Mn ore waste rock under rainfall leaching. Environ. Sci. Pollut. Res. 2022, 29, 5541–5551. [Google Scholar] [CrossRef]
- Shu, J.C.; Wu, H.P.; Liu, R.L.; Liu, Z.H.; Li, B.; Chen, M.J.; Tao, C.Y. Simultaneous stabilization/solidification of Mn2+ and NH4+-N from electrolytic Mn residue using MgO and different phosphate resource. Ecotoxicol. Environ. Saf. 2018, 148, 220–227. [Google Scholar] [CrossRef]
- Li, C.X.; Zhong, H.; Wang, S.; Xue, J.R. Leaching behavior and risk assessment of heavy metals in a landfill of electrolytic Mn residue in western Hunan, China. Hum. Ecol. Risk Assess. 2014, 20, 1249–1263. [Google Scholar] [CrossRef]
- Men, J.L.; Men, J.Y.; Zhang, Y.J.; Zhao, L.; Zhang, J.; Zhang, Z.H.; Zhang, D.; Shao, H.; Lu, Q.Y. Investigation on occupational hazards in 20 automobile manufacturing enterprises in Shandong Province. Zhonghua Laodong Weisheng Zhiyebing Zazhi 2021, 39, 198–202. [Google Scholar] [CrossRef]
- Moberly, A.H.; Czarnecki, L.A.; Pottackal, J.; Rubinstein, T.; Turkel, D.J.; Kass, M.D.; McGann, J.P. Intranasal exposure to Mn disrupts neurotransmitter release from glutamatergic synapses in the central nervous system in vivo. Neurotoxicology 2012, 33, 996–1004. [Google Scholar] [CrossRef]
- Sriram, K.; Jefferson, A.M.; Lin, G.X.; Afshari, A.; Zeidler-Erdely, P.C.; Meighan, T.G.; McKinney, W.; Jackson, M.; Cumpston, A.; Cumpston, J.L.; et al. Neurotoxicity following acute inhalation of aerosols generated during resistance spot weld-bonding of carbon steel. Inhal. Toxicol. 2014, 26, 720–732. [Google Scholar] [CrossRef][Green Version]
- Aschner, M.; Guilarte, T.R.; Schneider, J.S.; Zheng, W. Mn: Recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007, 221, 131–147. [Google Scholar] [CrossRef]
- Roth, J.A. Homeostatic and toxic mechanisms regulating Mn uptake, retention, and elimination. Biol. Res. 2006, 39, 45–57. [Google Scholar] [CrossRef]
- Pfeifer, G.D.; Roper, J.M.; Dorman, D.; Lynam, D.R. Health and environmental testing of Mn exhaust products from use of methylcyclopentadienyl Mn tricarbonyl in gasoline. Sci. Total Environ. 2004, 334, 397–408. [Google Scholar] [CrossRef]
- Zaw, M.; Chiswell, B. Iron and Mn dynamics in lake water. Water Res. 1999, 33, 1900–1910. [Google Scholar] [CrossRef]
- Peng, H.; Zheng, X.L.; Chen, L.; Wei, Y. Analysis of numerical simulations and influencing factors of seasonal Mn pollution in reservoirs. Environ. Sci. Pollut. Res. 2016, 23, 14362–14372. [Google Scholar] [CrossRef]
- Habib, E.; Ali, M.A.; Fattah, K.P. Modified sand for the removal of Mn and arsenic from groundwater. J. Environ. Eng. Sci. 2020, 15, 189–196. [Google Scholar] [CrossRef]
- Ngwenya, S.; Guyo, U.; Zinyama, N.P.; Chigondo, F.; Nyamunda, B.C.; Muchanyereyi, N. Response surface methodology for optimization of Cd (II) adsorption from wastewaters by fabricated tartaric acid-maize tassel magnetic hybrid sorbent. Biointerface Res. Appl. Chem. 2019, 9, 3996–4005. [Google Scholar] [CrossRef]
- Sidoryk-Wegrzynowicz, M. Impairment of glutamine/glutamate-gamma-aminobutyric acid cycle in Mn toxicity in the central nervous system. Folia Neuropathol. 2014, 52, 377–382. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Koller, W.C. The diagnosis of Mn-induced parkinsonism. Neurotoxicology 2006, 27, 340–346. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M. Mn metabolism in humans. Front. Biosci.-Landmark 2018, 23, 1655–1679. [Google Scholar] [CrossRef]
- Aschner, M.; Erikson, K.M.; Dorman, D.C. Mn dosimetry: Species differences and implications for neurotoxicity. Crit. Rev. Toxicol. 2005, 35, 1–32. [Google Scholar] [CrossRef]
- Nasir, A.; Cameron, S.F.; Niehaus, A.C.; Clemente, C.J.; von Hippel, F.A.; Wilson, R.S. Mn contamination affects the motor performance of wild northern quolls (Dasyurus hallucatus). Environ. Pollut. 2018, 241, 55–62. [Google Scholar] [CrossRef]
- Kula, E.; Martinek, P.; Chromcova, L.; Hedbavny, J. Development of Lymantria dispar affected by Mn in food. Environ. Sci. Pollut. Res. 2014, 21, 11987–11997. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.Y.; Xu, R.K. Distribution of Mn (II) chemical forms on Soybean roots and Mn (II) toxicity. Pedosphere 2019, 29, 656–664. [Google Scholar] [CrossRef]
- Alejandro, S.; Holler, S.; Meier, B.; Peiter, E. Mn in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 23. [Google Scholar] [CrossRef]
- Li, J.F.; Jia, Y.D.; Dong, R.S.; Huang, R.; Liu, P.D.; Li, X.Y.; Wang, Z.Y.; Liu, G.D.; Chen, Z.J. Advances in the mechanisms of plant tolerance to Mn toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef]
- Min, S.F.; Chang, Z.X.; Chen, L.; Feng, L.Z.; Tang, B.; Ping, Z.Z. Effects of Mn pollution on soil microbial community function and plant growth. J. Environ. Prot. Ecol. 2019, 20, 64–73. [Google Scholar]
- Winslow, J.W.W.; Limesand, K.H.; Zhao, N.N. The functions of ZIP8, ZIP14, and ZnT10 in the regulation of systemic Mn homeostasis. Int. J. Mol. Sci. 2020, 21, 3304. [Google Scholar] [CrossRef] [PubMed]
- Tallkvist, J.; Bowlus, C.L.; Lonnerdal, B. DMT1 gene expression and cadmium absorption in human absorptive enterocytes. Toxicol. Lett. 2001, 122, 171–177. [Google Scholar] [CrossRef]
- Li, H.Y.; Qian, Z.M. Transferrin/transferrin receptor-mediated drug delivery. Med. Res. Rev. 2002, 22, 225–250. [Google Scholar] [CrossRef]
- Nebert, D.W.; Galvez-Peralta, M.; Ben Hay, E.; Li, H.; Johansson, E.; Yin, C.; Wang, B.; He, L.; Soleimani, M. ZIP14 and ZIP8 zinc/bicarbonate symporters in Xenopus oocytes: Characterization of metal uptake and inhibition. Metallomics 2012, 4, 1218–1225. [Google Scholar] [CrossRef]
- Jeong, J.; Eide, D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013, 34, 612–619. [Google Scholar] [CrossRef]
- Tsunemi, T.; Hamada, K.; Krainc, D. ATP13A2/PARK9 regulates secretion of exosomes and alpha-Synuclein. J. Neurosci. 2014, 34, 15281–15287. [Google Scholar] [CrossRef]
- Singaraja, R.R.; Hadano, S.; Metzler, M.; Givan, S.; Wellington, C.L.; Warby, S.; Yanai, A.; Gutekunst, C.A.; Leavitt, B.R.; Yi, H.; et al. HIP14, a novel ankyrin domain-containing protein, links huntingtin to intracellular trafficking and endocytosis. Hum. Mol. Genet. 2002, 11, 2815–2828. [Google Scholar] [CrossRef]
- Du, J.Y.; Xie, J.; Zhang, Z.; Tsujikawa, H.; Fusco, D.; Silverman, D.; Liang, B.; Yue, L.X. TRPM7-Mediated Ca2+ Signals Confer Fibrogenesis in Human Atrial Fibrillation. Circ. Res. 2010, 106, 992–1003. [Google Scholar] [CrossRef]
- Long, L.Z.; Persson, D.P.; Duan, F.Y.; Jorgensen, K.; Yuan, L.X.; Schjoerring, J.K.; Pedas, P.R. The iron-regulated transporter 1 plays an essential role in uptake, translocation and grain-loading of Mn, but not iron, in barley. New Phytol. 2018, 217, 1640–1653. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Shang, C.; Huang, J.C. Co-removal of hexavalent chromium through copper precipitation in synthetic wastewater. Environ. Sci. Technol. 2003, 37, 4281–4287. [Google Scholar] [CrossRef] [PubMed]
- Abdolali, A.; Ngo, H.H.; Guo, W.S.; Lu, S.Y.; Chen, S.S.; Nguyen, N.C.; Zhang, X.B.; Wang, J.; Wu, Y. A breakthrough biosorbent in removing heavy metals: Equilibrium, kinetic, thermodynamic and mechanism analyses in a lab-scale study. Sci. Total Environ. 2016, 542, 603–611. [Google Scholar] [CrossRef]
- Alyuz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.H.; Cui, Z.J. A new indicator to evaluate the pollution of iron and Mn. RSC Adv. 2016, 6, 27963–27968. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.N.; Lv, M.; Chen, L.X. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Tebo, B.M.; Johnson, H.A.; McCarthy, J.K.; Templeton, A.S. Geomicrobiology of Mn (II) oxidation. Trends Microbiol. 2005, 13, 421–428. [Google Scholar] [CrossRef]
- Tang, W.W.; Gong, J.M.; Wu, L.J.; Li, Y.F.; Zhang, M.T.; Zeng, X.P. DGGE diversity of Mn mine samples and isolation of a Lysinibacillus sp. efficient in removal of high Mn (II) concentrations. Chemosphere 2016, 165, 277–283. [Google Scholar] [CrossRef]
- Toyoda, K.; Tebo, B.M. The effect of Ca2+ ions and ionic strength on Mn (II) oxidation by spores of the marine Bacillus sp. SG-1. Geochim. Cosmochim. Acta 2013, 101, 1–11. [Google Scholar] [CrossRef]
- Zhang, J.H.; Lion, L.W.; Nelson, Y.M.; Shuler, M.L.; Ghiorse, W.C. Kinetics of Mn (II) oxidation by Leptothrix discophora SS1. Geochim. Cosmochim. Acta 2002, 66, 773–781. [Google Scholar] [CrossRef]
- Tang, W.W.; Xia, J.; Zeng, X.P.; Wu, L.J.; Ye, G.Q. Biological characteristics and oxidation mechanism of a new Mn-oxidizing bacteria FM-2. Bio-Med. Mater. Eng. 2014, 24, 703–709. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Jin, L.; Deng, S.M.; Li, Z.; Zhang, C.Y. Removal of Mn (II) by a nitrifying bacterium Acinetobacter sp. AL-6: Efficiency and mechanisms. Environ. Sci. Pollut. Res. 2021, 28, 31218–31229. [Google Scholar] [CrossRef] [PubMed]
- Van, P.N.; Truong, H.T.H.; Pham, T.A.; Cong, T.L.; Le, T.; Nguyen, K.C.T. Removal of Mn and Copper from aqueous solution by Yeast Papiliotrema huenov. Mycobiology 2021, 49, 507–520. [Google Scholar] [CrossRef]
- Noszczynska, M.; Lakomy, K.; Nowacki, K.; Piotrowska-Seget, Z. A high Mn-tolerant Pseudomonas sp. strain isolated from Metallurgical waste heap can be a tool for enhancing Mn removal from contaminated soil. Appl. Sci. 2020, 10, 5717. [Google Scholar] [CrossRef]
- Cao, J.G.; Li, X.M.; Ouyang, Y.Z.; Zheng, W.; Yang, Q. Mn waste water treatment: Biosorption of Mn by Serratia sp. J. Environ. Prot. Ecol. 2011, 12, 661–666. [Google Scholar]
- Xu, Z.G.; Ding, Y.; Huang, H.M.; Wu, L.; Zhao, Y.L.; Yang, G.Y. Biosorption Characteristics of Mn (II) by Bacillus cereus Strain HM-5 Isolated from Soil Contaminated by Mn Ore. Pol. J. Environ. Stud. 2019, 28, 463–472. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 12. [Google Scholar] [CrossRef] [PubMed]
- Parvathi, K.; Nareshkumar, R.; Nagendran, R. Biosorption of Mn by Aspergillus niger and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 671–676. [Google Scholar] [CrossRef]
- Sekhar, K.C.; Subramanian, S.; Modak, J.M.; Natarajan, K.A. Removal of metal ions using an industrial biomass with reference to environmental control. Int. J. Miner. Process. 1998, 53, 107–120. [Google Scholar] [CrossRef]
- Silva, R.M.P.; Rodriguez, A.A.; De Oca, J.; Moreno, D.C. Biosorption of chromium, copper, Mn and zinc by Pseudomonas aeruginosa AT18 isolated from a site contaminated with petroleum. Bioresour. Technol. 2009, 100, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Baik, W.Y.; Bae, J.H.; Cho, K.M.; Hartmeier, W. Biosorption of heavy metals using whole mold mycelia and parts thereof. Bioresour. Technol. 2002, 81, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Dong, W.; Zhang, Y.; Yang, Z. Research on isolation and removal conditions of a Mn-resistant strain. China Rural. Water Hydropower 2014, 9, 35–38+42. [Google Scholar]
- Alluri, H.K.; Ronda, S.R.; Settalluri, V.S.; Singh, J.; Bondili, S.V.; Venkateshwar, P. Biosorption: An eco-friendly alternative for heavy metal removal. Afr. J. Biotechnol. 2007, 6, 2924–2931. [Google Scholar]
- Mangood, A.H.; Abdelfattah, I.; El-Saied, F.A.; Mansour, M.Z. Removal of heavy metals from polluted aqueous media using berry leaf. Int. J. Environ. Anal. Chem. 2021, 1–17. [Google Scholar] [CrossRef]
- Guechi, E.; Benabdesselam, S. Removal of cadmium and copper from aqueous media by biosorption on cattail (Typha angustifolia) leaves: Kinetic and isotherm studies. Desalin. Water Treat. 2020, 173, 367–382. [Google Scholar] [CrossRef]
- Ozdemir, S.; Kilinc, E.; Poli, A.; Nicolaus, B.; Guven, K. Cd, Cu, Ni, Mn and Zn resistance and bioaccumulation by thermophilic bacteria, Geobacillus toebii subsp decanicus and Geobacillus thermoleovorans subsp stromboliensis. World J. Microbiol. Biotechnol. 2012, 28, 155–163. [Google Scholar] [CrossRef]
- Madrid, Y.; Camara, C. Biological substrates for metal preconcentration and speciation. TrAC Trends Anal. Chem. 1997, 16, 36–44. [Google Scholar] [CrossRef]
- Vecchio, A.; Finoli, C.; Di Simine, D.; Andreoni, V. Heavy metal biosorption by bacterial cells. Fresenius’ J. Anal. Chem. 1998, 361, 338–342. [Google Scholar] [CrossRef]
- Zhu, F.K.; Qu, L.; Fan, W.X.; Qiao, M.Y.; Hao, H.L.; Wang, X.J. Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. Environ. Monit. Assess. 2011, 179, 191–199. [Google Scholar] [CrossRef]
- Yilmaz, E.I. Metal tolerance and biosorption capacity of Bacillus circulans strain EB1. Res. Microbiol. 2003, 154, 409–415. [Google Scholar] [CrossRef]
- Ozdemir, S.; Kilinc, E.; Nicolaus, B.; Poli, A. Resistance and bioaccumulation of Cd2+, Cu2+, Co2+ and Mn2+ by thermophilic bacteria, Geobacillus thermantarcticus and Anoxybacillus amylolyticus. Ann. Microbiol. 2013, 63, 1379–1385. [Google Scholar] [CrossRef]
- Huang, H.M.; Zhao, Y.L.; Xu, Z.G.; Ding, Y.; Zhou, X.M.; Dong, M. A high Mn (II)-tolerance strain, Bacillus thuringiensis HM7, isolated from Mn ore and its biosorption characteristics. PeerJ 2020, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Chaput, D.L.; Fowler, A.J.; Seo, O.; Duhn, K.; Hansel, C.M.; Santelli, C.M. Mn oxide formation by phototrophs: Spatial and temporal patterns, with evidence of an enzymatic superoxide-mediated pathway. Sci. Rep. 2019, 9, 18244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Kim, D.G.; Ko, S.O. Heavy metal adsorption with biogenic Mn oxides generated by Pseudomonas putida strain MnB1. J. Ind. Eng. Chem. 2015, 24, 132–139. [Google Scholar] [CrossRef]
- Templeton, A.S.; Staudigel, H.; Tebo, B.M. Diverse Mn (II)-oxidizing bacteria isolated from submarine basalts at Loihi Seamount. Geomicrobiol. J. 2005, 22, 127–139. [Google Scholar] [CrossRef]
- Das, A.P.; Sukla, L.B.; Pradhan, N.; Nayak, S. Mn biomining: A review. Bioresour. Technol. 2011, 102, 7381–7387. [Google Scholar] [CrossRef]
- Diem, D.; Stumm, W. Is dissolved Mn2+ being oxidized by O2 in absence of Mn-bacteria or surface catalysts? Geochim. Cosmochim. Acta 1984, 48, 1571–1573. [Google Scholar] [CrossRef]
- Emerson, D.; Ghiorse, W.C. Isolation, cultural maintenance, and taxonomy of a sheath-forming strain of Leptothrix-discophora and characterization of Mn-oxidizing activity associated with the sheath. Appl. Environ. Microbiol. 1992, 58, 4001–4010. [Google Scholar] [CrossRef]
- Emerson, D.; Ghiorse, W.C. Ultrastructure and chemical-composition of the sheath of Leptothrix discophora SP-6. J. Bacteriol. 1993, 175, 7808–7818. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A.I. Biological treatment of Mn (II) and Fe (II) containing groundwater: Kinetic considerations and product characterization. Water Res. 2004, 38, 1922–1932. [Google Scholar] [CrossRef]
- Burger, M.S.; Mercer, S.S.; Shupe, G.D.; Gagnon, G.A. Mn removal during bench-scale biofiltration. Water Res. 2008, 42, 4733–4742. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Muraleedharan, P.; George, R.P.; Sarvamangala, H.; Kalaichelvan, P.T.; Khatak, H.S.; Natarajan, K.A. Morphological differentiation observed in Mn oxidizing bacterial colonies. Natl. Acad. Sci. Lett.-India 2007, 30, 371–375. [Google Scholar]
- Learman, D.R.; Wankel, S.D.; Webb, S.M.; Martinez, N.; Madden, A.S.; Hansel, C.M. Coupled biotic-abiotic Mn (II) oxidation pathway mediates the formation and structural evolution of biogenic Mn oxides. Geochim. Cosmochim. Acta 2011, 75, 6048–6063. [Google Scholar] [CrossRef]
- Bohu, T.; Santelli, C.M.; Akob, D.M.; Neu, T.R.; Ciobota, V.; Rosch, P.; Popp, J.; Nietzsche, S.; Kusel, K. Characterization of pH dependent Mn (II) oxidation strategies and formation of a bixbyite-like phase by Mesorhizobium australicum T-G1. Front. Microbiol. 2015, 6, 15. [Google Scholar] [CrossRef]
- Su, J.M.; Bao, P.; Bai, T.L.; Deng, L.; Liu, F.; He, J. CotA, a multicopper oxidase from Bacillus pumilus WH4, exhibits Mn-oxidase activity. PLoS ONE 2013, 8, 13. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic Mn oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Brouwers, G.J.; Vijgenboom, E.; Corstjens, P.; De Vrind, J.P.M.; de Vrind-de Jong, E.W. Bacterial Mn2+ oxidizing systems and multicopper oxidases: An overview of mechanisms and functions. Geomicrobiol. J. 2000, 17, 1–24. [Google Scholar] [CrossRef]
- Nealson, K.H.; Tebo, B.M.; Rosson, R.A. Occurrence and mechanisms of microbial oxidation of Mn. Adv. Appl. Microbiol. 1988, 33, 279–318. [Google Scholar] [CrossRef]
- Francis, C.A.; Tebo, B.M. Enzymatic Mn (II) oxidation by metabolically dormant spores of diverse Bacillus species. Appl. Environ. Microbiol. 2002, 68, 874–880. [Google Scholar] [CrossRef]
- Lundell, T.; Hatakka, A. Participation of Mn (II) in the catalysis of laccase, Mn peroxidase and lignin peroxidase from Phlebia radiata. FEBS Lett. 1994, 348, 291–296. [Google Scholar] [CrossRef]
- Dick, G.J.; Torpey, J.W.; Beveridge, T.J.; Tebo, B.A. Direct identification of a bacterial Mn (II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species. Appl. Environ. Microbiol. 2008, 74, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Johnson, H.A.; Caputo, N.; Davis, R.E.; Torpey, J.W.; Tebo, B.M. Mn (II) oxidation is catalyzed by Heme Peroxidases in “Aurantimonas manganoxydans” strain SI85-9A1 and Erythrobacter sp. strain SD-21. Appl. Environ. Microbiol. 2009, 75, 4130–4138. [Google Scholar] [CrossRef]
- Lang, M.; Kanost, M.R.; Gorman, M.J. Multicopper oxidase-3 is a laccase associated with the peritrophic matrix of Anopheles gambiae. PLoS ONE 2012, 7, e33985. [Google Scholar] [CrossRef]
- Zhukhlistova, N.E.; Zhukova, Y.N.; Lyashenko, A.V.; Zaitsev, V.N.; Mikhailov, A.M. Three-dimensional organization of three-domain copper oxidases: A review. Crystallogr. Rep. 2008, 53, 92–109. [Google Scholar] [CrossRef]
- Kallio, J.P.; Auer, S.; Janis, J.; Andberg, M.; Kruus, K.; Rouvinen, J.; Koivula, A.; Hakulinen, N. Structure-function studies of a Melanocarpus albomyces laccase suggest a pathway for oxidation of phenolic compounds. J. Mol. Biol. 2009, 392, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Matera, I.; Gullotto, A.; Tilli, S.; Ferraroni, M.; Scozzafava, A.; Briganti, F. Crystal structure of the blue multicopper oxidase from the white-rot fungus Trametes trogii complexed with p-toluate. Inorg. Chim. Acta 2008, 361, 4129–4137. [Google Scholar] [CrossRef]
- Kallio, J.P.; Gasparetti, C.; Andberg, M.; Boer, H.; Koivula, A.; Kruus, K.; Rouvinen, J.; Hakulinen, N. Crystal structure of an ascomycete fungal laccase from Thielavia arenaria—common structural features of asco-laccases. FEBS J. 2011, 278, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Lee, F.S.; Farinas, E.T. Laboratory evolution of laccase for substrate specificity. J. Mol. Catal. B-Enzym. 2010, 62, 230–234. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Natasha; Mosa, A.; El-Naggar, A.; Hossain, M.F.; Abdelrahman, H.; Niazi, N.K.; Shahid, M.; Zhang, T.; Tsang, Y.F.; et al. Mn oxide-modified biochar: Production, characterization and applications for the removal of pollutants from aqueous environments a review. Bioresour. Technol. 2022, 346, 17. [Google Scholar] [CrossRef]
- Barboza, N.R.; Guerra-Sá, R.; Leão, V.A. Mechanisms of Mn bioremediation by microbes: An overview. J. Chem. Technol. Biotechnol. 2016, 91, 2733–2739. [Google Scholar] [CrossRef]
- Luther, G.W. The role of one- and two-electron transfer reactions in forming thermodynamically unstable intermediates as barriers in multi-electron redox reactions. Aquat. Geochem. 2010, 16, 395–420. [Google Scholar] [CrossRef]
- Su, J.M.; Deng, L.; Huang, L.B.; Guo, S.J.; Liu, F.; He, J. Catalytic oxidation of Mn (II) by multicopper oxidase CueO and characterization of the biogenic Mn oxide. Water Res. 2014, 56, 304–313. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Sakata, M.; Iwahori, K. Microbial Mn oxide formation and interaction with toxic metal ions. J. Biosci. Bioeng. 2007, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mayanna, S.; Peacock, C.L.; Schaffner, F.; Grawunder, A.; Merten, D.; Kothe, E.; Buchel, G. Biogenic precipitation of Mn oxides and enrichment of heavy metals at acidic soil pH. Chem. Geol. 2015, 402, 6–17. [Google Scholar] [CrossRef]
- Okazaki, M.; Sugita, T.; Shimizu, M.; Ohode, Y.; Iwamoto, K.; deVrinddeJong, E.W.; deVrind, J.P.M.; Corstjens, P. Partial purification and characterization of Mn-oxidizing factors of Pseudomonas fluorescens GB-1. Appl. Environ. Microbiol. 1997, 63, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Xu, Z.; Ma, H.Q.; Hursthouse, A.S. Removal of Mn (II) from acid mine wastewater: A review of the challenges and opportunities with special emphasis on Mn-oxidizing bacteria and microalgae. Water 2019, 11, 2493. [Google Scholar] [CrossRef]
- Huo, Y.L.; Mo, J.R.; He, Y.Y.; Twagirayezu, G.; Xue, L.G. Transcriptome analysis reveals manganese tolerance mechanisms in a novel native bacterium of Bacillus altitudinis strain HM-12. Sci. Total Environ. 2022, 846, 12. [Google Scholar] [CrossRef]
- Shu, J.; Wu, H.; Chen, M.; Peng, H.; Li, B.; Liu, R.; Liu, Z.; Wang, B.; Huang, T.; Hu, Z. Fractional removal of Mn and ammonia nitrogen from electrolytic metal Mn residue leachate using carbonate and struvite precipitation. Water Res. 2019, 153, 229–238. [Google Scholar] [CrossRef]
- Castro-Alonso, M.J.; Montanez-Hernandez, L.E.; Sanchez-Munoz, M.A.; Franco, M.R.M.; Narayanasamy, R.; Balagurusamy, N. Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: Microbiological and molecular concepts. Front. Mater. 2019, 6, 15. [Google Scholar] [CrossRef]
- Mwandira, W.; Nakashima, K.; Kawasaki, S.; Ito, M.; Sato, T.; Igarashi, T.; Chirwa, M.; Banda, K.; Nyambe, I.; Nakayama, S.; et al. Solidification of sand by Pb (II)-tolerant bacteria for capping mine waste to control metallic dust: Case of the abandoned Kabwe Mine, Zambia. Chemosphere 2019, 228, 17–25. [Google Scholar] [CrossRef]
- Duarte-Nass, C.; Rebolledo, K.; Valenzuela, T.; Kopp, M.; Jeison, D.; Rivas, M.; Azocar, L.; Torres-Aravena, A.; Ciudad, G. Application of microbe-induced carbonate precipitation for copper removal from copper-enriched waters: Challenges to future industrial application. J. Environ. Manag. 2020, 256, 10. [Google Scholar] [CrossRef] [PubMed]
- Song, H.W.; Kumar, A.; Zhang, Y.L. Microbial-induced carbonate precipitation prevents Cd2+ migration through the soil profile. Sci. Total Environ. 2022, 844, 8. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, Z.; Du, Y.; Wang, X.; Zhou, S.; Xu, L.; Jiang, X.; Liu, Y.; Lu, Q.; Yan, Z. The mineralization study of Pb (II), Cd (II)and Cr (VI)by induced calcite precipitation by urease producing strain Sporosarcina ureilytica ML-2. Acta Sci. Circumstantiae 2022, 42, 148–159. [Google Scholar]
- Cuaxinque-Flores, G.; Luis Aguirre-Noyola, J.; Hernandez-Flores, G.; Martinez-Romero, E.; Romero-Ramirez, Y.; Talavera-Mendoza, O. Bioimmobilization of toxic metals by precipitation of carbonates using Sporosarcina luteola: An in vitro study and application to sulfide-bearing tailings. Sci. Total Environ. 2020, 724, 138124. [Google Scholar] [CrossRef]
- Kang, B.; Zha, F.S.; Deng, W.H.; Wang, R.K.; Sun, X.G.; Lu, Z.T. Biocementation of pyrite tailings using microbially induced calcite carbonate precipitation. Molecules 2022, 27, 3608. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Larson, S.L.; Ballard, J.H.; Knotek-Smith, H.M.; Nie, J.; Hu, N.; Ding, D.X.; Han, F.X.X. Microbially induced carbonate precipitation techniques for the remediation of heavy metal and trace element-polluted soils and water. Water Air Soil Pollut. 2021, 232, 268. [Google Scholar] [CrossRef]
- Hatayama, K. Mn Carbonate precipitation induced by calcite-forming bacteria. Geomicrobiol. J. 2020, 37, 603–609. [Google Scholar] [CrossRef]
- Kataoka, K.; Komori, H.; Ueki, Y.; Konno, Y.; Kamitaka, Y.; Kurose, S.; Tsujimura, S.; Higuchi, Y.; Kano, K.; Seo, D.; et al. Structure and function of the engineered multicopper oxidase CueO from Escherichia coli—Deletion of the methionine-rich helical region covering the substrate-binding site. J. Mol. Biol. 2007, 373, 141–152. [Google Scholar] [CrossRef]
- Benini, S.; Rypniewski, W.R.; Wilson, K.S.; Miletti, S.; Ciurli, S.; Mangani, S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: Why urea hydrolysis casts two nickels. Structure 1999, 7, 205–216. [Google Scholar] [CrossRef]
- Zhang, J.; Su, P.; Li, Y.; Li, L. Environmental investigation of bio-modification of steel slag through microbially induced carbonate precipitation. J. Environ. Sci. 2021, 101, 282–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.K.; Qin, Z.Y.; Luo, P.H.; Ma, Z.; Tan, M.Y.; Kang, H.Y.; Huang, Z.N. A novel Mn oxidizing bacterium-Aeromonas hydrophila strain DS02: Mn (II) oxidization and biogenic Mn oxides generation. J. Hazard. Mater. 2019, 367, 539–545. [Google Scholar] [CrossRef]
- Song, F.H.; Zhang, G.L.; Xu, X.L.; Polyak, S.W.; Zhang, K.; Li, H.H.; Yang, N. Role of intracellular energy metabolism in Mn (II) removal by the novel bacterium Stenotrophomonas sp. MNB17. Chemosphere 2022, 308, 10. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Sigaud-Kutner, T.C.S.; Leitao, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal-induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Stuetz, R.M.; Greene, A.C.; Madgwick, J.C. Microalgal-facilitated bacterial oxidation of Mn. J. Ind. Microbiol. 1996, 16, 267–273. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Khan, Z.M.; Naz, I.; Sultan, M.; Ali, M.; Abbasi, I.A.; Islam, T.; Ye, T. Overview of microbes based fabricated biogenic nanoparticles for water and wastewater treatment. J. Environ. Manag. 2019, 230, 128–150. [Google Scholar] [CrossRef] [PubMed]
- Holguera, J.G.; Etui, I.D.; Jensen, L.H.S.; Peña, J. Contaminant loading and competitive access of Pb, Zn and Mn (III) to vacancy sites in biogenic MnO2. Chem. Geol. 2018, 502, 76–87. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yang, Y.; Wu, S.X.; Xia, F.; Han, X.; Xu, X.J.; Deng, S.; Jiang, Y.H. Insights into the synergistic removal mechanisms of thallium(I) by biogenic manganese oxides in a wide pH range. Sci. Total Environ. 2022, 831, 11. [Google Scholar] [CrossRef]
- Forrez, I.; Carballa, M.; Verbeken, K.; Vanhaecke, L.; Ternes, T.; Boon, N.; Verstraete, W. Diclofenac oxidation by biogenic Mn oxides. Environ. Sci. Technol. 2010, 44, 3449–3454. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Ding, Z.; Ruan, X.; Luo, J.; Chen, J.; Zheng, J.; Tang, J. Discovery of a novel native bacterium of Providencia sp. with high biosorption and oxidation ability of Mn for bioleaching of heavy metal contaminated soils. Chemosphere 2020, 241, 125039. [Google Scholar] [CrossRef]
- Yan, J.; Jetten, M.; Rang, J.L.; Hu, Y.Y. Comparison of the effects of different salts on aerobic ammonia oxidizers for treating ammonium-rich organic wastewater by free and sodium alginate immobilized biomass system. Chemosphere 2010, 81, 669–673. [Google Scholar] [CrossRef]
- Rezaee, A.; Godini, H.; Dehestani, S.; Yazdanbakhsh, A.R.; Mosavi, G.; Kazemnejad, A. Biological denitrification by Pseudomonas stutzeri immobilized on microbial cellulose. World J. Microbiol. Biotechnol. 2008, 24, 2397–2402. [Google Scholar] [CrossRef]
- An, Q.; Zhou, Y.; Zhao, B.; Huang, X.L. Efficient ammonium removal through heterotrophic nitrification-aerobic denitrification by Acinetobacter baumannii strain AL-6 in the presence of Cr (VI). J. Biosci. Bioeng. 2020, 130, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Chwastowski, J.; Bradlo, D.; Zukowski, W. Adsorption of cadmium, Mn and lead ions from aqueous solutions using spent coffee grounds and biochar produced by its pyrolysis in the fluidized bed reactor. Materials 2020, 13, 2782. [Google Scholar] [CrossRef] [PubMed]
- Youngwilai, A.; Kidkhunthod, P.; Jearanaikoon, N.; Chaiprapa, J.; Supanchaiyamat, N.; Hunt, A.J.; Ngernyen, Y.; Ratpukdi, T.; Khan, E.; Siripattanakul-Ratpukdi, S. Simultaneous Mn adsorption and biotransformation by Streptomyces violarus strain SBP1 cell-immobilized biochar. Sci. Total Environ. 2020, 713, 10. [Google Scholar] [CrossRef] [PubMed]
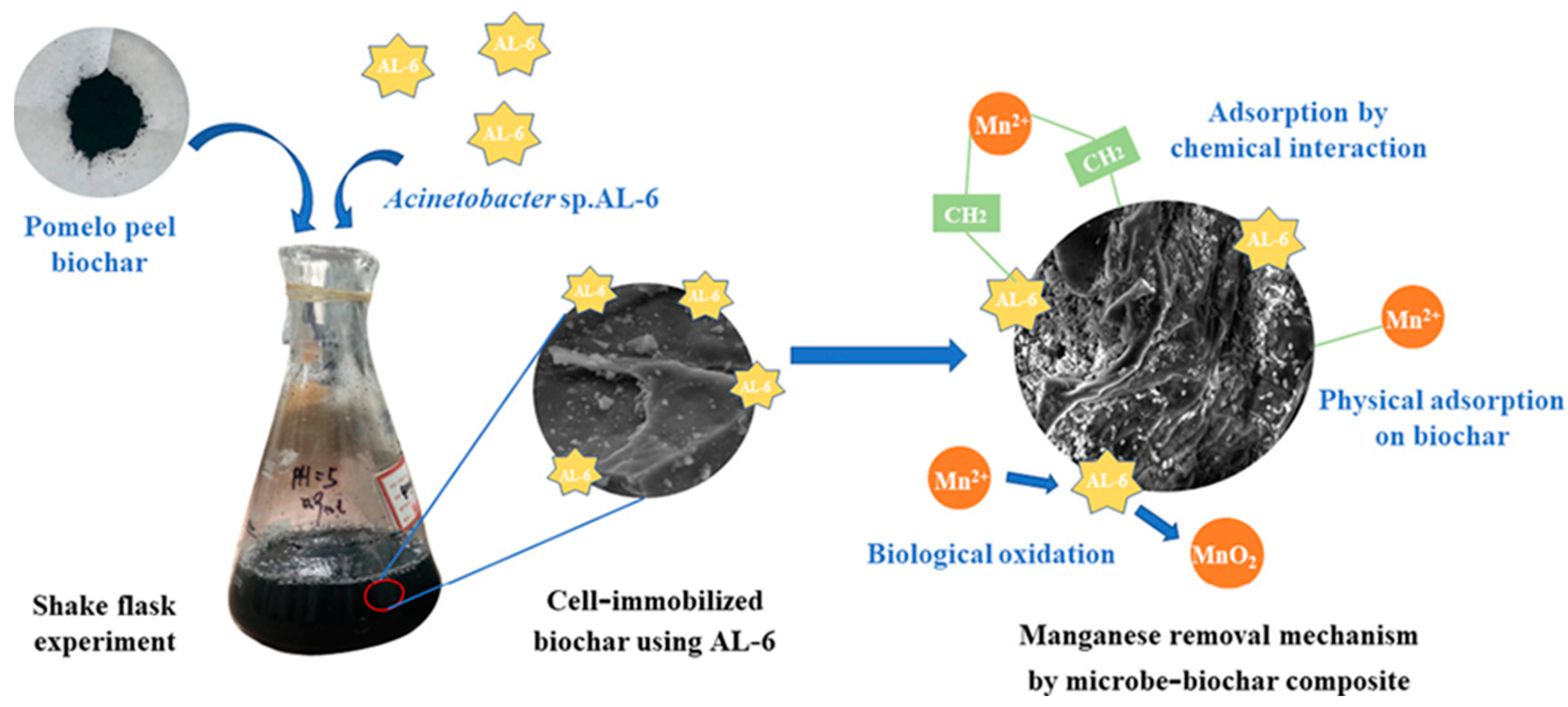
- An, Q.; Zhang, C.Y.; Zhao, B.; Li, Z.; Deng, S.M.; Wang, T.; Jin, L. Insight into synergies between Acinetobacter sp. AL-6 and pomelo peel biochar in a hybrid process for highly efficient Mn removal. Sci. Total Environ. 2021, 793, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, R. H2O2 treatment enhanced the heavy metals removal by manure biochar in aqueous solutions. Sci. Total Environ. 2018, 628–629, 1139–1148. [Google Scholar] [CrossRef]
- Huang, H.M.; Zhao, Y.L.; Xu, Z.G.; Zhang, W.; Jiang, K.K. Physiological responses of Broussonetia papyrifera to Mn stress, a candidate plant for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 181, 18–25. [Google Scholar] [CrossRef]
- Bai, Y.X.; Zhou, Y.C.; Gong, J.F. Physiological mechanisms of the tolerance response to manganese stress exhibited by Pinus massoniana, a candidate plant for the phytoremediation of Mn-contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 45422–45433. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Huang, B.; Xin, L. Effect of phytoremediation with Broussonetia papyrifera on the biological quality in soil contaminated with heavy metals. China Environ. Sci. 2018, 38, 2639–2645. [Google Scholar]
- Zhang, M.; Wu, Y.; Xing, D.; Zhao, K.; Yu, R. Rapid measurement of drought resistance in plants based on electrophysiological properties. Trans. ASABE 2015, 58, 1441–1446. [Google Scholar]
- Almaghrabi, O.A.; Massoud, S.I.; Abdelmoneim, T.S. Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J. Biol. Sci. 2013, 20, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Zhao, Y.L.; Fan, L.; Jin, Q.; Yang, G.Y.; Xu, Z.G. Improvement of Mn phytoremediation by Broussonetia papyrifera with two plant growth promoting (PGP) Bacillus species. Chemosphere 2020, 260, 12. [Google Scholar] [CrossRef] [PubMed]


| Methods | Advantages | Disadvantages |
|---|---|---|
| Electrochemical method | Accurate regulation of kinetics; High removal efficiency | High operating costs; Large power supply |
| Ion exchange method | Non-toxic and renewable; High removal efficiency; Meeting the needs of industrialization | Ion exchange materials being susceptible to organic contamination in wastewater |
| Adsorption method | Easy access to adsorbents, such as activated carbon, zeolite, etc. Adsorbents being regenerative | Great tendency to cause adsorbent sludge, resulting in secondary pollution |
| Chemical precipitation method | Simple operation; Low cost; High removal efficiency | Easy to cause secondary pollution; Accumulation of a large amount of sludge |
| Mechanisms | Species | Initial Mn2+ Concentration (mg/L) | Optimum Temperature | Optimum pH | Removal Efficiency | Experimental Time | Refs |
|---|---|---|---|---|---|---|---|
| Biological oxidation | Lysinibacillus sp. | 54.94 | 37 °C | 7.0 | 94.7% | 7 days | [70] |
| Biological oxidation | Bacillus sp. | 1.65 | 24 °C | 7.5 | >83.3% | - | [71] |
| Biological oxidation | Leptothrix discophora | 4.47 | 30 °C | 7.5 | 97.5% | 3.5 days | [72] |
| Biological oxidation | Citrobacter sp. | 53.0 | 27 °C | 7.0 | 76.2% | 4 days | [73] |
| Biological oxidation | Acinetobacter sp. | 200 | Not mentioned | Self-regulation | 99.1% | 6 days | [74] |
| Bioaccumulation | Papiliotrema huenov | 110 | 30 °C | 5 | 75.6% | 5 days | [75] |
| Bioaccumulation | Pseudomonas sp. | 43.5 | 28 °C | Self-regulation | 88% | 18 days | [76] |
| Biosorption | Serratia sp. | 500 | 34 °C | 6.0 | 96.8% | 76 h | [77] |
| Biosorption | Bacillus cereus | 600 | 35 °C | 6.0 | 60.3% | 5 days | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Yao, F.; Li, X.; Shi, C.; Zang, X.; Shu, X.; Liu, H.; Zhang, W. Manganese Pollution and Its Remediation: A Review of Biological Removal and Promising Combination Strategies. Microorganisms 2022, 10, 2411. https://doi.org/10.3390/microorganisms10122411
Wu R, Yao F, Li X, Shi C, Zang X, Shu X, Liu H, Zhang W. Manganese Pollution and Its Remediation: A Review of Biological Removal and Promising Combination Strategies. Microorganisms. 2022; 10(12):2411. https://doi.org/10.3390/microorganisms10122411
Chicago/Turabian StyleWu, Rongrong, Fangting Yao, Xiaoya Li, Chongjing Shi, Xue Zang, Xiao Shu, Hengwei Liu, and Wenchao Zhang. 2022. "Manganese Pollution and Its Remediation: A Review of Biological Removal and Promising Combination Strategies" Microorganisms 10, no. 12: 2411. https://doi.org/10.3390/microorganisms10122411
APA StyleWu, R., Yao, F., Li, X., Shi, C., Zang, X., Shu, X., Liu, H., & Zhang, W. (2022). Manganese Pollution and Its Remediation: A Review of Biological Removal and Promising Combination Strategies. Microorganisms, 10(12), 2411. https://doi.org/10.3390/microorganisms10122411







