Protozoa-Derived Extracellular Vesicles on Intercellular Communication with Special Emphasis on Giardia lamblia
Abstract
1. Introduction
2. Extracellular Vesicles
3. Protozoa EVs in Intercellular Communication
3.1. Trichomonas vaginalis
3.2. Leishmania spp.
3.3. Trypanosoma spp.
3.4. Entamoeba histolytica
3.5. Plasmodium spp.
3.6. Toxoplasma gondii
3.7. Cryptosporidium parvum
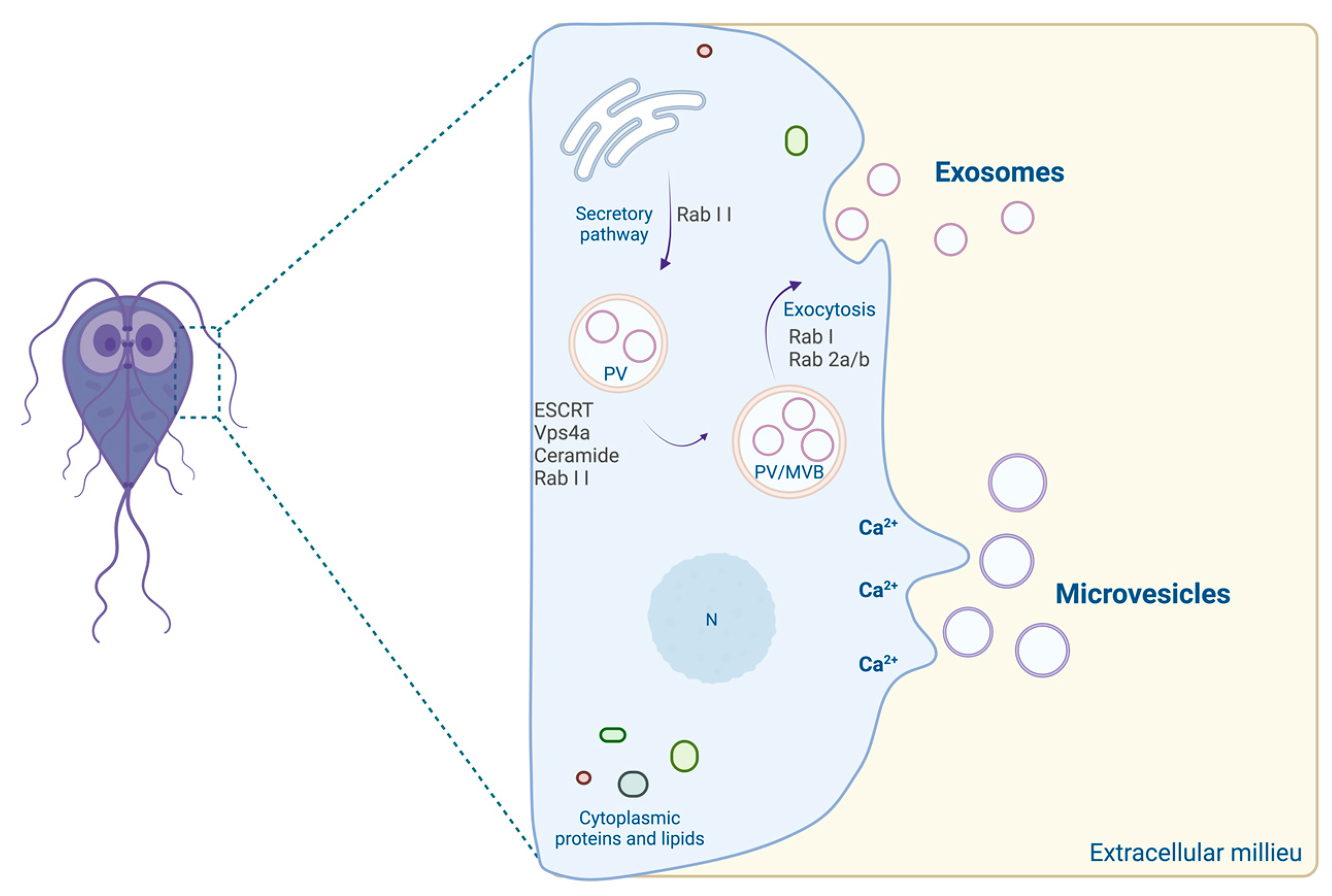
4. Giardia Extracellular Vesicles
| EVs’ Source | Type | Main Cargo | Actions | References |
|---|---|---|---|---|
| Culture medium of G.lamblia trophozoites, isolate WB clone C6 | Microvesicles | α-tubulin; ADI; β-tubulin; Enolase; Giardin; HSP70; OCT; Ribossomal protein; rRNA; VSPs; ZFD protein | Increase trophozoite adhesion to Caco-2 cells | [101] |
| Activation and allostimulation of human DCs | ||||
| Culture medium of G.lamblia trophozoites, isolate WB clone C6 | Large and small EVs | ADI; α-1 giardin; Ankyrin; Arginine-metabolizing enzymes; Cathepsin B; FixW protein; Giardins; Katanin; OCT; Peroxiredoxin-1; PFOR; VSPs | Increase trophozoite attachment to Caco-2 cells (only large EVs) | [103] |
| Culture medium of G.lamblia trophozoites, isolate NF | Extracellular vesicles | Cathepsin B cysteine proteases; VSPs; ADI; Tenascins; Tubulin β-chain; Pyruvate phosphate dikinase; PFOR; EF-α; HSP90; OCT; Tubulin α- chain; NADH oxidase; EF-γ; Phosphoglycerate kinase; EF-2; Cluster of kinase activity; NADH oxidase; Peroxiredoxins | Disruption of tight junctional proteins, Zonula occludins-1, and claudin-4 of SCBN cells; | [104] |
| Antibacterial effect on Escherichia coli (HB101 strain) and Enterobacter cloacae | ||||
| Culture medium of G. lamblia trophozoites, isolate WB clone C6 | Extracellular vesicles | 14-3-3 proteins; ADI; α-tubulin; Giardin; HSPs; OCT; VSPs | Proinflammatory response in murine macrophages | [105] |
| Activation of TLR2 and NLRP3 inflammasome signaling pathways | ||||
| Culture medium of G.lamblia trophozoites, isolate WB clone C6 | Extracellular vesicles | NA | Activation of the p38, ERK, and NF-κB signaling proinflammatory pathways in murine macrophages | [106] |
| Culture medium of G.lamblia trophozoites, isolate WB clone C6 | Extracellular vesicles | NA | Therapeutic effects on experimental murine colitis | [107] |
| Culture medium of G.lamblia trophozoites, isolate WB clone C6 | Large and small EVs | Alteration of proteome profile by nystatin and oseltamivir treatment | Decrease in attachment and encystation | [108] |
EVs: Future Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Wang, L.; Li, J.; Wang, L.; Wu, Z.; Sun, X. Extracellular Vesicle-Mediated Communication within Host-Parasite Interactions. Front. Immunol. 2019, 9, 3066. [Google Scholar] [CrossRef] [PubMed]
- Hailegebriel, T. Prevalence of intestinal parasitic infections and associated risk factors among students at Dona Berber primary school, Bahir Dar, Ethiopia. BMC Infect. Dis. 2017, 17, 362. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, H.S.; Ekubagewargies, D.T. Prevalence and factors associated with intestinal parasites among under-five children attending Woreta Health Center, Northwest Ethiopia. BMC Infect. Dis. 2019, 19, 256. [Google Scholar] [CrossRef] [PubMed]
- Certad, G.; Viscogliosi, E.; Chabé, M.; Cacciò, S.M. Pathogenic Mechanisms of Cryptosporidium and Giardia. Trends Parasitol. 2017, 33, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.L.C.; Rebello, K.M. An Overview of Mucosa-Associated Protozoa: Challenges in Chemotherapy and Future Perspectives. Front. Cell. Infect. Microbiol. 2022, 12, 860442. [Google Scholar] [CrossRef] [PubMed]
- Short, E.E.; Caminade, C.; Thomas, B.N. Climate Change Contribution to the Emergence or Re-Emergence of Parasitic Diseases. Infect. Dis. 2017, 10, 1178633617732296. [Google Scholar] [CrossRef]
- Thompson, R.C. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet. Parasitol. 2004, 126, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, L. Mucosal defences against Giardia. Parasite Immunol. 2003, 25, 259–270. [Google Scholar] [CrossRef]
- Partida-Rodríguez, O.; Serrano-Vázquez, A.; Nieves-Ramírez, M.E.; Moran, P.; Rojas-Velázquez, L.; Portillo, T.; González, E.; Hernandez, E.G.; Finlay, B.B.; Ximenez, C. Human Intestinal Microbiota: Interaction between Parasites and the Host Immune Response. Arch. Med. Res. 2017, 48, 690–700. [Google Scholar] [CrossRef]
- Wensaas, K.A.; Langeland, N.; Hanevik, K.; Mørch, K.; Eide, G.E.; Rortveit, G. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: Historic cohort study. Gut 2012, 61, 214–219. [Google Scholar] [CrossRef]
- Halliez, M.C.M.; Buret, A.G. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J. Gastroenterol. 2013, 19, 8974–8985. [Google Scholar] [CrossRef] [PubMed]
- Allain, T.; Buret, A.G. Pathogenesis and post-infectious complications in giardiasis. Adv. Parasitol. 2020, 107, 173–199. [Google Scholar] [PubMed]
- Coakley, G.; Maizels, R.M.; Buck, A.H. Exosomes and Other Extracellular Vesicles: The New Communicators in Parasite Infections. Trends Parasitol. 2015, 31, 477–489. [Google Scholar] [CrossRef]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Lindo, M.V. Extracellular Vesicles and the Parasite-Host Interaction: Giardia lamblia, a Case of Study. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2019. Available online: http://hdlhandlenet/10316/88371 (accessed on 7 October 2022).
- Deolindo, P.; Evans-Osses, I.; Ramirez, M.I. Microvesicles and exosomes as vehicles between protozoan and host cell communication. Biochem, Soc. Trans. 2013, 41, 252–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tatischeff, I. Dictyostelium: A Model for Studying the Extracellular Vesicle Messengers Involved in Human Health and Disease. Cells 2019, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xing, D.; Zhu, Y.; Dong, S.; Zhao, B. The State of Exosomes Research: A Global Visualized Analysis. BioMed Res. Int. 2019, 2019, 1495130. [Google Scholar] [CrossRef] [PubMed]
- Lässer, C. Exosomes in Diagnostic and Therapeutic Applications: Biomarker, Vaccine and RNA Interference Delivery Vehicle. Expert Opin. Biol. Ther. 2015, 15, 103–117. [Google Scholar] [CrossRef]
- Twu, O.; Johnson, P.J. Parasite extracellular vesicles: Mediators of intercellular communication. PLoS Pathog. 2014, 10, e1004289. [Google Scholar] [CrossRef]
- Nawaz, M.; Malik, M.I.; Hameed, M.; Zhou, J. Research progress on the composition and function of parasite-derived exosomes. Acta Trop. 2019, 196, 30–36. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Sedger, L.M. Intercellular Vesicular Transfer by Exosomes, Microparticles and Oncosomes—Implications for Cancer Biology and Treatments. Front. Oncol. 2019, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.; Sims, B.; Matthews, Q. Biological Function of Exosomes as Diagnostic Markers and Therapeutic Delivery Vehicles in Carcinogenesis and Infectious Diseases. In Nanomedicines; IntechOpen: London, UK, 2018; pp. 1–32. [Google Scholar]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell. Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef]
- Edgar, J.R.; Eden, E.R.; Futter, C.E. Hrs- and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic 2014, 15, 197–211. [Google Scholar] [CrossRef]
- Williams, R.L.; Urbé, S. The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell. Biol. 2007, 8, 355–368. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell. 2011, 21, 77–91. [Google Scholar] [CrossRef]
- Gavinho, B.; Rossi, I.V.; Evans-Osses, I.; Inal, J.; Ramirez, M.I. A New Landscape of Host-Protozoa Interactions Involving the Extracellular Vesicles World. Parasitology 2018, 145, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Twu, O.; de Miguel, N.; Lustig, G.; Stevens, G.C.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host: Parasite interactions. PLoS Pathog. 2013, 9, e1003482. [Google Scholar] [CrossRef] [PubMed]
- Nievas, Y.R.; Coceres, V.M.; Midlej, V.; de Souza, W.; Benchimol, M.; Pereira-Neves, A.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J.; de Miguel, N. Membrane-shed vesicles from the parasite Trichomonas vaginalis: Characterization and their association with cell interaction. Cell. Mol. Life Sci. 2018, 75, 2211–2226. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Ortiz, L.M.; Barajas-Mendiola, M.A.; Barrios-Rodiles, M.; Castellano, L.E.; Arias-Negrete, S.; Avila, E.E.; Cuéllar-Mata, P. Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected mice. Parasite Immunol. 2017, 39, e12426. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Clos, J.; De’Oliveira, C.C.; Shirvani, O.; Fang, Y.; Wang, C.; Foster, L.J.; Reiner, N.E. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J. Cell. Sci. 2010, 123, 842–852. [Google Scholar] [CrossRef]
- Barbosa, F.M.C.; Dupin, T.V.; Toledo, M.D.S.; Reis, N.F.D.C.; Ribeiro, K.; Cronemberger-Andrade, A.; Rugani, J.N.; De Lorenzo, B.H.P.; E Brito, R.R.N.; Soares, R.P.; et al. Extracellular Vesicles Released by Leishmania (Leishmania) amazonensis Promote Disease Progression and Induce the Production of Different Cytokines in Macrophages and B-1 Cells. Front. Microbiol. 2018, 9, 3056. [Google Scholar] [CrossRef]
- Nogueira, P.M.; De Menezes-Neto, A.; Borges, V.M.; Descoteaux, A.; Torrecilhas, A.C.; Xander, P.; Revach, O.-Y.; Regev-Rudzki, N.; Soares, R.P. Immunomodulatory Properties of Leishmania Extracellular Vesicles During Host-Parasite Interaction: Differential Activation of TLRs and NF-κB Translocation by Dermotropic and Viscerotropic Species. Front. Cell. Infect. Microbiol. 2020, 10, 380. [Google Scholar] [CrossRef]
- Silverman, J.M.; Clos, J.; Horakova, E.; Wang, A.Y.; Wiesgigl, M.; Kelly, I.; Lynn, M.A.; McMaster, W.R.; Foster, L.J.; Levings, M.; et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 2010, 185, 5011–5022. [Google Scholar] [CrossRef]
- Cronemberger-Andrade, A.; Aragão-França, L.; de Araujo, C.F.; Rocha, V.J.; Borges-Silva, M.D.C.; Figueiras, C.P.; Oliveira, P.R.; de Freitas, L.A.R.; Veras, P.S.T.; Pontes-De-Carvalho, L. Extracellular vesicles from Leishmania-infected macrophages confer an anti-infection cytokine-production profile to naïve macrophages. PLoS Negl. Trop. Dis. 2014, 8, e3161. [Google Scholar] [CrossRef]
- Dupin, T.V.; Reis, N.F.C.; Perez, E.C.; Soares, R.P.; Torrecilhas, A.C.; Xander, P. Long-Term In Vitro Passaging Had a Negligible Effect on Extracellular Vesicles Released by Leishmania amazonensis and Induced Protective Immune Response in BALB/c Mice. J. Immunol. Res. 2021, 2021, 7809637. [Google Scholar] [CrossRef]
- Atayde, V.D.; Aslan, H.; Townsend, S.; Hassani, K.; Kamhawi, S.; Olivier, M. Exosome Secretion by the Parasitic Protozoan Leishmania within the Sand Fly Midgut. Cell. Rep. 2015, 13, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Trocoli Torrecilhas, A.; Tonelli, R.R.; Pavanelli, W.R.; Da Silva, J.S.; Schumacher, R.I.; De Souza, W.; Esilva, N.; Abrahamsohn, I.D.A.; Colli, W.; Mansoalves, M.; et al. Trypanosoma cruzi: Parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 2009, 11, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Silva, M.R.; das Neves, R.F.C.; Cabrera-Cabrera, F.; Sanguinetti, J.; Medeiros, L.C.; Robello, C.; Naya, H.; Fernandez-Calero, T.; Souto-Padron, T.; de Souza, W.; et al. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol. Res. 2014, 113, 285–304. [Google Scholar] [CrossRef]
- Nogueira, P.M.; Ribeiro, K.; Silveira, A.C.O.; Campos, J.H.; Martins-Filho, O.A.; Bela, S.R.; Campos, M.A.; Pessoa, N.L.; Colli, W.; Alves, M.J.M.; et al. Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune responses. J. Extracell. Vesicles 2015, 4, 28734. [Google Scholar] [CrossRef]
- Cronemberger-Andrade, A.; Xander, P.; Soares, R.P.; Pessoa, N.L.; Campos, M.A.; Ellis, C.C.; Grajeda, B.; Ofir-Birin, Y.; Almeida, I.C.; Regev-Rudzki, N.; et al. Trypanosoma cruzi-Infected Human Macrophages Shed Proinflammatory Extracellular Vesicles That Enhance Host-Cell Invasion via Toll-like Receptor 2. Front. Cell. Infect. Microbiol. 2020, 10, 99. [Google Scholar] [CrossRef]
- Madeira, R.P.; Dal’Mas Romera, L.M.; de Cássia Buck, P.; Mady, C.; Ianni, B.M.; Torrecilhas, A.C. New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response. J. Immunol. Res. 2021, 2021, 6650670. [Google Scholar] [CrossRef]
- Szempruch, A.J.; Sykes, S.E.; Kieft, R.; Dennison, L.; Becker, A.C.; Gartrell, A.; Martin, W.J.; Nakayasu, E.S.; Almeida, I.C.; Hajduk, S.L.; et al. Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell 2016, 164, 246–257. [Google Scholar] [CrossRef]
- Dias-Guerreiro, T.; Palma-Marques, J.; Mourata-Gonçalves, P.; Alexandre-Pires, G.; Valério-Bolas, A.; Gabriel, Á.; Nunes, T.; Antunes, W.; da Fonseca, I.P.; Sousa-Silva, M.; et al. African Trypanosomiasis: Extracellular Vesicles Shed by Trypanosoma brucei brucei Manipulate Host Mononuclear Cells. Biomedicines 2021, 9, 1056. [Google Scholar] [CrossRef]
- Sharma, M.; Morgado, P.; Zhang, H.; Ehrenkaufer, G.; Manna, D.; Singh, U. Characterization of Extracellular Vesicles from Entamoeba histolytica Identifies Roles in Intercellular Communication That Regulates Parasite Growth and Development. Infect. Immun. 2020, 88, e00349-20. [Google Scholar] [CrossRef]
- Díaz-Godínez, C.; Ríos-Valencia, D.G.; García-Aguirre, S.; Martínez-Calvillo, S.; Carrero, J.C. Immunomodulatory effect of extracellular vesicles from Entamoeba histolytica trophozoites: Regulation of NETs and respiratory burst during confrontation with human neutrophils. Front. Cell. Infect. Microbiol. 2022, 12, 1018314. [Google Scholar] [CrossRef]
- Martin-Jaular, L.; Nakayasu, E.S.; Ferrer, M.; Almeida, I.C.; Del Portillo, H.A. Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS ONE 2011, 6, e26588. [Google Scholar] [CrossRef]
- Regev-Rudzki, N.; Wilson, D.W.; Carvalho, T.G.; Sisquella, X.; Coleman, B.M.; Rug, M.; Bursac, D.; Angrisano, F.; Gee, M.; Hill, A.F.; et al. Cell-cell Communication Between Malaria-Infected Red Blood Cells via Exosome-like Vesicles. Cell 2013, 153, 1120–1133. [Google Scholar] [CrossRef]
- Mantel, P.-Y.; Hoang, A.N.; Goldowitz, I.; Potashnikova, D.; Hamza, B.; Vorobjev, I.; Ghiran, I.; Toner, M.; Irimia, D.; Ivanov, A.R.; et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 2013, 13, 521–534. [Google Scholar] [CrossRef]
- Mantel, P.-Y.; Hjelmqvist, D.; Walch, M.; Kharoubi-Hess, S.; Nilsson, S.; Ravel, D.; Ribeiro, M.; Grüring, C.; Ma, S.; Padmanabhan, P.; et al. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat. Commun. 2016, 7, 12727. [Google Scholar] [CrossRef]
- Sampaio, N.G.; Emery, S.J.; Garnham, A.L.; Tan, Q.Y.; Sisquella, X.; Pimentel, M.A.; Jex, A.R.; Regev-Rudzki, N.; Schofield, L.; Eriksson, E.M. Extracellular vesicles from early stage Plasmodium falciparum-infected red blood cells contain PfEMP1 and induce transcriptional changes in human monocytes. Cell Microbiol. 2018, 20, e12822. [Google Scholar] [CrossRef]
- Vimonpatranon, S.; Roytrakul, S.; Phaonakrop, N.; Lekmanee, K.; Atipimonpat, A.; Srimark, N.; Sukapirom, K.; Chotivanich, K.; Khowawisetsut, L.; Pattanapanyasat, K. Extracellular Vesicles Derived from Early and Late Stage Plasmodium falciparum-Infected Red Blood Cells Contain Invasion-Associated Proteins. J. Clin. Med. 2022, 11, 4250. [Google Scholar] [CrossRef]
- Aline, F.; Bout, D.; Amigorena, S.; Roingeard, P.; Dimier-Poisson, I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect. Immun. 2004, 72, 4127–4137. [Google Scholar] [CrossRef]
- Beauvillain, C.; Juste, M.O.; Dion, S.; Pierre, J.; Dimier-Poisson, I. Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine 2009, 27, 1750–1757. [Google Scholar] [CrossRef]
- Li, Y.; Xiu, F.; Mou, Z.; Xue, Z.; Du, H.; Zhou, C.; Li, Y.; Shi, Y.; He, S.; Zhou, H. Exosomes derived from Toxoplasma gondii stimulate an inflammatory response through JNK signaling pathway. Nanomedicine 2018, 13, 1157–1168. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Xiu, F.; Wang, J.; Cong, H.; He, S.; Shi, Y.; Wang, X.; Li, X.; Zhou, H. Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses. Int. J. Nanomed. 2018, 13, 467–477. [Google Scholar] [CrossRef]
- Maia, M.M.; da Cruz, A.B.; Taniwaki, N.N.; Namiyama, G.M.; Gava, R.; Gomes, A.H.S.; Kanamura, C.T.; Barbo, M.L.P.; Pereira-Chioccola, V.L. Immunization with extracellular vesicles excreted by Toxoplasma gondii confers protection in murine infection, activating cellular and humoral responses. Int. J. Parasitol. 2021, 51, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.O.; Maia, M.M.; Torrecilhas, A.C.; Taniwaki, N.N.; Namiyama, G.M.; Oliveira, K.C.; Ribeiro, K.S.; Toledo, M.D.S.; Xander, P.; Pereira-Chioccola, V.L. Extracellular vesicles isolated from Toxoplasma gondii induce host immune response. Parasite Immunol. 2018, 40, e12571. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Gong, A.-Y.; Roth, A.L.; Huang, B.Q.; Ward, H.D.; Zhu, G.; LaRusso, N.F.; Hanson, N.D.; Chen, X.-M. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013, 9, e1003261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Y.; Liu, H.; Yin, J.; Zhang, X.-T.; Gong, A.-Y.; Chen, X.; Chen, S.; Mathy, N.W.; Cao, J.; et al. Induction of Inflammatory Responses in Splenocytes by Exosomes Released from Intestinal Epithelial Cells following Cryptosporidium parvum Infection. Infect. Immun. 2019, 87, e00705-18. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Hu, D.; Zhang, L.; Tang, P. Role of extracellular vesicles in rheumatoid arthritis. Mol. Immunol. 2018, 93, 125–132. [Google Scholar] [CrossRef]
- de Miguel, N.; Lustig, G.; Twu, O.; Chattopadhyay, A.; Wohlschlegel, J.A.; Johnson, P.J. Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol. Cell. Proteom. 2010, 9, 1554–1566. [Google Scholar] [CrossRef]
- De Miguel, N.; Riestra, A.; Johnson, P.J. Reversible association of tetraspanin with Trichomonas vaginalis flagella upon adherence to host cells. Cell. Microbiol. 2012, 14, 1797–1807. [Google Scholar] [CrossRef]
- Salas, N.; Coceres, V.M.; Melo, T.D.S.; Pereira-Neves, A.; Maguire, V.G.; Rodriguez, T.M.; Sabatke, B.; Ramirez, M.I.; Sha, J.; Wohlschlegel, J.A.; et al. VPS32, a member of the ESCRT complex, modulates adherence to host cells in the parasite Trichomonas vaginalis by affecting biogenesis and cargo sorting of released extracellular vesicles. Cell Mol. Life Sci. 2021, 79, 11. [Google Scholar] [CrossRef]
- Rai, A.K.; Johnson, P.J. Trichomonas vaginalis extracellular vesicles are internalized by host cells using proteoglycans and caveolin-dependent endocytosis. Proc. Natl. Acad. Sci. USA 2019, 116, 21354–21360. [Google Scholar] [CrossRef]
- Govender, Y.; Chan, T.; Yamamoto, H.S.; Budnik, B.; Fichorova, R.N. The Role of Small Extracellular Vesicles in Viral-Protozoan Symbiosis: Lessons from Trichomonasvirus in an Isogenic Host Parasite Model. Front. Cell. Infect. Microbiol. 2020, 10, 591172. [Google Scholar] [CrossRef]
- Rada, P.; Hrdý, I.; Zdrha, A.; Narayanasamy, R.K.; Smutná, T.; Horáčková, J.; Harant, K.; Beneš, V.; Ong, S.-C.; Tsai, C.-Y.; et al. Double-Stranded RNA Viruses Are Released from Trichomonas vaginalis Inside Small Extracellular Vesicles and Modulate the Exosomal Cargo. Front. Microbiol. 2022, 13, 893692. [Google Scholar] [CrossRef] [PubMed]
- Atayde, V.D.; Hassani, K.; Filho, A.D.S.L.; Borges, A.R.; Adhikari, A.; Martel, C.; Olivier, M. Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cell. Immunol. 2016, 309, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Reiner, N.E. Leishmania exosomes deliver preemptive strikes to create an environment permissive for early infection. Front. Cell. Infect. Microbiol. 2011, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Colineau, L.; Clos, J.; Moon, K.-M.; Foster, L.J.; Reiner, N.E. Leishmania donovani chaperonin 10 regulates parasite internalization and intracellular survival in human macrophages. Med. Microbiol. Immunol. 2017, 206, 235–257. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.A.; Contreras, I.; Halle, M.; Tremblay, M.L.; McMaster, R.W.; Olivier, M. Leishmania GP63 alters host signaling through cleavage-activated protein tyrosine phosphatases. Sci. Signal. 2009, 2, ra58. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Bose, M.; Roy, S.; Bhattacharyya, S.N. Leishmania donovani targets Dicer1 to downregulate miR-122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe 2013, 13, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Bayer-Santos, E.; Aguilar-Bonavides, C.; Rodrigues, S.P.; Cordero, E.M.; Marques, A.F.; Varela-Ramirez, A.; Choi, H.; Yoshida, N.; da Silveira, J.F.; Almeida, I.C. Proteomic analysis of Trypanosoma cruzi secretome: Characterization of two populations of extracellular vesicles and soluble proteins. J. Proteome Res. 2013, 12, 883–897. [Google Scholar] [CrossRef]
- Osorio, L.; Ríos, I.; Gutiérrez, B.; González, J. Virulence factors of Trypanosoma cruzi: Who is who? Microbes Infect. 2012, 14, 1390–1402. [Google Scholar] [CrossRef]
- Lovo-Martins, M.I.; Malvezi, A.D.; Zanluqui, N.G.; Lucchetti, B.F.C.; Tatakihara, V.L.H.; Mörking, P.A.; de Oliveira, A.G.; Goldenberg, S.; Wowk, P.F.; Pinge-Filho, P. Extracellular Vesicles Shed By Trypanosoma cruzi Potentiate Infection and Elicit Lipid Body Formation and PGE2 Production in Murine Macrophages. Front. Immunol. 2018, 9, 896. [Google Scholar] [CrossRef]
- Cortes-Serra, N.; Mendes, M.T.; Mazagatos, C.; Segui-Barber, J.; Ellis, C.C.; Ballart, C.; Garcia-Alvarez, A.; Gállego, M.; Gascon, J.; Almeida, I.C.; et al. Plasma-Derived Extracellular Vesicles as Potential Biomarkers in Heart Transplant Patient with Chronic Chagas Disease. Emerg. Infect. Dis. 2020, 26, 1846–1851. [Google Scholar] [CrossRef]
- Madeira, R.P.; Meneghetti, P.; de Barros, L.A.; Buck, P.D.C.; Mady, C.; Ianni, B.M.; Fernandez-Becerra, C.; Torrecilhas, A.C. Isolation and molecular characterization of circulating extracellular vesicles from blood of chronic Chagas disease patients. Cell. Biol. Int. 2022, 46, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Nten, C.M.A.; Sommerer, N.; Rofidal, V.; Hirtz, C.; Rossignol, M.; Cuny, G.; Peltier, J.-B.; Geiger, A. Excreted/secreted proteins from trypanosome procyclic strains. J. Biomed. Biotechnol. 2010, 2010, 212817. [Google Scholar]
- Eliaz, D.; Kannan, S.; Shaked, H.; Arvatz, G.; Tkacz, I.D.; Binder, L.; Ben-Asher, H.W.; Okalang, U.; Chikne, V.; Cohen-Chalamish, S.; et al. Exosome secretion affects social motility in Trypanosoma brucei. PLoS Pathog. 2017, 13, e1006245. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.C.; Reyes-López, M.; Serrano-Luna, J.; Shibayama, M.; Unzueta, J.; León-Sicairos, N.; de la Garza, M. Intestinal amoebiasis: 160 years of its first detection and still remains as a health problem in developing countries. Int. J. Med. Microbiol. 2020, 310, 151358. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.M.; Franklin, B.S.; Teixeira-Carvalho, A.; Filho, A.L.; de Paula, S.C.; Fontes, C.J.; Brito, C.F.; Carvalho, L.H. Augmented plasma microparticles during acute Plasmodium vivax infection. Malar J. 2010, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Nantakomol, D.; Dondorp, A.M.; Krudsood, S.; Udomsangpetch, R.; Pattanapanyasat, K.; Combes, V.; Grau, G.E.; White, N.J.; Viriyavejakul, P.; Day, N.P.; et al. Circulating red cell-derived microparticles in human malaria. J. Infect. Dis. 2011, 203, 700–706. [Google Scholar] [CrossRef]
- Mfonkeu, J.B.P.; Gouado, I.; Kuaté, H.F.; Zambou, O.; Zollo, P.H.A.; Grau, G.E.R.; Combes, V. Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS ONE 2010, 5, e13415. [Google Scholar]
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007, 110, 3234–3244. [Google Scholar] [CrossRef]
- Pope, S.M.; Lässer, C. Toxoplasma gondii infection of fibroblasts causes the production of exosome-like vesicles containing a unique array of mRNA and miRNA transcripts compared to serum starvation. J. Extracell. Vesicles 2013, 2, 22484. [Google Scholar] [CrossRef]
- Ryan, U.; Zahedi, A.; Paparini, A. Cryptosporidium in humans and animals-a one health approach to prophylaxis. Parasite Immunol. 2016, 38, 535–547. [Google Scholar] [CrossRef]
- Chin, A.C.; Teoh, D.A.; Scott, K.G.; Meddings, J.B.; Macnaughton, W.K.; Buret, A.G. Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect. Immun. 2002, 70, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.; Hardin, J.A.; Olson, M.E.; Gall, D.G. Pathophysiology of small intestinal malabsorption in gerbils infected with Giardia lamblia. Gastroenterology 1992, 103, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.G.; Logan, M.R.; Klammer, G.M.; Teoh, D.A.; Buret, A.G. Jejunal brush border microvillous alterations in Giardia muris-infected mice: Role of T lymphocytes and interleukin. Infect. Immun. 2000, 68, 3412–3418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scott, K.G.-E.; Yu, L.C.H.; Buret, A.G. Role of CD8+ and CD4+ T lymphocytes in jejunal mucosal injury during murine giardiasis. Infect. Immun. 2004, 72, 3536–3542. [Google Scholar] [CrossRef]
- Konrad, C.; Spycher, C.; Hehl, A.B. Selective condensation drives partitioning and sequential secretion of cyst wall proteins in differentiating Giardia lamblia. PLoS Pathog. 2010, 6, e1000835. [Google Scholar] [CrossRef]
- Wampfler, P.B.; Tosevski, V.; Nanni, P.; Spycher, C.; Hehl, A.B. Proteomics of secretory and endocytic organelles in Giardia lamblia. PLoS ONE 2014, 9, e94089. [Google Scholar] [CrossRef]
- Evans-Osses, I.; Reichembach, L.H.; Ramirez, M.I. Exosomes or microvesicles? Two kinds of extracellular vesicles with different routes to modify protozoan-host cell interaction. Parasitol. Res. 2015, 114, 3567–3575. [Google Scholar] [CrossRef]
- Barteneva, N.S.; Maltsev, N.; Vorobjev, I.A. Microvesicles and intercellular communication in the context of parasitism. Front. Cell. Infect. Microbiol. 2013, 3, 49. [Google Scholar] [CrossRef]
- Evans-Osses, I.; Mojoli, A.; Monguió-Tortajada, M.; Marcilla, A.; Aran, V.; Amorim, M.; Inal, J.; Borràs, F.E.; Ramirez, M.I. Microvesicles released from Giardia intestinalis disturb host-pathogen response in vitro. Eur. J. Cell Biol. 2017, 96, 131–142. [Google Scholar] [CrossRef]
- Gavinho, B.; Sabatke, B.; Feijoli, V.; Rossi, I.V.; Da Silva, J.M.; Evans-Osses, I.; Palmisano, G.; Lange, S.; Ramirez, M.I. Characterization of the Giardia intestinalis secretome during interaction with human intestinal epithelial cells: The impact on host cells. PLoS Negl. Trop. Dis. 2017, 11, e0006120. [Google Scholar]
- Gavinho, B.; Sabatke, B.; Feijoli, V.; Rossi, I.V.; Da Silva, J.M.; Evans-Osses, I.; Palmisano, G.; Lange, S.; Ramirez, M.I. Peptidylarginine Deiminase Inhibition Abolishes the Production of Large Extracellular Vesicles from Giardia intestinalis, Affecting Host-Pathogen Interactions by Hindering Adhesion to Host Cells. Front. Cell. Infect. Microbiol. 2020, 10, 417. [Google Scholar] [CrossRef]
- Siddiq, A. The Role of Extracellular Vesicles in Giardia Microbiota Interactions. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2021. Available online: http://hdl.handle.net/1880/113521 (accessed on 5 October 2022).
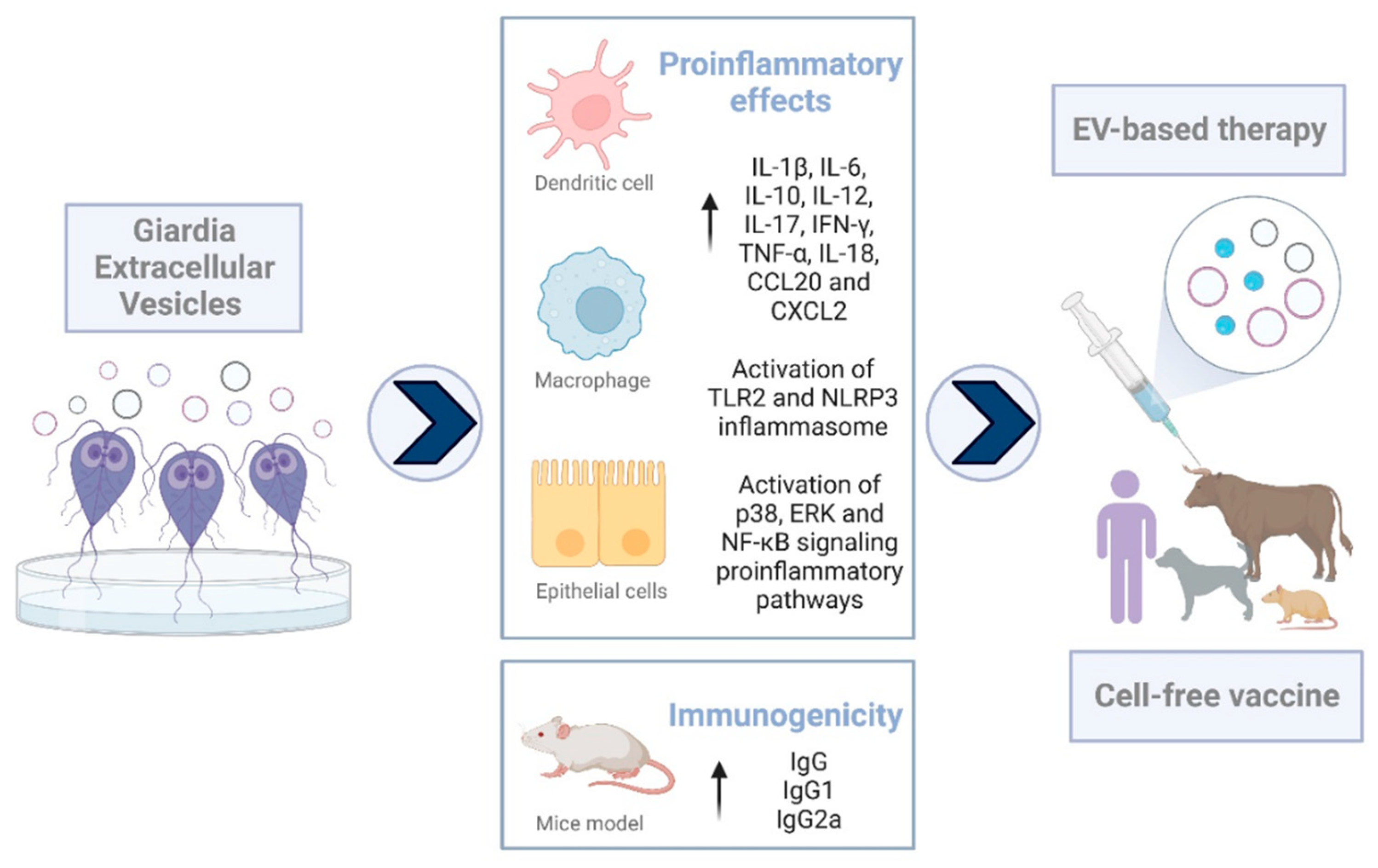
- Zhao, P.; Cao, L.; Wang, X.; Dong, J.; Zhang, N.; Li, X.; Li, J.; Zhang, X.; Gong, P. Extracellular vesicles secreted by Giardia duodenalis regulate host cell innate immunity via TLR2 and NLRP3 inflammasome signaling pathways. PLoS Negl. Trop. Dis. 2021, 15, e0009304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cao, L.; Wang, X.; Li, J.; Dong, J.; Zhang, N.; Li, X.; Li, S.; Sun, M.; Zhang, X.; et al. Giardia duodenalis extracellular vesicles regulate the proinflammatory immune response in mouse macrophages in vitro via the MAPK, AKT and NF-κB pathways. Parasit. Vectors 2021, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, Y.J.; Back, S.O.; Cho, S.H.; Lee, H.I.; Lee, M.R. Treatment with Extracellular Vesicles from Giardia lamblia Alleviates Dextran Sulfate Sodium-Induced Colitis in C57BL/6 Mice. Korean J. Parasitol. 2022, 60, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Grajeda, B.I.; De Chatterjee, A.; Villalobos, C.M.; Pence, B.C.; Ellis, C.C.; Enriquez, V.; Roy, S.; Roychowdhury, S.; Neumann, A.K.; Almeida, I.C.; et al. Giardial lipid rafts share virulence factors with secreted vesicles and participate in parasitic infection in mice. Front. Cell. Infect. Microbiol. 2022, 12, 974200. [Google Scholar] [CrossRef] [PubMed]
- Moyano, S.; Musso, J.; Feliziani, C.; Zamponi, N.; Frontera, L.S.; Ropolo, A.S.; Lanfredi-Rangel, A.; Lalle, M.; Touz, M.C. Exosome Biogenesis in the Protozoa Parasite Giardia lamblia: A Model of Reduced Interorganellar Crosstalk. Cells 2019, 8, 1600. [Google Scholar] [CrossRef]
- Serradell, M.C.; Saura, A.; Rupil, L.L.; Gargantini, P.R.; Faya, M.I.; Furlan, P.J.; Lujan, H.D. Vaccination of domestic animals with a novel oral vaccine prevents Giardia infections, alleviates signs of giardiasis and reduces transmission to humans. NPJ Vaccines 2016, 1, 16018. [Google Scholar] [CrossRef]
- Jiménez, J.C.; Fontaine, J.; Creusy, C.; Fleurisse, L.; Grzych, J.-M.; Capron, M.; Dei-Cas, E. Antibody and cytokine responses to Giardia excretory/secretory proteins in Giardia intestinalis-infected BALB/c mice. Parasitol. Res. 2014, 113, 2709–2718. [Google Scholar] [CrossRef]
- Jenikova, G.; Hruz, P.; Andersson, M.K.; Tejman-Yarden, N.; Ferreira, P.C.; Andersen, Y.S.; Davids, B.J.; Gillin, F.D.; Svärd, S.G.; Curtiss, R.; et al. A1-giardin based live heterologous vaccine protects against Giardia lamblia infection in a murine model. Vaccine 2011, 29, 9529–9537. [Google Scholar] [CrossRef]
- Davids, B.J.; Liu, C.M.; Hanson, E.M.; Le, C.H.Y.; Ang, J.; Hanevik, K.; Fischer, M.; Radunovic, M.; Langeland, N.; Ferella, M.; et al. Identification of Conserved Candidate Vaccine Antigens in the Surface Proteome of Giardia lamblia. Infect. Immun. 2019, 87, e00219-19. [Google Scholar] [CrossRef]
- Lanfredi-Rangel, A.; Attias, M.; Reiner, D.S.; Gillin, F.D.; De Souza, W. Fine structure of the biogenesis of Giardia lamblia encystation secretory vesicles. J. Struct. Biol. 2003, 143, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Romero, G.; Quintero, J.; Astiazaran-Garcia, H.; Velazquez, C. Host defences against Giardia lamblia. Parasite Immunol. 2015, 37, 394–406. [Google Scholar] [CrossRef]
- Dubourg, A.; Xia, D.; Winpenny, J.P.; Al Naimi, S.; Bouzid, M.; Sexton, D.; Wastling, J.M.; Hunter, P.; Tyler, K.M. Giardia secretome highlights secreted tenascins as a key component of pathogenesis. Gigascience 2018, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma’ayeh, S.; Peirasmaki, D.; Lundström-Stadelmann, B.; Hellman, L.; Svärd, S.G. Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines. Virulence 2018, 9, 879–894. [Google Scholar] [CrossRef]
- Faria, C.P.; Lourenço, A.; Neves, B.M.; Girão, H.; Rodrigues, T.R.; Cruz, M.T.; Sousa, M.C. Unveiling the role of extracelullar vesicles secreted by Giardia lamblia on innate immune response. In Proceedings of the First Meeting of the Portuguese Network on Extracellular Vesicles (PNEV) Abstract Congress, Porto, Portugal, 13–14 September 2021. [Google Scholar]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Tandoh, K.Z.; Hagan, O.C.; Wilson, M.D.; Quashie, N.B.; Duah-Quashie, N.O. Transcriptome-module phenotype association study implicates extracellular vesicles biogenesis in Plasmodium falciparum artemisinin resistance. Front. Cell. Infect. Microbiol. 2022, 12, 886728. [Google Scholar] [CrossRef] [PubMed]
| Disease | EVs’ Source | Type | Actions |
|---|---|---|---|
| Phylum Sarcomastigophora | |||
| Trichomoniasis | Trophozoite of T. vaginalis | Exosomes | Modulate host immune response and increase parasite attachment [34] |
| Microvesicle-like structures and large vesicles | Modulate cell interactions [35] | ||
| Exosome like vesicles | Increase IL-10, IL-6, and TNF- α secretion in mouse macrophages; Increase IL-10 and decrease of IL-17 production in infected murine model [36] | ||
| Leishmaniasis | Promastigotes of L. donovani, L. major, and L. mexicana | Exosomes | Delivery of proteins in mouse macrophages and increase of IL-8 [37] |
| Promastigotes of L. amazonensis | Extracellular vesicles | Stimulate bone-marrow-derived macrophages and peritoneal B-1 cells [38] | |
| Promastigotes of L. amazonensis, L. braziliensis, and L. infantum | Extracellular vesicles | Activation of macrophage immunomodulatory response (only EVs of L. amazonensis) [39] | |
| Promastigotes of L. donovani | Exosomes | Immunosuppressive effect [40] | |
| L. amazonensis-infected mouse macrophages | Extracellular vesicles | Increase in IL-12, IL-1β, and TNF-α [41] | |
| Long-term and in-vivo-derived promastigotes of L.amazonensis | Extracellular vesicles | Partial protective effect in an immunization model [42] | |
| Lavages of midgut of sandflies-infected L. infantum and L. major | Exosomes | Participation in pathogenesis and modulate monocyte cytokine response [43] | |
| Chagas Disease | Trypomastigotes | Extracellular vesicles | Increase tissue parasitism and inflammation; Increase in IL-10 and IL-4 secretion [44] |
| Extracellular vesicles | Promotes metacyclogenesis [45] | ||
| Extracellular vesicles | Activation of macrophages Production of TNF-α by dendritic cells (mice) [46] | ||
| Infected human macrophages | Extracellular vesicles | Interact with TLR2 and stimulate the translocation of NF-κB [47] | |
| Plasma of chronic patients | Extracellular vesicles | Increase in IFN-y and decrease in IL-17 production [48] | |
| African Trypanosomiasis | Trypomastigotes | Extracellular vesicles | Mediate transfer of virulence factor and induce anemia [49] |
| Extracellular vesicles | Induce differentiation of M1- and M2- macrophages; Generation of MHCI+, MHCII+ and MHCI+, MHCII+ macrophages; Expression of CD3 and nuclear factor FoxP3 [50] | ||
| Amebiasis | Encysting parasites | Extracellular vesicles | Promoted encystation [51] |
| E. histolytica trophozoites | Extracellular vesicles | Immunomodulatory effects on macrophages [52] | |
| Phylum Apicomplexa | |||
| Malaria | P. yoelii-infected reticulocytes | Exosomes | Induce immune protection [53] |
| P. falciparum-infected erythrocyte | Exosomes-like vesicles | Delivery of genes [54] | |
| Microvesicles | Immunostimulatory and act as messengers between iRBCs [55] | ||
| Extracellular vesicles | Alter vascular function [56] | ||
| Extracellular vesicles | Modulate monocyte cytokine response [57] | ||
| Extracellular vesicles | Decresase of parasite invasion [58] | ||
| Toxoplasmosis | Dendritic-cell-derived pulsed ex vivo with T. gondii antigens | Exosomes | Induce immune protection [59] |
| Splenic dendritic cell pulsed in vitro with T. gondii-derived antigen | Exosomes | Induce immune protection [60] | |
| Tachyzoites | Exosomes/Microvesicles | Modulate macrophage activation; induce immune protection [61,62,63] | |
| Extracellular vesicles | Induce IL-10, TNF-α, and iNOS secretion [64] | ||
| Cryptosporidiosis | C. parvum-infected epithelial cells | Exosomes | Anti-parasitic activity [65] |
| C. parvum-infected epithelial cells | Exosomes | Activate immune cells [66] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, B.; Lourenço, Á.; Sousa, M.d.C. Protozoa-Derived Extracellular Vesicles on Intercellular Communication with Special Emphasis on Giardia lamblia. Microorganisms 2022, 10, 2422. https://doi.org/10.3390/microorganisms10122422
Ferreira B, Lourenço Á, Sousa MdC. Protozoa-Derived Extracellular Vesicles on Intercellular Communication with Special Emphasis on Giardia lamblia. Microorganisms. 2022; 10(12):2422. https://doi.org/10.3390/microorganisms10122422
Chicago/Turabian StyleFerreira, Bárbara, Ágata Lourenço, and Maria do Céu Sousa. 2022. "Protozoa-Derived Extracellular Vesicles on Intercellular Communication with Special Emphasis on Giardia lamblia" Microorganisms 10, no. 12: 2422. https://doi.org/10.3390/microorganisms10122422
APA StyleFerreira, B., Lourenço, Á., & Sousa, M. d. C. (2022). Protozoa-Derived Extracellular Vesicles on Intercellular Communication with Special Emphasis on Giardia lamblia. Microorganisms, 10(12), 2422. https://doi.org/10.3390/microorganisms10122422





