Characterization of Human Papillomavirus 16 from Kinshasa (Democratic Republic of the Congo)—Implications for Pathogenicity and Vaccine Effectiveness
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Collection
2.3. HPV Molecular Detection
2.4. DNA Extraction for HPV16 Characterizatin
2.5. Primers Design and HPV16 Amplification
2.6. Sequencing and Interpretation
2.7. Ethics
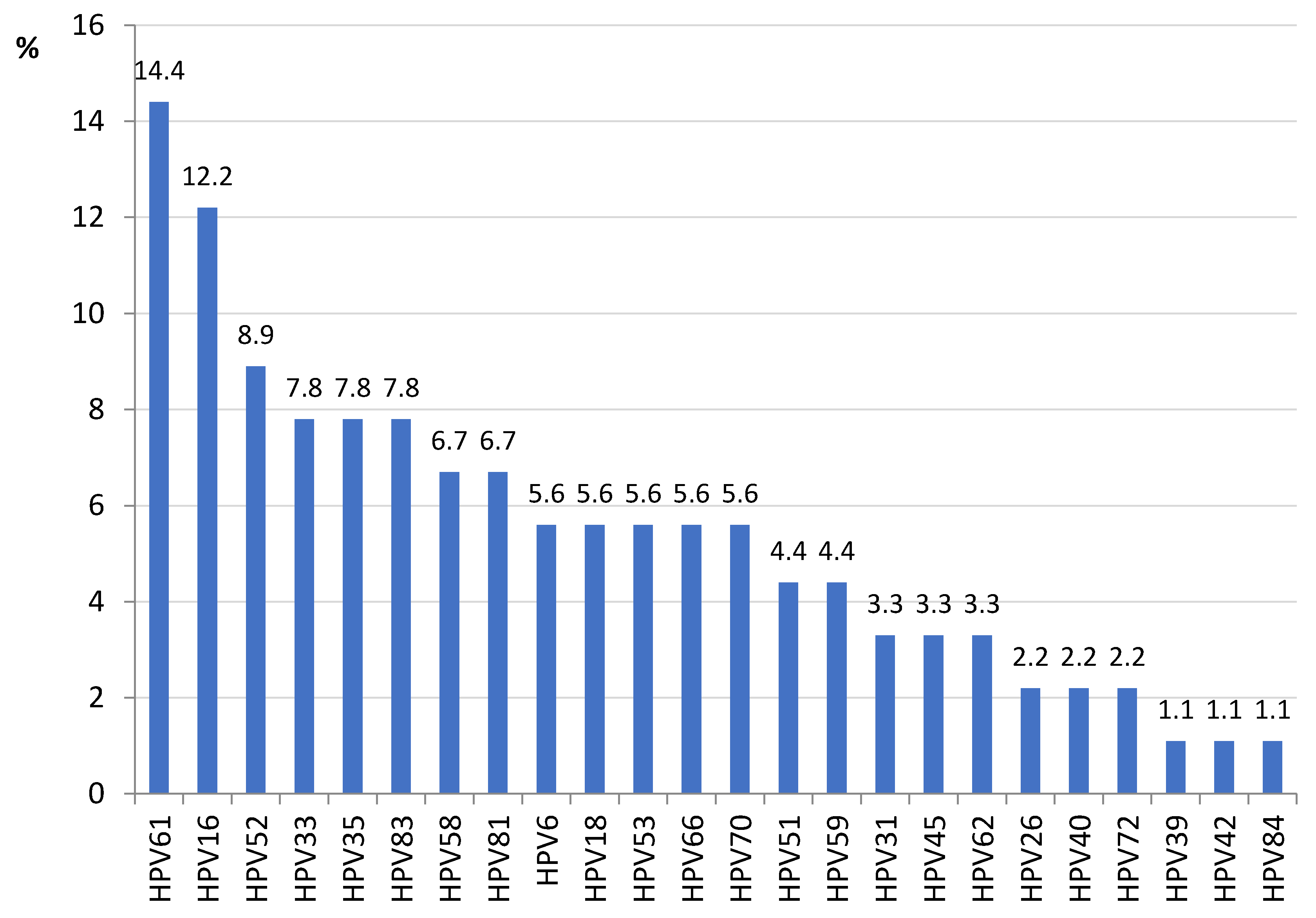
3. Results
3.1. HPV16 Lineage Distribution
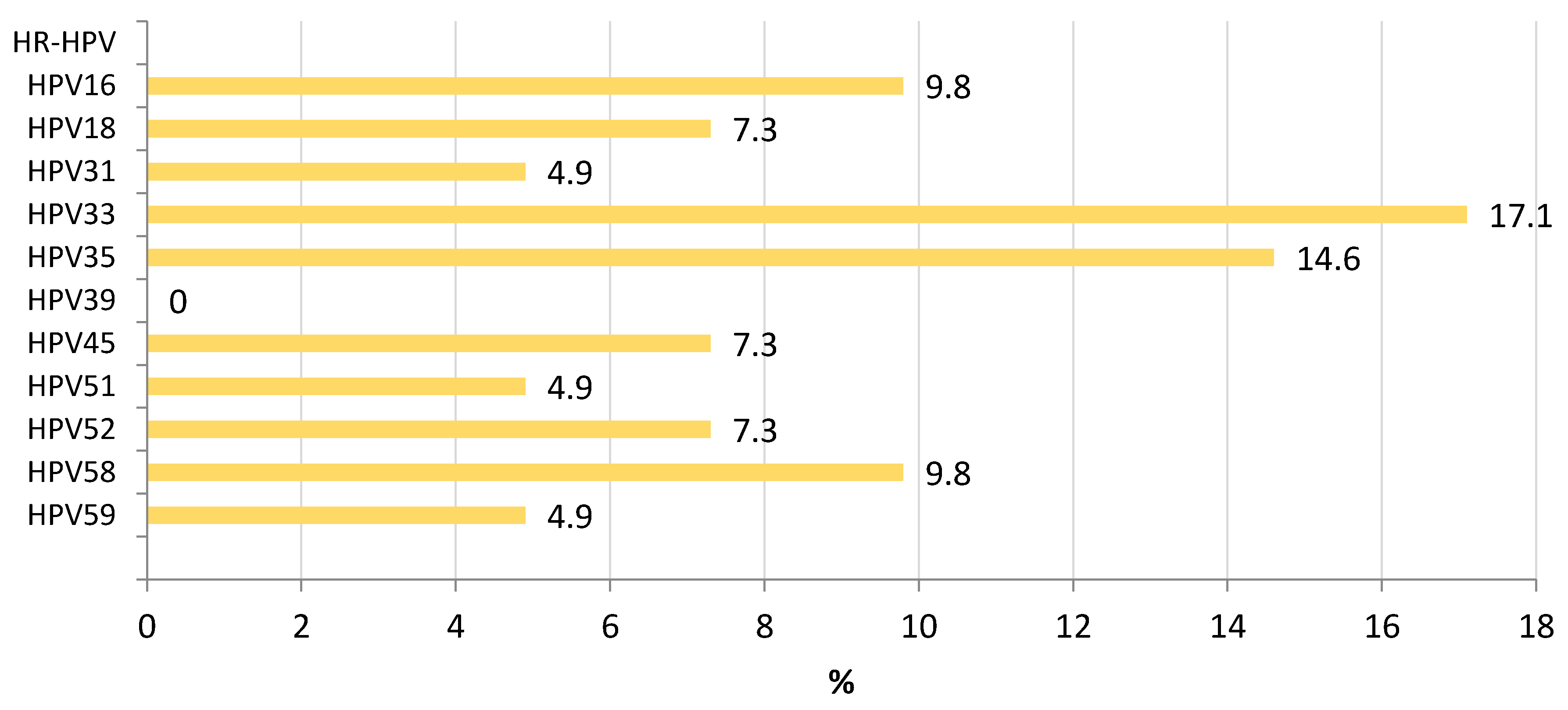
3.2. L1 Genomic Polymorphisms
3.3. LCR Genomic Polymorphisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chelimo, C.; Wouldes, T.A.; Cameron, L.D.; Elwood, J.M. Risk Factors for and Prevention of Human Papillomaviruses (HPV), Genital Warts and Cervical Cancer. J. Infect. 2013, 66, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Cachay, E.; Ocampo, A.; Poveda, E. Update on the Epidemiological Features and Clinical Implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) Coinfection. Microorganisms 2022, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Zayats, R.; Murooka, T.T.; McKinnon, L.R. HPV and the Risk of HIV Acquisition in Women. Front. Cell. Infect. Microbiol. 2022, 12, 814948. [Google Scholar] [CrossRef] [PubMed]
- CDC Genital HPV Infection—Basic Fact Sheet. Available online: https://www.cdc.gov/std/hpv/stdfact-hpv.htm (accessed on 22 November 2022).
- Lorenzoni, V.; Chaturvedi, A.K.; Vignat, J.; Laversanne, M.; Bray, F.; Vaccarella, S. The Current Burden of Oropharyngeal Cancer: A Global Assessment Based on GLOBOCAN 2020. Cancer Epidemiol. Biomark. Prev. 2022, 31, 2054–2062. [Google Scholar] [CrossRef]
- Mix, J.M.; Gopalani, S.V.; Simko, S.; Saraiya, M. Trends in HPV- and Non-HPV-Associated Vulvar Cancer Incidence, United States, 2001–2017. Prev. Med. 2022, 164, 107302. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Bosi, S.; Bassi, C.; Ferracin, M.; Lanza, G.; Gafà, R.; Magri, E.; Selvatici, R.; Torresani, S.; Marci, R.; et al. Gene Expression Changes in Progression of Cervical Neoplasia Revealed by Microarray Analysis of Cervical Neoplastic Keratinocytes. J. Cell. Physiol. 2015, 230, 806–812. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, H.; Tian, M.; Wang, D.; He, J.; Xu, T. DNA Methylation and Hydroxymethylation in Cervical Cancer: Diagnosis, Prognosis and Treatment. Front. Genet. 2020, 11, 347. [Google Scholar] [CrossRef]
- Ghissassi, F.E.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. Special Report: Policy A Review of Human Carcinogens—Part B: Biological Agents. Lancet Oncol. 2009, 10, 751–752. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, W.; Dong, X.; Liu, Y.; Tan, X. Infection Status and Survival Impact of High-Risk Human Papillomavirus in Cervical Adenocarcinomas: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2022, 167, 129–136. [Google Scholar] [CrossRef]
- Cancer Research UK. Cervical Cancer. Risks and Causes. Available online: https://www.cancerresearchuk.org/about-cancer/cervical-cancer/risks-causes (accessed on 22 November 2022).
- International Agency for Research on Cancer (IARC). IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 22 November 2022).
- Mulongo, M.; Chibwesha, C.J. Prevention of Cervical Cancer in Low-Resource African Settings. Obstet. Gynecol. Clin. N. Am. 2022, 49, 771–781. [Google Scholar] [CrossRef]
- Huang, J.; Deng, Y.; Boakye, D.; Tin, M.S.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Global Distribution, Risk Factors, and Recent Trends for Cervical Cancer: A Worldwide Country-Level Analysis. Gynecol. Oncol. 2022, 164, 85–92. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical Human Papillomavirus Prevalence in 5 Continents: Meta-Analysis of 1 Million Women with Normal Cytological Findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.; Gómez, D.; Muñoz, J.; Bosch, F.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 22 October 2021. Available online: www.hpvcentre.com (accessed on 30 October 2022).
- Mutombo, A.B.; Benoy, I.; Tozin, R.; Bogers, J.; Van geertruyden, J.-P.; Jacquemyn, Y. Prevalence and Distribution of Human Papillomavirus Genotypes Among Women in Kinshasa, The Democratic Republic of the Congo. J. Glob. Oncol. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC) Estimated Age-Standardized Incidence and Mortality Rates (World) in 2020, Breast, Females, All Ages. Available online: https://gco.iarc.fr/ (accessed on 22 November 2022).
- Ogembo, R.K.; Gona, P.N.; Seymour, A.J.; Park, H.S.M.; Bain, P.A.; Maranda, L.; Ogembo, J.G. Prevalence of Human Papillomavirus Genotypes among African Women with Normal Cervical Cytology and Neoplasia: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0122488. [Google Scholar] [CrossRef] [PubMed]
- Brianti, P.; De Flammineis, E.; Mercuri, S.R. Review of HPV-Related Diseases and Cancers. New Microbiol. 2017, 40, 80–85. [Google Scholar]
- Mac, M.; Moody, C.A. Epigenetic Regulation of the Human Papillomavirus Life Cycle. Pathogens 2020, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Day, P.M.; Trus, B.L. The Papillomavirus Major Capsid Protein L1. Virology 2013, 445, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Chen, J.; Xu, M.; Yang, H.; Luo, J.; Pan, Y.; Wang, X.; Qiu, L.; Yang, J.; Sun, Q. Genetic Variability and Functional Implication of the Long Control Region in HPV-16 Variants in Southwest China. PLoS ONE 2017, 12, e0182388. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Jiao, Y.; Wu, C. High-Risk Human Papillomavirus Oncogenic E6/E7 MRNAs Splicing Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 929666. [Google Scholar] [CrossRef]
- Burk, R.D.; Harari, A.; Chen, Z. Human Papillomavirus Genome Variants. Virology 2013, 445, 232–243. [Google Scholar] [CrossRef]
- Pientong, C.; Wongwarissara, P.; Ekalaksananan, T.; Swangphon, P.; Kleebkaow, P.; Kongyingyoes, B.; Siriaunkgul, S.; Tungsinmunkong, K.; Suthipintawong, C. Association of Human Papillomavirus Type 16 Long Control Region Mutation and Cervical Cancer. Virol. J. 2013, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Cornet, I.; Gheit, T.; Franceschi, S.; Vignat, J.; Burk, R.D.; Sylla, B.S.; Tommasino, M.; Clifford, G.M. Human Papillomavirus Type 16 Genetic Variants: Phylogeny and Classification Based on E6 and LCR. J. Virol. 2012, 86, 6855–6861. [Google Scholar] [CrossRef] [PubMed]
- Boumba, L.M.A.; Assoumou, S.Z.; Hilali, L.; Mambou, J.V.; Moukassa, D.; Ennaji, M.M. Genetic Variability in E6 and E7 Oncogenes of Human Papillomavirus Type 16 from Congolese Cervical Cancer Isolates Cancer Centers in Low- and Middle-Income Countries. Infect. Agent. Cancer 2015, 10, 15. [Google Scholar] [CrossRef]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; Hausen, H.Z.; de Villiers, E.M. Classification of Papillomaviruses (PVs) Based on 189 PV Types and Proposal of Taxonomic Amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef]
- Pande, S.; Jain, N.; Prusty, B.K.; Bhambhani, S.; Gupta, S.; Sharma, R.; Batra, S.; Das, B.C. Human Papillomavirus Type 16 Variant Analysis of E6, E7, and L1 Genes and Long Control Region in Biopsy Samples from Cervical Cancer Patients in North India. J. Clin. Microbiol. 2008, 46, 1060–1066. [Google Scholar] [CrossRef]
- WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention; WHO: Geneva, Switzerland, 2013; Volume 60.
- Tendobi, C.; Fernandez-Marques, M.; Carlos, S.; Amann, M.; Ndaye, M.; Ngoya, L.; Segura, G.; Nuñez, L.; Oliver, D.; Oiz, I.; et al. Validation of a Sustainable Internationally Monitored Cervical Cancer Screening System Using a Visual Smartphone Inspection in Kinshasa, Democratic Republic of Congo. Int. J. Gynecol. Cancer 2022, 32, 1244–1249. [Google Scholar] [CrossRef]
- Genomica. CLART HPV4 Genotyping of Human Papillomavirus via Genomic Identification for In Vitro Diagnosis. 2021, pp. 1–30. Available online: https://genomica.com/wp-content/uploads/2022/03/CLART-HPV4-HPV-4S.-v13-Octubre2021-ENGLISH.pdf (accessed on 5 December 2022).
- Frati, E.; Bianchi, S.; Colzani, D.; Zappa, A.; Orlando, G.; Tanzi, E. Genetic Variability in the Major Capsid L1 Protein of Human Papillomavirus Type 16 (HPV-16) and 18 (HPV-18). Infect. Genet. Evol. 2011, 11, 2119–2124. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for MacOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for Inferring Very Large Phylogenies by Using the Neighbor-Joining Method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.M.; Sangwa-Lugoma, G.; Nasr, S.H.; Kayembe, P.K.; Tozin, R.R.; Drouin, P.; Lorincz, A.; Ferenczy, A.; Franco, E.L. Comparison of Human Papillomavirus Testing and Cytology for Cervical Cancer Screening in a Primary Health Care Setting in the Democratic Republic of the Congo. Gynecol. Oncol. 2012, 124, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ali-Risasi, C.; Verdonck, K.; Padalko, E.; Vanden Broeck, D.; Praet, M. Prevalence and Risk Factors for Cancer of the Uterine Cervix among Women Living in Kinshasa, the Democratic Republic of the Congo: A Cross-Sectional Study. Infect. Agent. Cancer 2015, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Bishop, B.; Dasgupta, J.; Klein, M.; Garcea, R.L.; Christensen, N.D.; Zhao, R.; Chen, X.S. Crystal Structures of Four Types of Human Papillomavirus L1 Capsid Proteins: Understanding the Specificity of Neutralizing Monoclonal Antibodies. J. Biol. Chem. 2007, 282, 31803–31811. [Google Scholar] [CrossRef] [PubMed]
- Ning, T.; Wolfe, A.; Nie, J.; Huang, W.; Chen, X.S.; Wang, Y. Naturally Occurring Single Amino Acid Substitution in the L1 Major Capsid Protein of Human Papillomavirus Type 16: Alteration of Susceptibility to Antibody-Mediated Neutralization. J. Infect. Dis. 2017, 216, 867–876. [Google Scholar] [CrossRef]
- Mane, A.; Patil, L.; Limaye, S.; Nirmalkar, A.; Kulkarni-Kale, U. Characterization of Major Capsid Protein (L1) Variants of Human Papillomavirus Type 16 by Cervical Neoplastic Status in Indian Women: Phylogenetic and Functional Analysis. J. Med. Virol. 2020, 92, 1303–1308. [Google Scholar] [CrossRef]
- Pillai, M.R.; Hariharan, R.; Babu, J.M.; Lakshmi, S.; Chiplunkar, S.V.; Patkar, M.; Tongaonkar, H.; Dinshaw, K.; Jayshree, R.S.; Reddy, B.K.M.; et al. Molecular Variants of HPV-16 Associated with Cervical Cancer in Indian Population. Int. J. Cancer 2009, 125, 91–103. [Google Scholar] [CrossRef]
- El Aliani, A.; El Abid, H.; Kassal, Y.; Khyatti, M.; Attaleb, M.; Ennaji, M.M.; El Mzibri, M. HPV16 L1 Diversity and Its Potential Impact on the Vaccination-Induced Immunity. Gene 2020, 747, 144682. [Google Scholar] [CrossRef]
- Tawe, L.; Choga, W.T.; Paganotti, G.M.; Bareng, O.T.; Ntereke, T.D.; Ramatlho, P.; Ditshwanelo, D.; Gaseitsiwe, S.; Kasvosve, I.; Ramogola-Masire, D.; et al. Genetic Diversity in L1 ORF of Human Papillomavirus in Women with Cervical Cancer with and without Human Immunodeficiency Virus in Botswana and Kenya. BMC Infect. Dis. 2022, 22, 95. [Google Scholar] [CrossRef]
- Kammer, C.; Warthorst, U.; Torrez-Martinez, N.; Wheeler, C.M.; Pfister, H. Sequence Analysis of the Long Control Region of Human Papillomavirus Type 16 Variants and Functional Consequences for P97 Promoter Activity. J. Gen. Virol. 2000, 81, 1975–1981. [Google Scholar] [CrossRef]
- Villa, L.L.; Sichero, L.; Rahal, P.; Caballero, O.; Ferenczy, A.; Rohan, T.; Franco, E.L. Molecular Variants of Human Papillomavirus Types 16 and 18 Preferentially Associated with Cervical Neoplasia. J. Gen. Virol. 2000, 81, 2959–2968. [Google Scholar] [CrossRef]
- Awua, A.K.; Adanu, R.M.K.; Wiredu, E.K.; Afari, E.A.; Zubuch, V.A.; Asmah, R.H.; Severini, A. Unique LCR Variations among Lineages of HPV16, 18 and 45 Isolates from Women with Normal Cervical Cytology in Ghana. Virol. J. 2017, 14, 85. [Google Scholar] [CrossRef]
- Kurvinen, K.; Yliskoski, M.; Saarikoski, S.; Syrjänen, K.; Syrjänen, S. Variants of the Long Control Region of Human Papillomavirus Type 16. Eur. J. Cancer 2000, 36, 1402–1410. [Google Scholar] [CrossRef]
- Kim, Y.B.; Song, Y.S.; Jeon, Y.T.; Park, J.S.; Um, S.J.; Kim, J.W.; Park, N.H.; Kang, S.B.; Lee, H.P. Sequence Variation and the Transcriptional Activity of the Upstream Regulatory Region in Human Papillomavirus 16 E7 Variants in Cervical Cancer of Korean Women. Oncol. Rep. 2005, 14, 459–464. [Google Scholar] [CrossRef][Green Version]
- Wang, H.Y.; Lian, P.; Zheng, P.S. SOX9, a Potential Tumor Suppressor in Cervical Cancer, Transactivates P21WAF1/CIP1 and Suppresses Cervical Tumor Growth. Oncotarget 2015, 6, 20711–20722. [Google Scholar] [CrossRef]
- May, M.; Dong, X.P.; Beyer-Finkler, E.; Stubenrauch, F.; Fuchs, P.G.; Pfister, H. The E6/E7 Promoter of Extrachromosomal HPV16 DNA in Cervical Cancers Escapes from Cellular Repression by Mutation of Target Sequences for YY1. EMBO J. 1994, 13, 1460–1466. [Google Scholar] [CrossRef]
- Marongiu, L.; Godi, A.; Parry, J.V.; Beddows, S. Human Papillomavirus Type 16 Long Control Region and E6 Variants Stratified by Cervical Disease Stage. Infect. Genet. Evol. 2014, 26, 8–13. [Google Scholar] [CrossRef]
- Ishiji, T.; Lace, M.J.; Parkkinen, S.; Anderson, R.D.; Haugen, T.H.; Cripe, T.P.; Xiao, J.H.; Davidson, I.; Chambon, P.; Turek, L.P. Transcriptional Enhancer Factor (TEF)-1 and Its Cell-Specific Co-Activator Activate Human Papillomavirus-16 E6 and E7 Oncogene Transcription in Keratinocytes and Cervical Carcinoma Cells. EMBO J. 1992, 11, 2271–2281. [Google Scholar] [CrossRef]
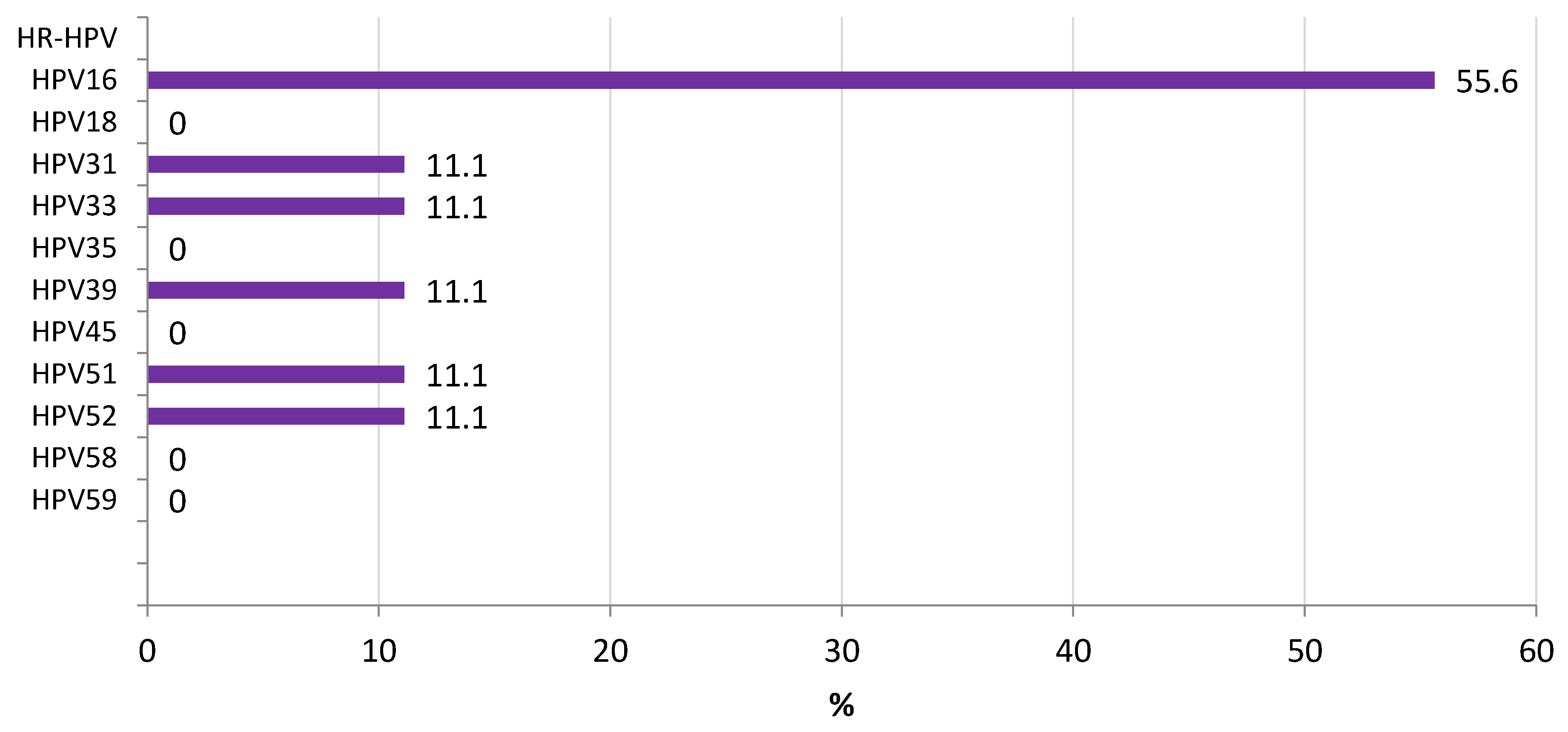


| Target Region | Primer Name * | Sequence | Amplicon Size (bp) |
|---|---|---|---|
| L1 | L1_16_1F | ATGTCTCTTTGGCTGCCTAG | 911 |
| L1 | L1_16_1R | GCATCAGAGGTAACCATAGAAC | |
| L1 | L1_16_2F | CTATGGACTTTACTACATTACAGGCTA | 879 |
| L1 | L1_16_2R | GTTTAGCAGTTGTAGAGGTAGATGA | |
| LCR | LCR_1F | ACCCACCACCTCATCTACCTCTACAA | 460 |
| LCR | LCR_1R | ATTTGGCACGCATGGCAAGCAGGAA | |
| LCR | LCR_2F | CATGCTTTTTGGCACAAAATGTGTTTT | 621 |
| LCR | LCR_2R | ATATCATGTATAGTTGTTTGCAGCTCT |
| Lineage | Sequence ID | Nucleotide Mutation Sites | Amino Acid Mutation Sites | |
|---|---|---|---|---|
| A1 | P114_L1 | None | None | |
| A1 | P154_L1.2 | A796G | T266A | |
| C | P44_L1 | G60A | C921T | H76Y |
| C226T | A1057C | T176N | ||
| T273C | G1083A | N181T | ||
| C527A | C1332T | T266A | ||
| A542C | C1216T | S282P | ||
| T609C | C1227T | T353P | ||
| A678G | G1356A | L474F | ||
| A796G | G1422T | |||
| T844C | ||||
| C | P232_L1.2 | A678G | G1083A | T266A |
| A796G | C1216T | S282P | ||
| T844C | C1227T | T353P | ||
| C921T | C1332T | |||
| A1057C | G1356A | |||
| C | P408_L1.2 | A678G | C1216T | T266A |
| A796G | C1227T | S282P | ||
| T844C | C1332T | T353P | ||
| C921T | G1356A | L474F | ||
| A1057C | G1422T | |||
| G1083A | ||||
| Lineage | Sequence ID | Nucleotide Mutation Sites | |
|---|---|---|---|
| A1 | P114_LCR | C7430T | |
| C7876A | |||
| A1 | P154_LCR | G7193T | |
| T7416A | |||
| G7521A | |||
| C7533G | |||
| B1 | P366_LCR | G7193T | C7764T |
| A7232G | C7786T | ||
| T7293G | A7797T | ||
| G7489A | G7834T | ||
| G7521A | C7876A | ||
| C7689A | G28T | ||
| T7714A | TT31C | ||
| C | P44_LCR | G7193T | C7764T |
| A7233C | C7786T | ||
| G7387C | A7826G | ||
| G7435A | G7834T | ||
| A7485C | A7837C | ||
| G7489A | A7839G | ||
| G7521A | T31C | ||
| C7669T | T109C | ||
| C7689A | |||
| C | P216_LCR | G7435A | A7826G |
| G7436C | G7834T | ||
| A7485C | A7837C | ||
| G7489A | A7839G | ||
| G7521A | T31C | ||
| C7669T | T109C | ||
| C7689A | G132T | ||
| T7700G | C143G | ||
| C7764T | G145T | ||
| C7786T | |||
| C | P408_LCR | A7156C | C7786T |
| A7159G | A7826G | ||
| G7193T | G7834T | ||
| A7233C | A7837C | ||
| G7387C | A7839G | ||
| G7435A | T31C | ||
| A7485C | T109C | ||
| G7489A | G122C | ||
| G7521A | G124C | ||
| C7669T | G132T | ||
| C7689A | C143G | ||
| C7764T | G145T | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, P.; Tendobi, C.; Carlos, S.; Lozano, M.D.; Barquín, D.; Chiva, L.; Reina, G. Characterization of Human Papillomavirus 16 from Kinshasa (Democratic Republic of the Congo)—Implications for Pathogenicity and Vaccine Effectiveness. Microorganisms 2022, 10, 2492. https://doi.org/10.3390/microorganisms10122492
Iglesias P, Tendobi C, Carlos S, Lozano MD, Barquín D, Chiva L, Reina G. Characterization of Human Papillomavirus 16 from Kinshasa (Democratic Republic of the Congo)—Implications for Pathogenicity and Vaccine Effectiveness. Microorganisms. 2022; 10(12):2492. https://doi.org/10.3390/microorganisms10122492
Chicago/Turabian StyleIglesias, Paula, Celine Tendobi, Silvia Carlos, Maria D. Lozano, David Barquín, Luis Chiva, and Gabriel Reina. 2022. "Characterization of Human Papillomavirus 16 from Kinshasa (Democratic Republic of the Congo)—Implications for Pathogenicity and Vaccine Effectiveness" Microorganisms 10, no. 12: 2492. https://doi.org/10.3390/microorganisms10122492
APA StyleIglesias, P., Tendobi, C., Carlos, S., Lozano, M. D., Barquín, D., Chiva, L., & Reina, G. (2022). Characterization of Human Papillomavirus 16 from Kinshasa (Democratic Republic of the Congo)—Implications for Pathogenicity and Vaccine Effectiveness. Microorganisms, 10(12), 2492. https://doi.org/10.3390/microorganisms10122492








