Prebiotic Functions of Konjac Root Powder in Chocolate Milk Enriched with Free and Encapsulated Lactic Acid Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Culture Activation
2.2.2. Encapsulation
2.2.3. Preparation of Chocolate Milk Samples
2.2.4. Analyses of Physio-Chemical and Microbial Characteristics of the Prepared Chocolate Milk Samples
pH
Viscosity
Titratable Acidity
LAB Bacteria Count
Statistical Analysis
3. Results
3.1. Effect of Added KRP on the Physio-Chemical Properties Chocolate Milk
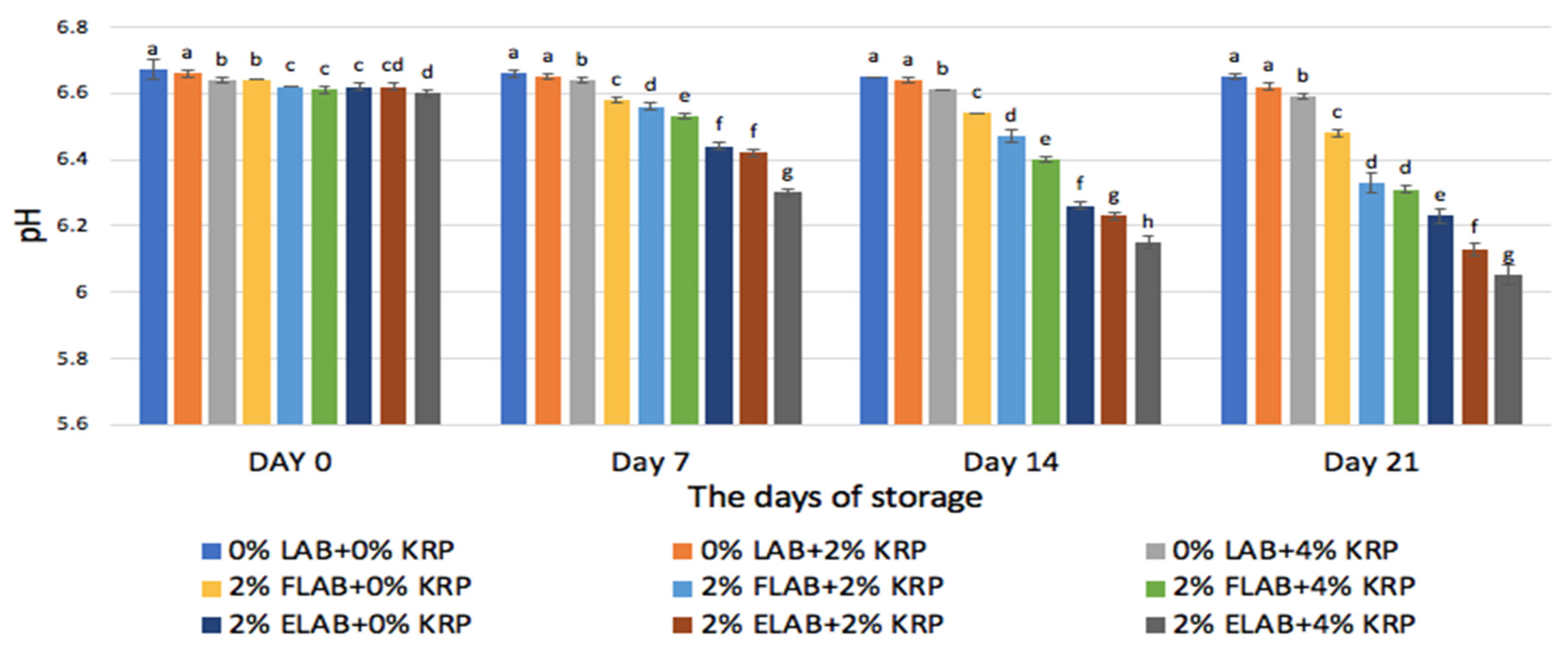
3.1.1. pH
3.1.2. Titratable Acidity
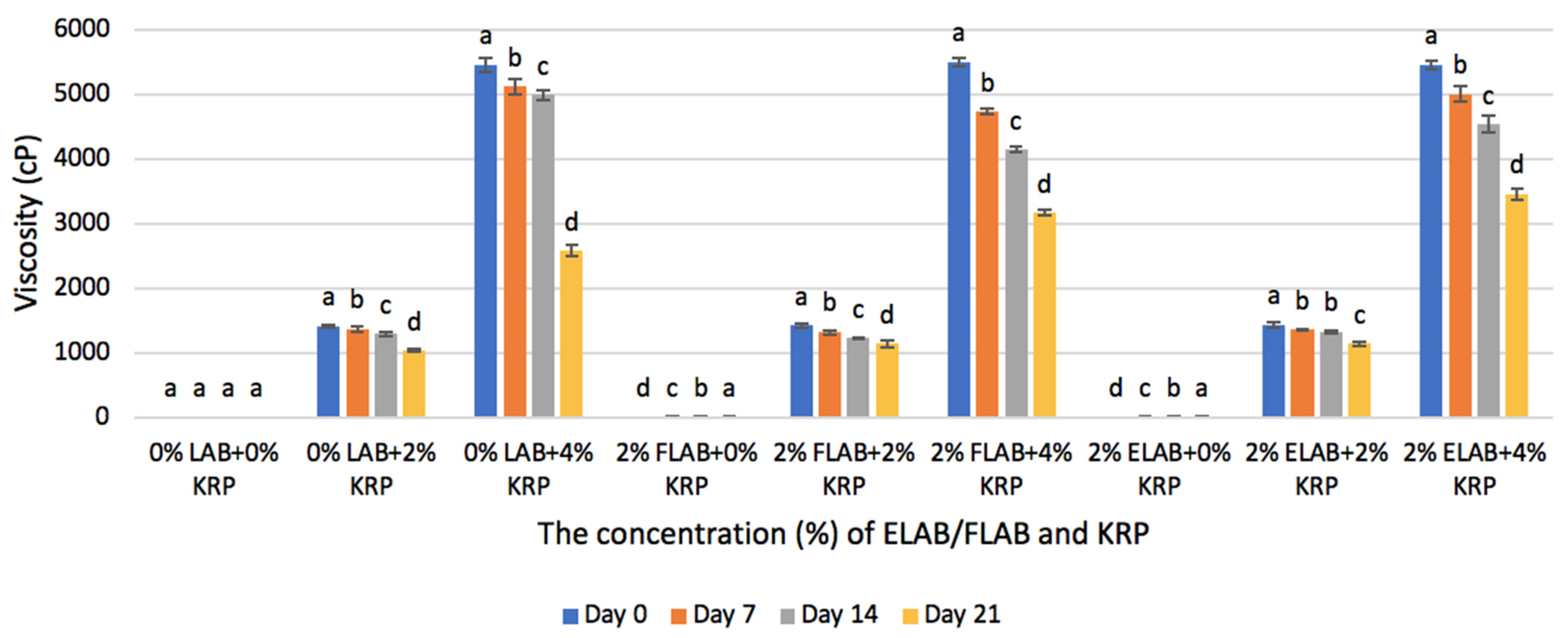
3.1.3. Viscosity
3.2. Effect of Added KRP on LAB Counts in Chocolate Milk
4. Discussion
4.1. Effect of Added KRP on the Physio-Chemical Properties Chocolate Milk
4.1.1. pH
4.1.2. Titratable Acidity
4.1.3. Viscosity
4.2. Effect of Added KRP on LAB Counts in Chocolate Milk
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turkmen, N.; Akal, C.; Ozer, B. Probiotic dairy-based beverages: A review. J. Funct. Foods 2019, 53, 62–75. [Google Scholar] [CrossRef]
- Brown, A.C.; Valiere, A. Probiotics and medical nutrition therapy. Nutr. Clin. Care 2004, 7, 56–68. [Google Scholar] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles, V.; Fang, Y.; Brent, D.P. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 2008, 14, 1585–1596. [Google Scholar]
- Girardin, M.; Seidman, E.G. Indications for the use of probiotics in gastrointestinal diseases. Dig. Dis. 2011, 29, 574–587. [Google Scholar] [CrossRef]
- Karamese, M.; Aydin, H.; Sengul, E.; Gelen, V.; Sevim, C.; Ustek, D.; Karakus, E. The Immunostimulatory Effect of Lactic Acid Bacteria in a Rat Model. Iran. J. Immunol. 2016, 13, 220–228. [Google Scholar]
- Rosa, C.M.; Carmo, R.S.M.; Balthazar, F.C.; Guimarães, T.J.; Esmerino, A.E.; Freitas, Q.M.; Silva, C.M.; Pimentel, C.T.; Cruz, G.A. Dairy products with prebiotics: An overview of the health benefits, technological and sensory properties. Int. Dairy J. 2021, 117, 105009. [Google Scholar] [CrossRef]
- Gobbetti, M.; di Cagno, R.; de Angelis, M. Functional microorganisms for functional food quality. Crit. Rev. Food Sci. Nutr. 2010, 50, 716–727. [Google Scholar] [CrossRef]
- Hajfathalian, M.; Ghelichi, S.; Garcia-Moreno, P.J.; Sorensen, A.D.M.; Jacobsen, C. Peptides: Production, bioactivity, functionality, and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 3097–3129. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, M.B. What is beneficial for health? The concept of functional food. Food Chem. Toxicol. 1999, 37, 1039–1041. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Castaneda, P.C.; Garcia-Gonzalez, A.; Bencomo-Alvarez, A.E.; Barros-Nunez, P.; Gaxiola-Robles, R.; Mendez-Rodriguez, L.C.; Zenteno-Savin, T. Micronutrient content and antioxidant enzyme activities in human breast milk. J. Trace Elem. Med. Biol. 2019, 51, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Savoini, G.; Farina, G.; Dell’Orto, V.; Cattaneo, D. Through ruminant nutrition to human health: Role of fatty acids. Adv. Anim. Biosci. 2016, 7, 200–207. [Google Scholar] [CrossRef]
- Reshma, B.; Nambiar, S.; Sellamuthu, P.; Bbabu Perumal, A. Development of milk chocolate supplemented with microencapsulated Lactobacillus plantarum HM47 and to determine the safety in a Swiss albino mice model. Food Control 2018, 94, 300–306. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Presctt, L.S.; Reimer, A.R.; Salmine, J.S.; Scott, K.; Stanton, C.; Swanson, S.K.; Cani, D.P.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Do, G.Y.; Kwon, E.-Y.; Kim, Y.J.; Han, Y.; Kim, S.-B.; Kim, Y.H.; Choi, M.S. Supplementation of non-dairy creamer-enriched high-fat diet with D-allulose ameliorated blood glucose and body fat accumulation in C57BL/6J mice. Appl. Sci. 2019, 9, 2750. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta glucan: A valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Budi, D.S.; Munawar, B.S. Forest community empowerment through the increasing role of productive crop-based smis around forest: A study on porgan plants in east Java, Indonesia. Russ. J. Agric. Socio-Econ. Sci. 2018, 84, 260–274. [Google Scholar] [CrossRef]
- Shang, W.; Li, H.; Strappe, P.; Zhou, Z.; Blanchard, C. Konjac glucomannans attenuate diet-induced fat accumulation on livers and its regulation pathway. J. Funct. Foods 2019, 52, 258–265. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, J.; Zhang, Y.; Men, Y.; Zhang, B.; Sun, Y. Prebiotic, immunomodulating, and antifatigue effects of konjac oligosaccharide. J. Food Sci. 2018, 83, 3110–3117. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, Y.; Tu, K. Effect of konjac glucomannan coating on antioxidant capacity and phenolic metabolism in fresh-cut lotus roots. J. Food Process. Preserv. 2018, 42, e13759. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, S.; Cruz, G.A.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Brookfield Dial Viscometer. Available online: https://www.brookfieldengineering.com/-/media/ametekbrookfield/manuals/obsolete%20manuals/dial%20m85-150-p700.pdf?la=en (accessed on 8 July 2019).
- Chuah, L.; Shamila-Syuhada, A.K.; Liong, M.T.; Rosma, A.; Kwai Lin Thong, K.L.; Rusul, G. Physio-chemical, microbiological properties of tempoyak and molecular characterisation of lactic acid bacteria isolated from tempoyak. Food Microbiol. 2016, 58, 95–104. [Google Scholar] [CrossRef]
- James, W. Spot Plating Assay for the Determination of Survival and Plating Efficiency of Escherichia coli in sub-MIC Levels of Antibiotics. Department of Microbiology and Immunology, University of British Columbia. Available online: https://jemi.microbiology.ubc.ca/sites/default/files/Wang%20et%20al%20JEMI-methods%20Vol%201%20pg%2026-29_0.pdf (accessed on 5 July 2019).
- Daneshi, M.; Ehsani, M.R.; Razavi, S.H.; Labbafi, M. Effect of refrigerated storage on the probiotic survival and sensory properties of milk/carrot juice mix drink. Electron. J. Biotechnol. 2013, 16, 5. [Google Scholar] [CrossRef]
- Valencia, M.S.; Salgado, S.M.; Andrade, S.A.C.; Padilha, V.M.; Livera, A.V.S.; Stamford, T.L.M. Development of creamy milk chocolate dessert added with fructo-oligosaccharide and Lactobacillus paracasei subsp. paracasei LBC 81. LWT 2016, 69, 104–109. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Schillinger, U. Introduction to pre- and probiotics. Food Res. Int. 2002, 35, 109–116. [Google Scholar] [CrossRef]
- Rajaie Azarkhavarani, P.; Ziaee, E.; Hashem Hosseini, S.M. Effect of encapsulation on the stability and survivability of Enterococcus faecium in a non-dairy probiotic beverage. Food Sci. Technol. Int. 2019, 25, 233–242. [Google Scholar] [CrossRef]
- Yoshimura, M.; Nishinari, K. Dynamic viscoelastic study on the gelation of konjac glucomannan with different molecular weights. Food Hydrocoll. 1999, 13, 227–233. [Google Scholar] [CrossRef]
- Rong, S.; Shasha, T.; Bin, L.; Ling, W. Effects of konjac oligosaccharides on yogurt quality and population of lactic acid bacteria during storage. Food Ferment. Ind. 2019, 45, 93–97. [Google Scholar]
- Tobin, J.T.; Fitzsimons, S.M.; Kelly, A.L.; Fenelon, M.A. The effect of native and modified konjac on the physical attributes of pasteurized and UHT-treated skim milk. Int. Dairy J. 2011, 21, 790–797. [Google Scholar] [CrossRef]
- Chenlo, F.; Moreira, R.; Silva, C. Rheological behaviour of aqueous systems of tragacanth and guar gums with storage time. J. Food Eng. 2010, 96, 107–113. [Google Scholar] [CrossRef]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ghazzewi, F.H.; Khanna, S.; Tester, R.F.; Piggott, J. The potential use of hydrolysed konjac glucomannan as a prebiotic. J. Sci. Food Agric. 2007, 87, 1758–1766. [Google Scholar] [CrossRef]
- Crispin-Isidro, G.; Lobato-Calleros, C.; Espinosa-Andrews, H.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of inulin and agave fructans addition on the rheological, microstructural and sensory properties of reduced-fat stirred yogurt. LWT 2015, 62 Pt 2, 438–444. [Google Scholar] [CrossRef]
| % of Added LAB | Type of Added LAB | Amounts of Added KRP (%) | Storage Time (Days) | |||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | |||
| 0 | N/A | 0 | ||||
| 2 | ||||||
| 4 | ||||||
| 2 | FLAB | 0 | ||||
| 2 | ||||||
| 4 | ||||||
| ELAB | 0 | |||||
| 2 | ||||||
| 4 | ||||||
| Amounts of Added LAB (%) | Type of Added LAB | Amounts of Added KRP (%) | Storage Time (Days) | |||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | |||
| 0 | None (Control) | 0 | <100 | <100 | <100 | <100 |
| 2 | <100 | <100 | <100 | <100 | ||
| 4 | <100 | <100 | <100 | <100 | ||
| 2 | FLAB | 0 | 5.20 | 5.12 | 5.01 | 4.73 |
| 2 | 5.19 | 5.11 | 5.05 | 4.82 | ||
| 4 | 5.19 | 5.15 | 5.11 | 4.91 | ||
| ELAB | 0 | 5.19 | 5.05 | 4.97 | 3.85 | |
| 2 | 5.18 | 5.09 | 5.02 | 4.91 | ||
| 4 | 5.17 | 5.09 | 5.06 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajlouni, S.; Hossain, M.N.; Tang, Z. Prebiotic Functions of Konjac Root Powder in Chocolate Milk Enriched with Free and Encapsulated Lactic Acid Bacteria. Microorganisms 2022, 10, 2433. https://doi.org/10.3390/microorganisms10122433
Ajlouni S, Hossain MN, Tang Z. Prebiotic Functions of Konjac Root Powder in Chocolate Milk Enriched with Free and Encapsulated Lactic Acid Bacteria. Microorganisms. 2022; 10(12):2433. https://doi.org/10.3390/microorganisms10122433
Chicago/Turabian StyleAjlouni, Said, Md. Nur Hossain, and Ziqian Tang. 2022. "Prebiotic Functions of Konjac Root Powder in Chocolate Milk Enriched with Free and Encapsulated Lactic Acid Bacteria" Microorganisms 10, no. 12: 2433. https://doi.org/10.3390/microorganisms10122433





