Toxicological and Safety Pharmacological Profiling of the Anti-Infective and Anti-Inflammatory Peptide Pep19-2.5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptides
2.2. Stimulation of Mononuclear Cells (MNC) by LPS
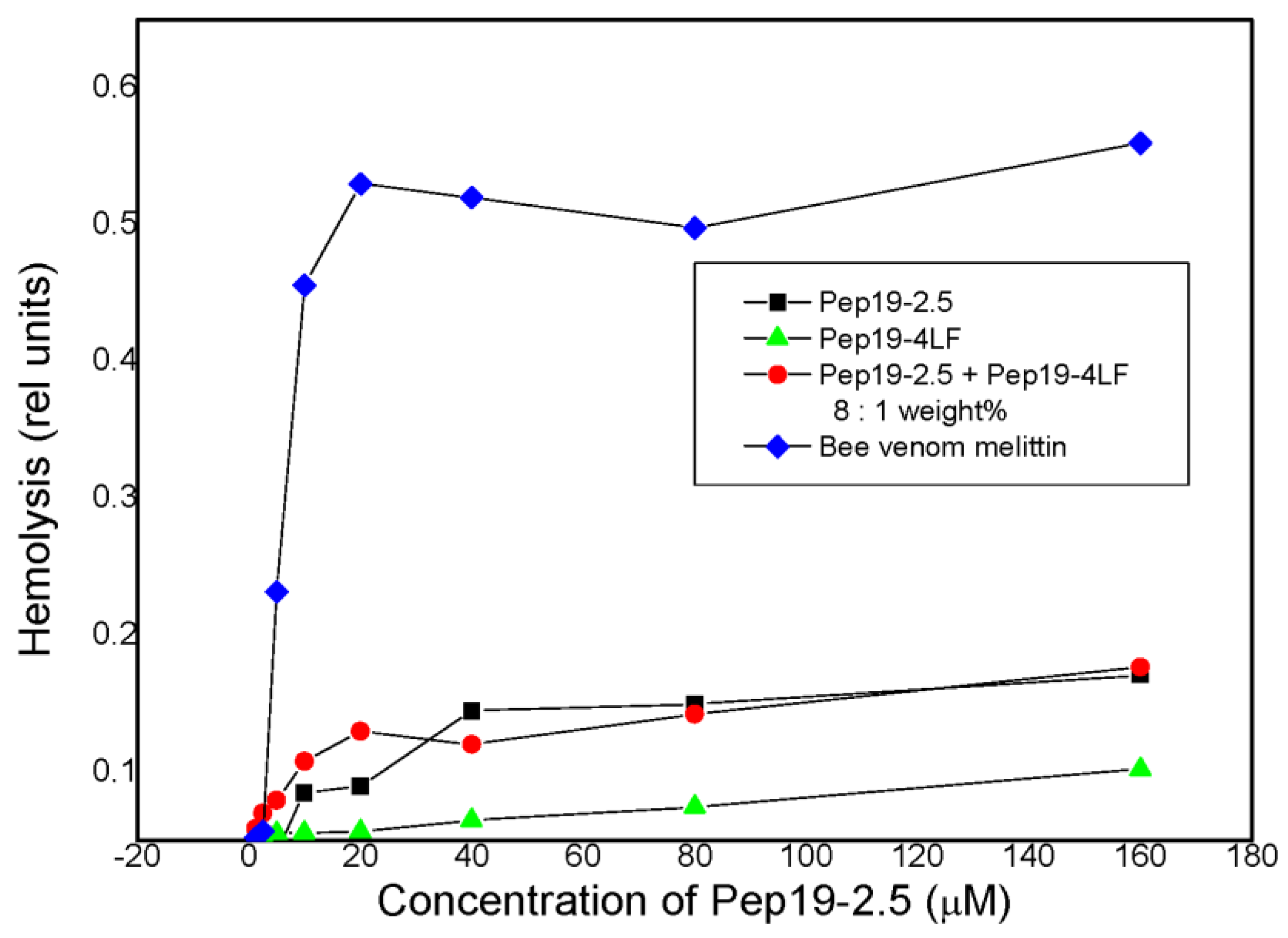
2.3. Cytotoxicity Assays and Hemolysis
2.4. Hemolysis Assay
2.5. Chip-Based Cellular System (BIONAS)
2.6. Modelling of Peptide Structure
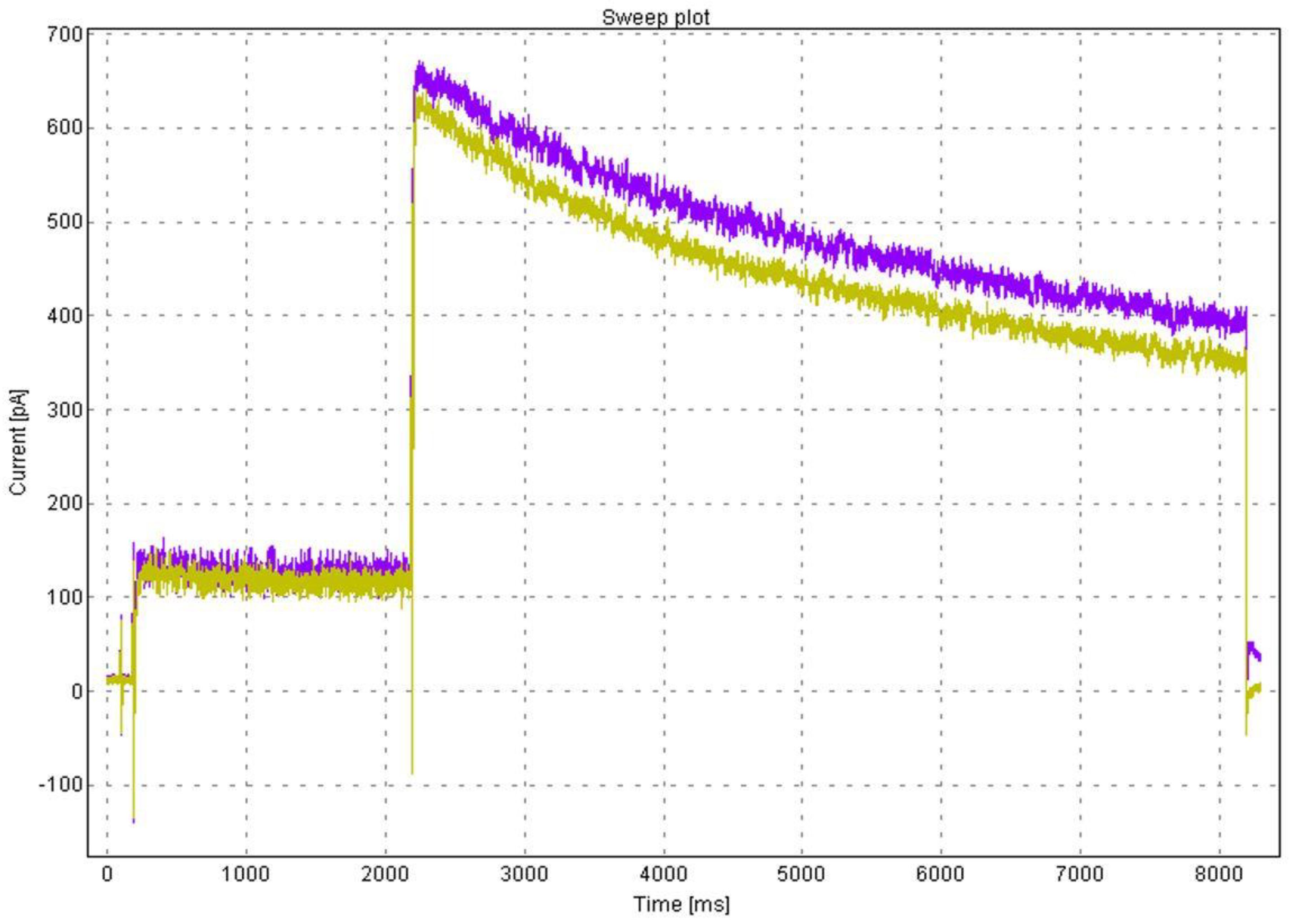
2.7. Electrophysiology
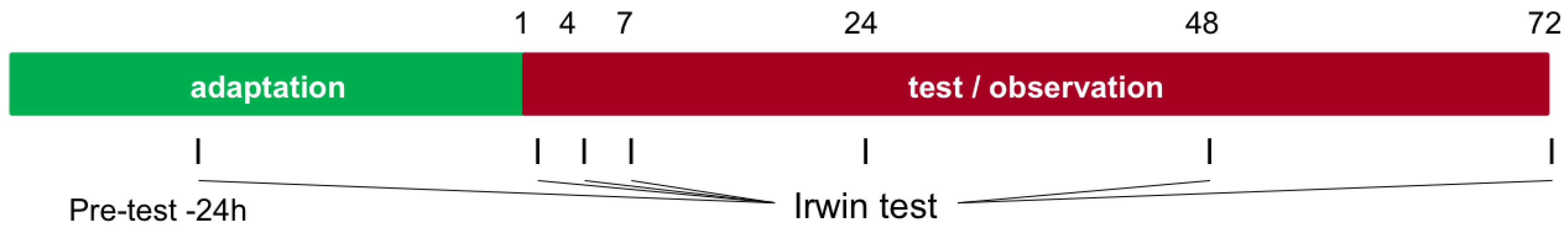
2.8. Irwin Test (Neurobehavioral Activity)
- Mortality: twice daily during the observation period
- Clinical observations: on the day of treatment before application
- Body weight development: on the day of delivery and on the day of treatment before application
- Modified Irwin test: at the time points pre-test (24 h prior to administration), 1 h, 4 h, 7 h, 24 h, 48 h, and 72 h after application.
2.9. Local Lymph Node Assay (LLNA)
- Test item concentrations were based on the results of the formulation evaluation and preliminary irritation/toxicity tests according to relevant guidelines (for example, S7A).
- The maximum attainable test concentration based on solubility was 5% (w/v) in aqueous 1% Pluronic®PE9200 (aqueous 1% Pluronic) using concentration series recommended by relevant guidelines
- Since no adverse effect of the test item was observed during the preliminary irritation/toxicity test up to this maximum concentration, Pep 19-2.5 was tested in the main test at 5%, 2.5%, 1%, and 0.5% (w/v) in aqueous 1% Pluronic
- Male (day 6: 208–259 g; day 1: 244–314 g) Wistar rats
- Five animals per group treated with one single i.v. dose of the respective formulation, application volume: 2 mL/kg
2.10. Repeated Dose Toxicology in Rats
2.11. Fluorescence Resonance Energy Transfer Spectroscopy (FRET)
3. Results
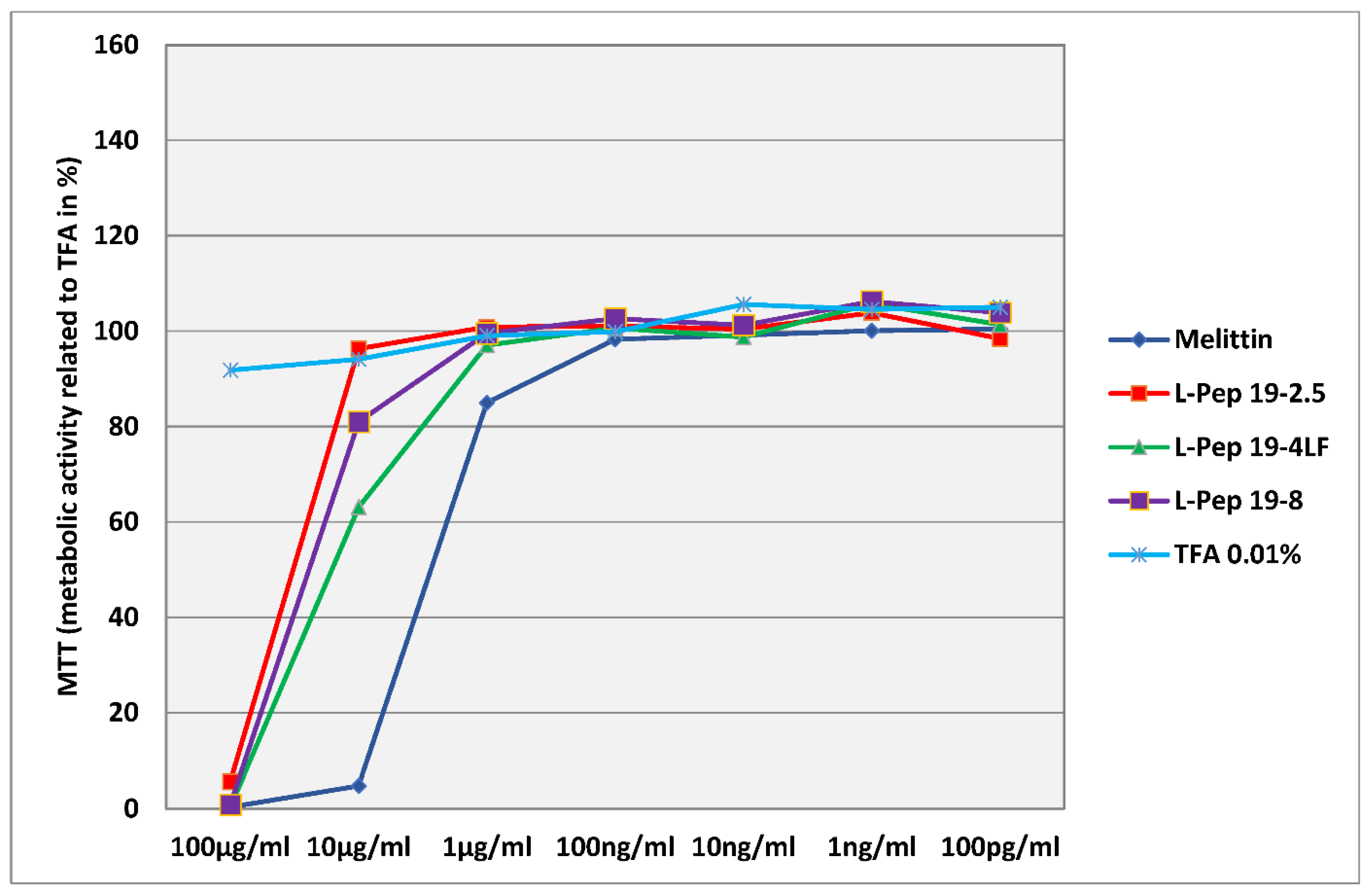
3.1. General In Vitro Toxicology
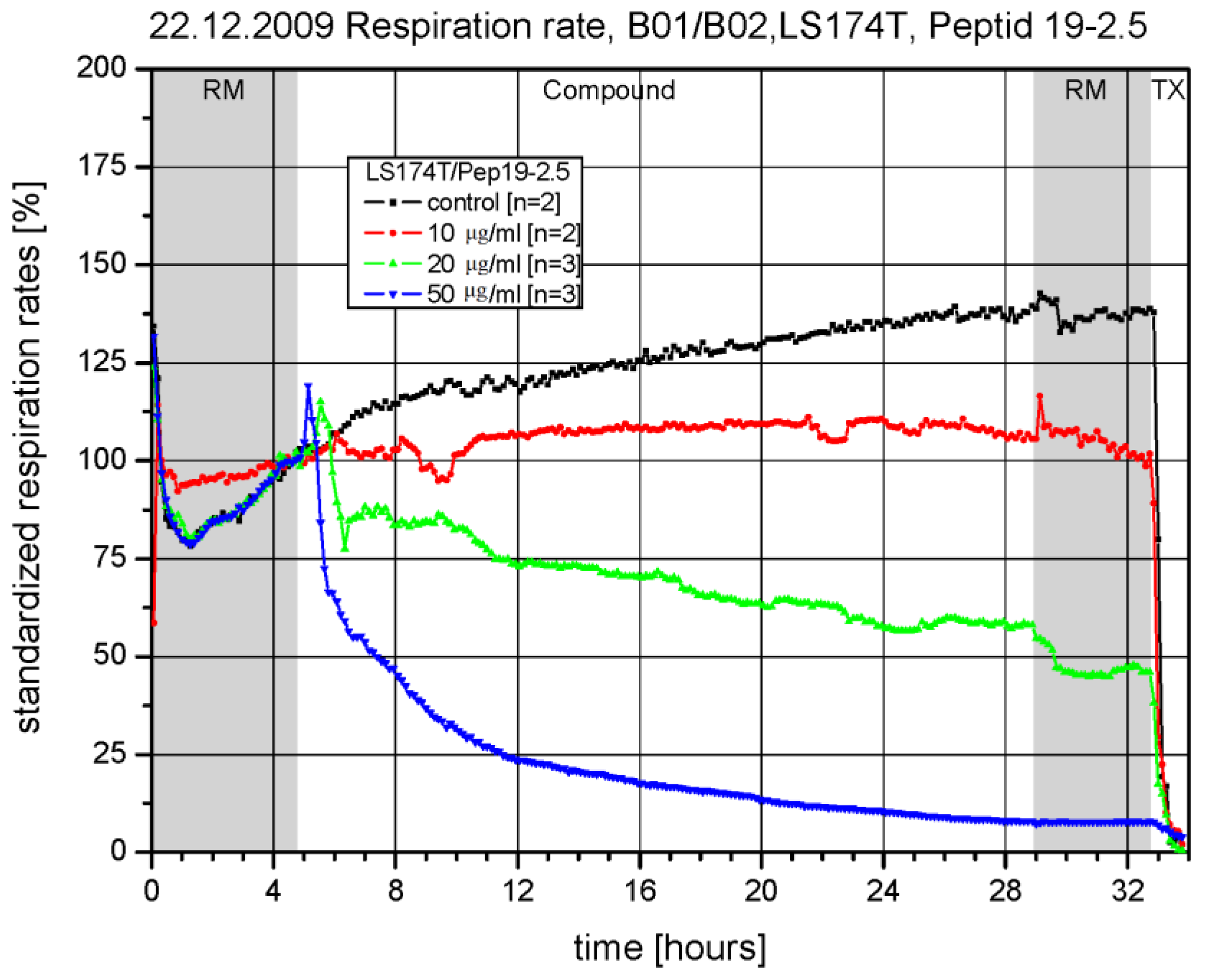
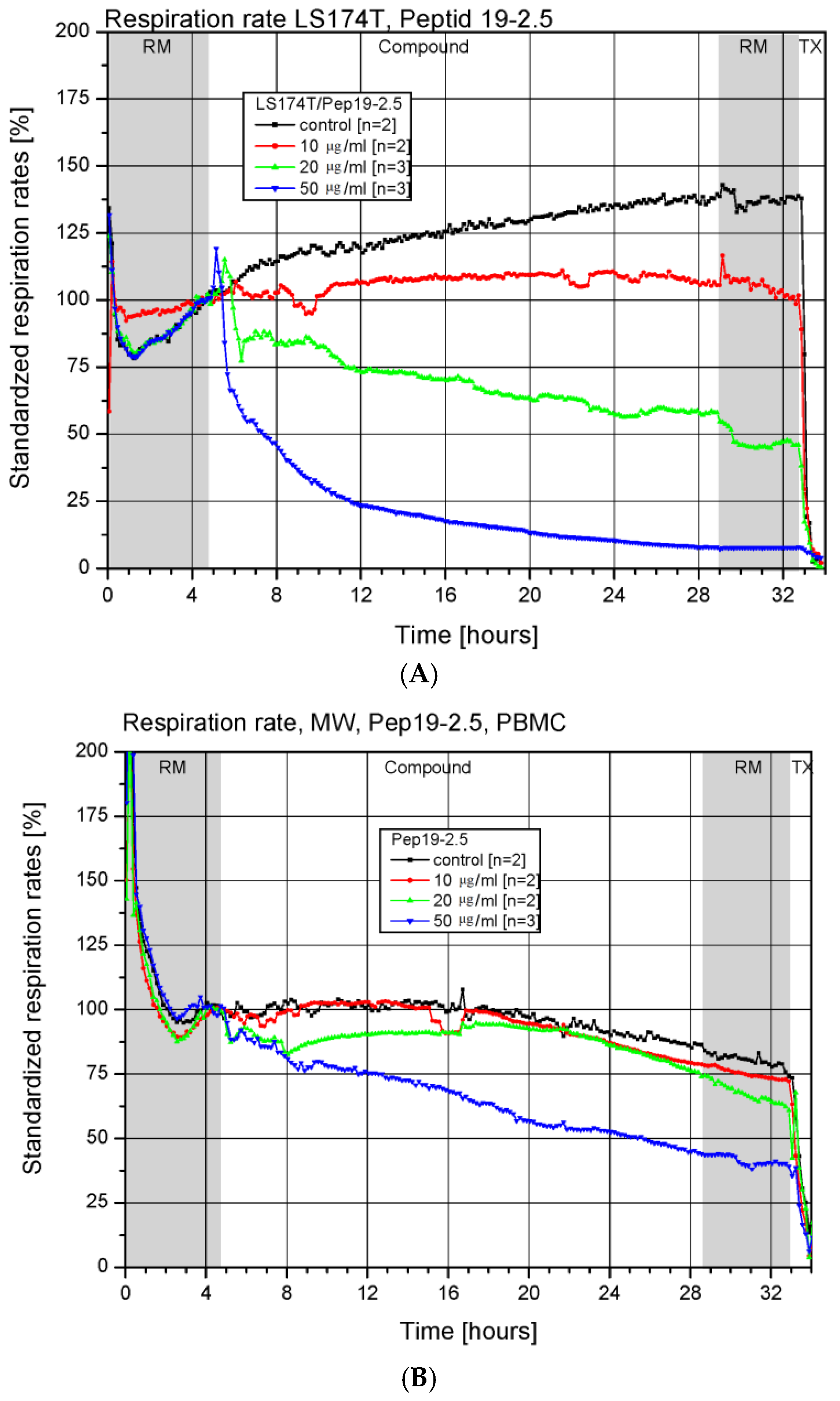
3.2. Toxicity in a Chip-Based System
3.3. Molecular Modeling of Pep19-2.5
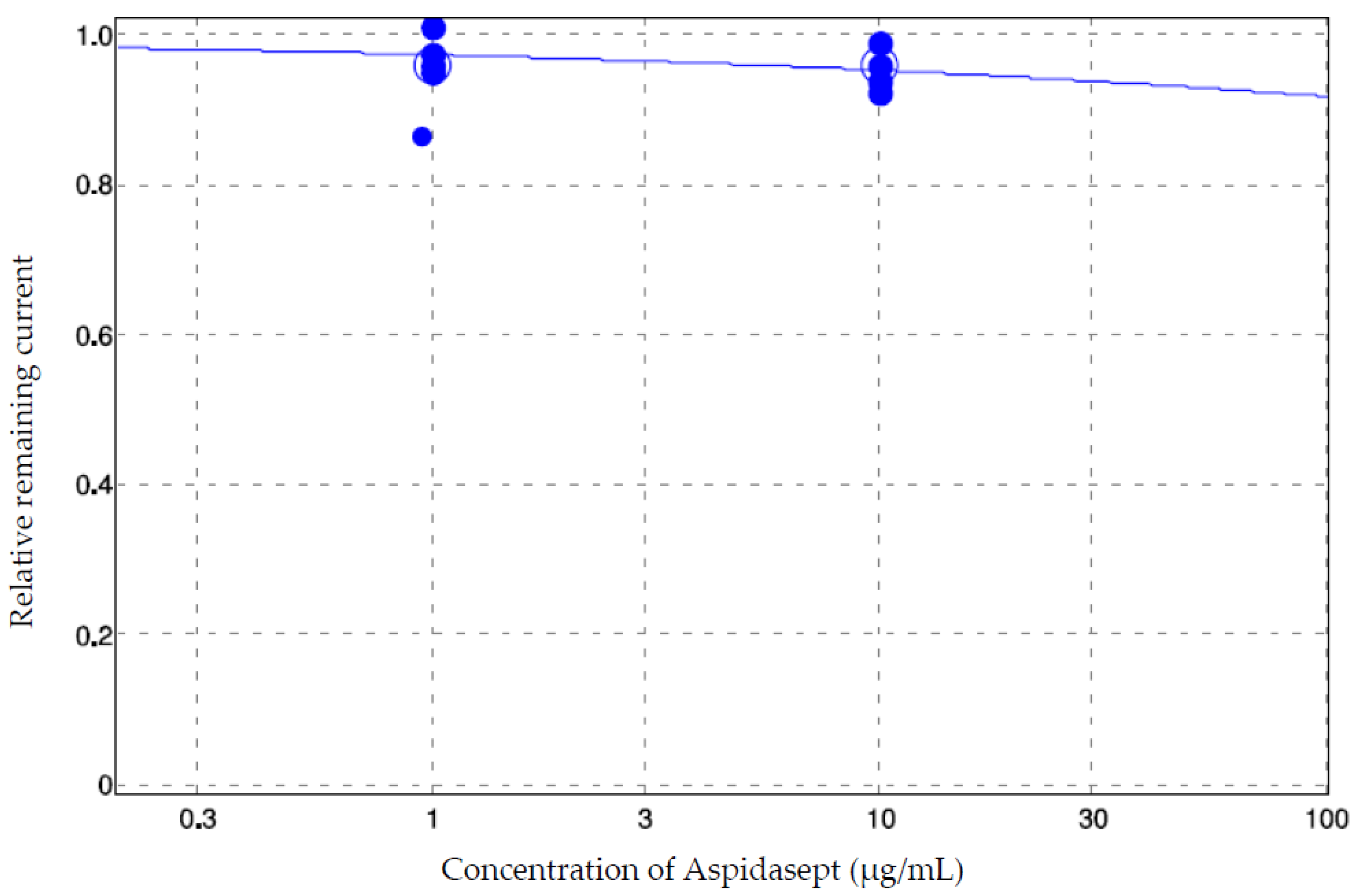
3.4. Electrophysiology
3.5. Safety Pharmacology: Neurological Behaviour
3.6. Irwin Test
Statistical Analysis
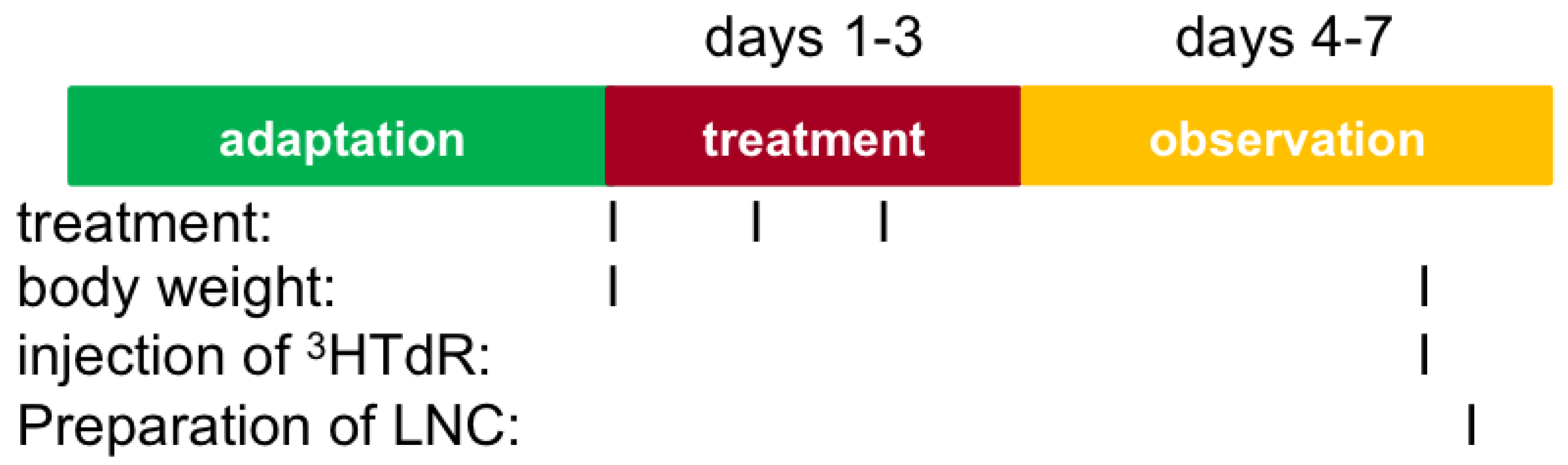
3.7. Local Lymph Node Assay (LLNA)
- Application of substance on external surface of each ear (25 µL/ear) for 3 consecutive days
- On day 6, intravenous injection of tritiated methyl thymidine (3HTdR) in tail vein, and after about 6 h, sacrification and preparation of lymph node cell
- Cell proliferation in lymph node measured by incorporation of 3HTdR
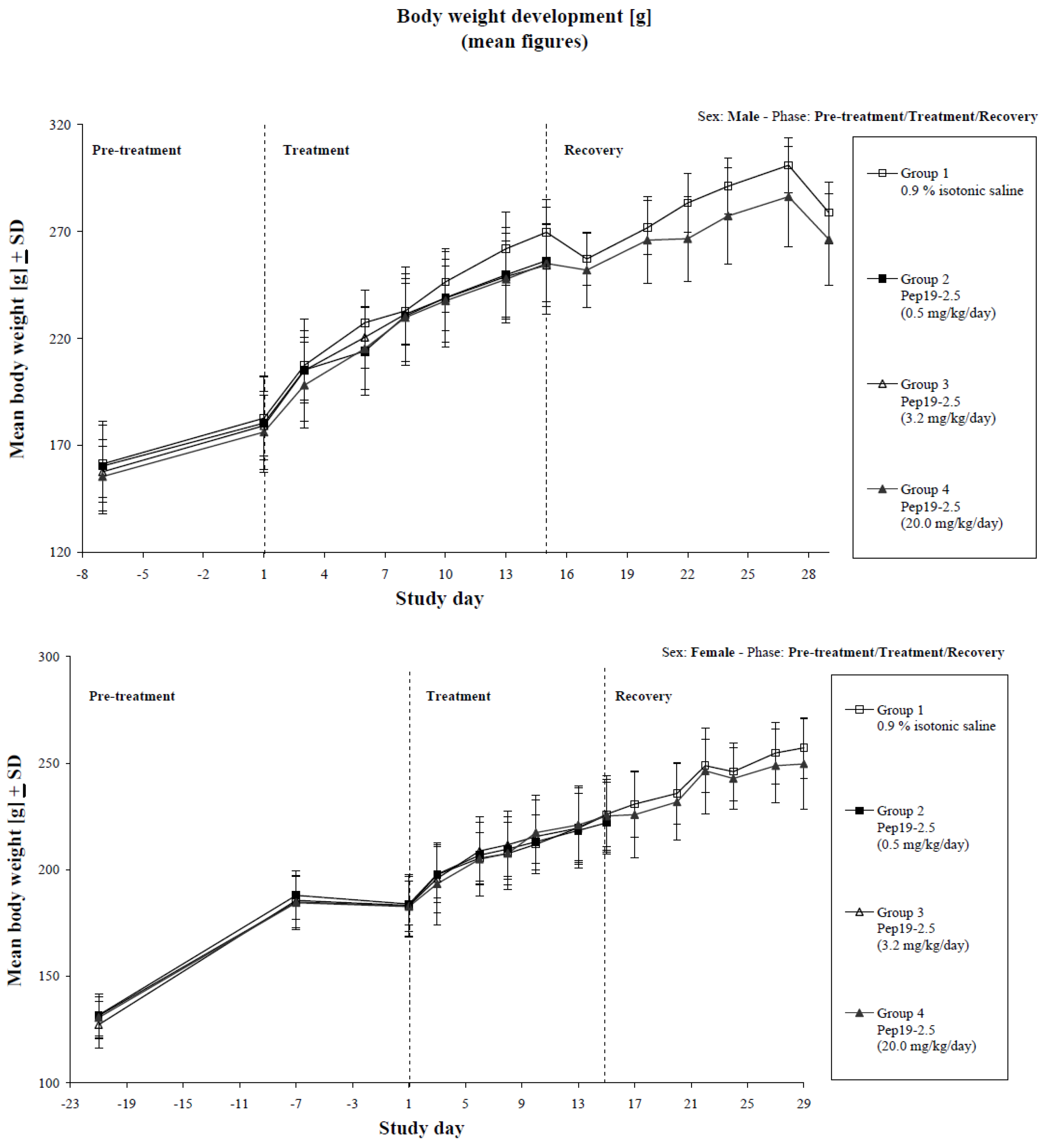
3.8. Repeated Dose Toxicology in Rats
3.9. Origin of the Toxicology of the Pep19-2.5 Series
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kempker, J.A.; Martin, G.S. The Changing Epidemiology and Definitions of Sepsis. Clin. Chest Med. 2016, 37, 165–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenburg, K.; Andrä, J.; Garidel, P.; Gutsmann, T. Peptide-based treatment of sepsis. Appl. Microbiol. Biotechnol. 2011, 90, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Wiese, A. Endotoxins: Relationships between structure, function, and activity. Curr. Top. Med. Chem. 2004, 4, 1127–1146. [Google Scholar] [CrossRef] [PubMed]
- Heinbockel, L.; Sánchez-Gómez, S.; Martinez de Tejada, G.; Dömming, S.; Brandenburg, J.; Kaconis, Y.; Hornef, M.; Dupont, A.; Marwitz, S.; Goldmann, T.; et al. Preclinical investigations reveal the broad-spectrum neutralizing activity of peptide Pep19-2.5 on bacterial pathogenicity factors. Antimicrob. Agents Chemother. 2013, 57, 1480–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaconis, Y.; Kowalski, I.; Howe, J.; Brauser, A.; Richter, W.; Razquin-Olazarán, I.; Inigo-Pestana, M.; Garidel, P.; Rössle, M.; de Tejada, G.M.; et al. Biophysical mechanisms of endotoxin neutralization by cationic amphiphilic peptides. Biophys. J. 2011, 100, 2652–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutsmann, T.; Razquin-Olazarán, I.; Kowalski, I.; Kaconis, Y.; Howe, J.; Bartels, R.; Hornef, M.; Schürholz, T.; Rössle, M.; Sanchez-Gómez, S.; et al. New antiseptic peptides to protect against endotoxin-mediated shock. Antimicrob. Agents Chemother. 2010, 54, 3817–3824. [Google Scholar] [CrossRef] [Green Version]
- De Tejada, G.M.; Heinbockel, L.; Ferrer-Espada, R.; Heine, H.; Alexander, C.; Bárcena-Varela, S.; Goldmann, T.; Correa, W.; Wiesmüller, K.H.; Gisch, N.; et al. Lipoproteins/peptides are sepsis-inducing toxins from bacteria that can be neutralized by synthetic anti-endotoxin peptides. Sci. Rep. 2015, 5, 14292. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; De Santis, R.; Koczera, P.; Simons, N.; Haase, H.; Heinbockel, L.; Brandenburg, K.; Marx, G.; Schuerholz, T. The Synthetic Antimicrobial Peptide 19-2.5 Interacts with Heparanase and Heparan Sulfate in Murine and Human Sepsis. PLoS ONE 2015, 10, e0143583. [Google Scholar] [CrossRef] [Green Version]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Heinbockel, L.; Su, Q.; Brandenburg, K.; Weindl, G. Synthetic anti-endotoxin peptides inhibit cytoplasmic LPS-mediated responses. Biochem. Pharmacol. 2017, 140, 64–72. [Google Scholar] [CrossRef]
- Redfern, W.S.; Carlsson, L.; Davis, A.S.; Lynch, W.G.; MacKenzie, I.L.; Palethorpe, S.; Siegl, P.K.S.; Strang, I.; Sullivan, A.T.; Wallis, R.; et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: Evidence for a provisional safety margin in drug development. Cardiovasc. Res. 2003, 58, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Sun, J.; Wang, Y.; Xu, Y. Cardiac hERG K+ Channel as Safety and Pharmacological Target. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Do, N.; Weindl, G.; Grohmann, L.; Salwiczek, M.; Koksch, B.; Korting, H.C.; Schäfer-Korting, M. Cationic membrane-active peptides-anticancer and antifungal activity as well as penetration into human skin. Exp. Dermatol. 2014, 23, 326–331. [Google Scholar] [CrossRef]
- Maupetit, J.; Derreumaux, P.; Tufféry, P. A fast method for large-scale de novo peptide and miniprotein structure prediction. J. Comput. Chem. 2010, 31, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Maupetit, J.; Tuffery, P.; Derreumaux, P. A coarse-grained protein force field for folding and structure prediction. Proteins 2007, 69, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Thevenet, P.; Shen, Y.; Maupetit, J.; Guyon, F.; Derreumaux, P.; Tuffery, P. PEP-FOLD: An updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012, 40, W288–W293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, A.J.; Möller, C.; Heifetz, A.; Mazanetz, M.P.; Law, R.J.; Ebneth, A.; Gemkow, M.J. Using electrophysiology and in silico three-dimensional modeling to reduce human Ether-à-go-go related gene K(+) channel inhibition in a histamine H3 receptor antagonist program. Assay Drug Dev. Technol. 2010, 8, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Korolkova, Y.V.; Tseng, G.N.; Grishin, E.V. Unique interaction of scorpion toxins with the hERG channel. J. Mol. Recognit. JMR 2004, 17, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflug. Arch. Eur. J. Physiol. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 18 November 2021).
- Danker, T.; Möller, C. Early identification of hERG liability in drug discovery programs by automated patch clamp. Front. Pharmacol. 2014, 5, 203. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.A.; Garbati, N.; Zhang, H.; Large, C.H. Automated patch clamping using the QPatch. Methods Mol. Biol. 2009, 565, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Milligan, C.J.; Möller, C. Automated planar patch-clamp. Methods Mol. Biol. 2013, 998, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Thedinga, E.; Kob, A.; Holst, H.; Keuer, A.; Drechsler, S.; Niendorf, R.; Baumann, W.; Freund, I.; Lehmann, M.; Ehret, R. Online monitoring of cell metabolism for studying pharmacodynamic effects. Toxicol. Appl. Pharmacol. 2007, 220, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Maupetit, J.; Derreumaux, P.; Tuffery, P. PEP-FOLD: An online resource for de novo peptide structure prediction. Nucleic Acids Res. 2009, 37, W498–W503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Fang, M.; Gao, B.; Chui, R.W.; Vargas, H.M. BeKm-1, a peptide inhibitor of human ether-a-go-go-related gene potassium currents, prolongs QTc intervals in isolated rabbit heart. J. Pharmacol. Exp. Ther. 2011, 337, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Yi, H.; Cao, Z.; Yin, S.; Dai, C.; Wu, Y.; Li, W. Interaction simulation of hERG K+ channel with its specific BeKm-1 peptide: Insights into the selectivity of molecular recognition. J. Proteome Res. 2007, 6, 611–620. [Google Scholar] [CrossRef]
- Zhang, M.; Korolkova, Y.V.; Liu, J.; Jiang, M.; Grishin, E.V.; Tseng, G.N. BeKm-1 Is a HERG-Specific Toxin that Shares the Structure with ChTx but the Mechanism of Action with ErgTx1. Biophys. J. 2003, 84, 3022–3036. [Google Scholar] [CrossRef] [Green Version]
- Krepstakies, M.; Lucifora, J.; Nagel, C.H.; Zeisel, M.B.; Holstermann, B.; Hohenberg, H.; Kowalski, I.; Gutsmann, T.; Baumert, T.F.; Brandenburg, K.; et al. A new class of synthetic peptide inhibitors blocks attachment and entry of human pathogenic viruses. J. Infect. Dis. 2012, 205, 1654–1664. [Google Scholar] [CrossRef] [Green Version]
- Pfalzgraff, A.; Heinbockel, L.; Su, Q.; Gutsmann, T.; Brandenburg, K.; Weindl, G. Synthetic antimicrobial and LPS-neutralising peptides suppress inflammatory and immune responses in skin cells and promote keratinocyte migration. Sci. Rep. 2016, 6, 31577. [Google Scholar] [CrossRef]
- Ando, F.; Kuruma, A.; Kawano, S. Synergic effects of β-estradiol and erythromycin on hERG currents. J. Membr. Biol. 2011, 241, 31–38. [Google Scholar] [CrossRef]
- Nilsson, M.F.; Webster, W.S. Effects of macrolide antibiotics on rat embryonic heart function in vitro. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2014, 101, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Stanat, S.J.; Carlton, C.G.; Crumb, W.J.; Agrawal, K.C.; Clarkson, C.W. Characterization of the inhibitory effects of erythromycin and clarithromycin on the HERG potassium channel. Mol. Cell Biochem. 2003, 254, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Volberg, W.A.; Koci, B.J.; Su, W.; Lin, J.; Zhou, J. Blockade of human cardiac potassium channel human ether-a-go-go-related gene (HERG) by macrolide antibiotics. J. Pharmacol. Exp. Ther. 2002, 302, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisialowski, T.; Crimin, K.; Engtrakul, J.; O’Donnell, J.; Fermini, B.; Fossa, A.A. Differentiation of arrhythmia risk of the antibacterials moxifloxacin, erythromycin, and telithromycin based on analysis of monophasic action potential duration alternans and cardiac instability. J. Pharmacol. Exp. Ther. 2006, 318, 352–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, H.; Horie, M.; Ito, M.; Imoto, K. Arrhythmogenesis in the short-QT syndrome associated with combined HERG channel gating defects: A simulation study. Circ. J. Off. J. Jpn. Circ. Soc. 2006, 70, 502–508. [Google Scholar] [CrossRef]



| HepG2 | Human Hepatom Cell Line | Cell Lines Service, Eppelheim Germany |
|---|---|---|
| LS-174T | Human colon adenocarcinoma cell line | Cell Lines Service |
| Jurkat | Human acute lymphocytic leukemia cell line | Cell Lines Service |
| PBMC | Human peripheral blood mononuclear cells [hPBMCs] | ProBioGen, Berlin Germany |
| Group | No. of Animals | Treatment | Single Dose in [mg/kg] |
|---|---|---|---|
| Negative control | 6 males | 0.9% isotonic saline | - |
| Positive control | 6 males | chlorpromazine | 3.00 |
| Low dose | 6 males | Pep19-2.5 | 0.02 |
| Mid dose | 6 males | Pep19-2.5 | 0.20 |
| High dose | 6 males | Pep19-2.5 | 2.00 |
| Peptide | Sequence | Molecular Weight |
|---|---|---|
| Pep19-2.5 | GCKKYRRFRWKFKGKFWFWG | 2711 |
| Pep19-2.5AK | KKYRRFRWKFKGKFWFWCG | 2654 |
| Pep19-Re1 | GKKYRWKFKGKFWFWG | 2149 |
| Pep19-2.5M2 | GCKKYRRFRWKFK | 1802 |
| Pep19-2.5M3 | GCKKYRRFRW | 1399 |
| Pep19-2.5M4 | KFKGKFWFWG | 1330 |
| Pep19-2.5M5 | YRRFRWKFKG | 1443 |
| Pep19-2.5M6 | YRRFRWK | 1110 |
| Aspidasept (Manual Patch-Clamp) (Rel. Rem. Current ± SD) | Aspidasept (QPatch) (Rel. Rem. Current ± SD) | Aspidasept (QPatch, 20 min Application) (Rel. Rem. Current) | |
|---|---|---|---|
| 1 µg/mL | 1.00 ± 0.04 | 0.97 ± 0.02 | Not tested |
| 10 µg/mL | Beyond detection limit of the assay (no successful experiments (n = 7)) | 0.95 ± 0.02 | 0.92 |
| 100 µg/mL | Beyond detection limit of the assay (not tested) | Beyond detection limit of the assay (no successful experiments (n = 6)) | Not tested |
| Group | No. of Animals | Treatment | Single Dose in [% (w/v)] |
|---|---|---|---|
| Negative control | 5 females | aqueous 1% Pluronic | - |
| Low dose | 5 females | Pep19-2.5 in aq. 1% Pluronic | 0.5 |
| Mid dose I | 5 females | Pep19-2.5 in aq. 1% Pluronic | 1.0 |
| Mid dose II | 5 females | Pep19-2.5 in aq. 1% Pluronic | 2.5 |
| High dose | 5 females | Pep19-2.5 in aq. 1% Pluronic | 5.0 |
| Positive control | 5 females | α-Hexylcinnamaldehyde in AOO | 25 |
| Neg. control of pos. control | 5 females | AOO (4:1 v/v acetone/olive oil) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, C.; Heinbockel, L.; Garidel, P.; Gutsmann, T.; Mauss, K.; Weindl, G.; Fukuoka, S.; Loser, D.; Danker, T.; Brandenburg, K. Toxicological and Safety Pharmacological Profiling of the Anti-Infective and Anti-Inflammatory Peptide Pep19-2.5. Microorganisms 2022, 10, 2412. https://doi.org/10.3390/microorganisms10122412
Möller C, Heinbockel L, Garidel P, Gutsmann T, Mauss K, Weindl G, Fukuoka S, Loser D, Danker T, Brandenburg K. Toxicological and Safety Pharmacological Profiling of the Anti-Infective and Anti-Inflammatory Peptide Pep19-2.5. Microorganisms. 2022; 10(12):2412. https://doi.org/10.3390/microorganisms10122412
Chicago/Turabian StyleMöller, Clemens, Lena Heinbockel, Patrick Garidel, Thomas Gutsmann, Karl Mauss, Günther Weindl, Satoshi Fukuoka, Dominik Loser, Timm Danker, and Klaus Brandenburg. 2022. "Toxicological and Safety Pharmacological Profiling of the Anti-Infective and Anti-Inflammatory Peptide Pep19-2.5" Microorganisms 10, no. 12: 2412. https://doi.org/10.3390/microorganisms10122412








