Molecular Typing of Mastadenoviruses in Simultaneously Collected Nasopharyngeal Swabs and Stool Samples from Children Hospitalized for Acute Bronchiolitis, Acute Gastroenteritis, and Febrile Seizures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Preparation and Nucleic Acid Extraction
2.3. Detection of Other Viruses
2.4. HAdV Sequencing and Characterization
2.5. Statistical Analysis
3. Results
3.1. Patients and Controls
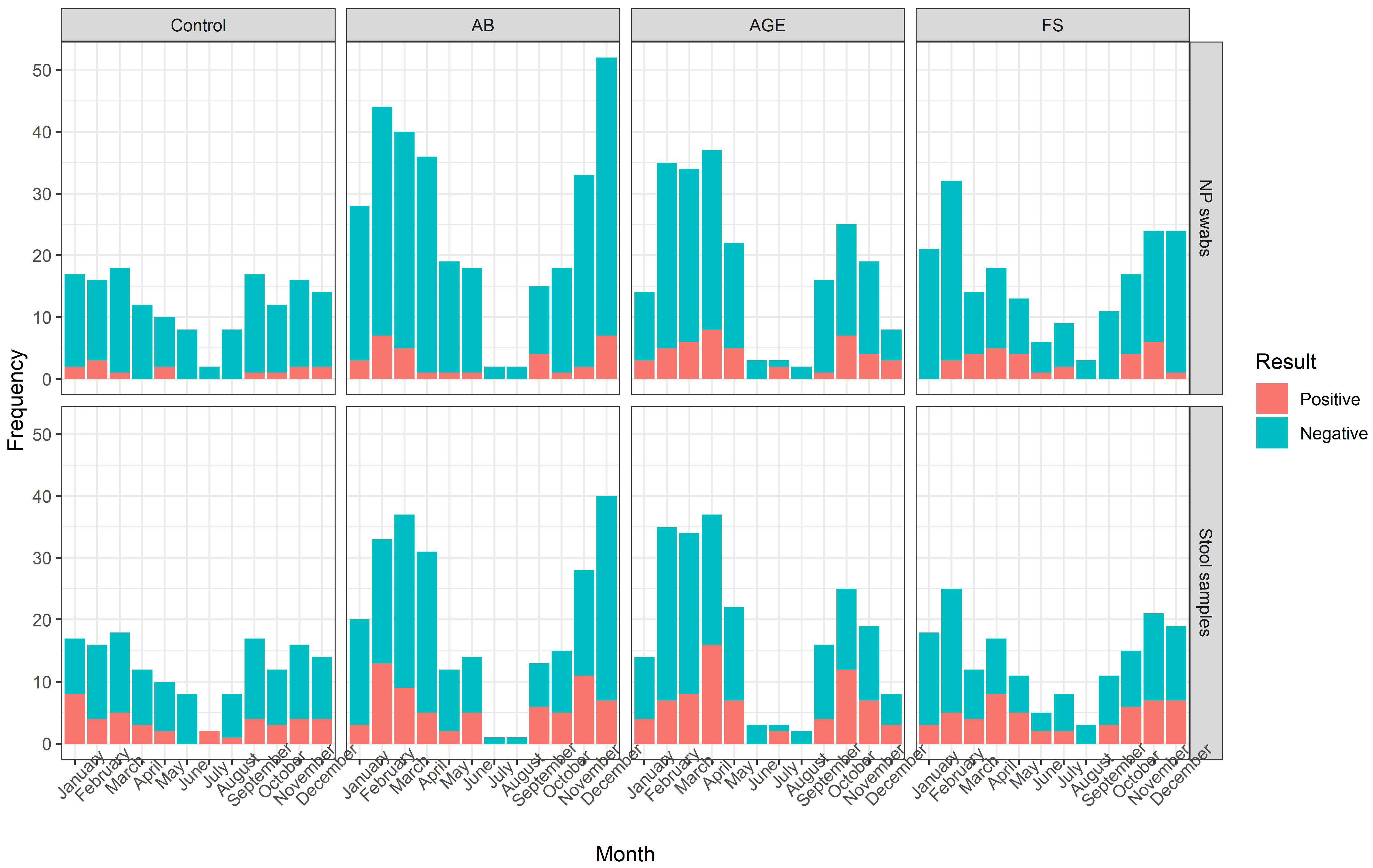
3.2. Samples
3.3. HAdV Detection
3.4. Presence of Additional Viruses
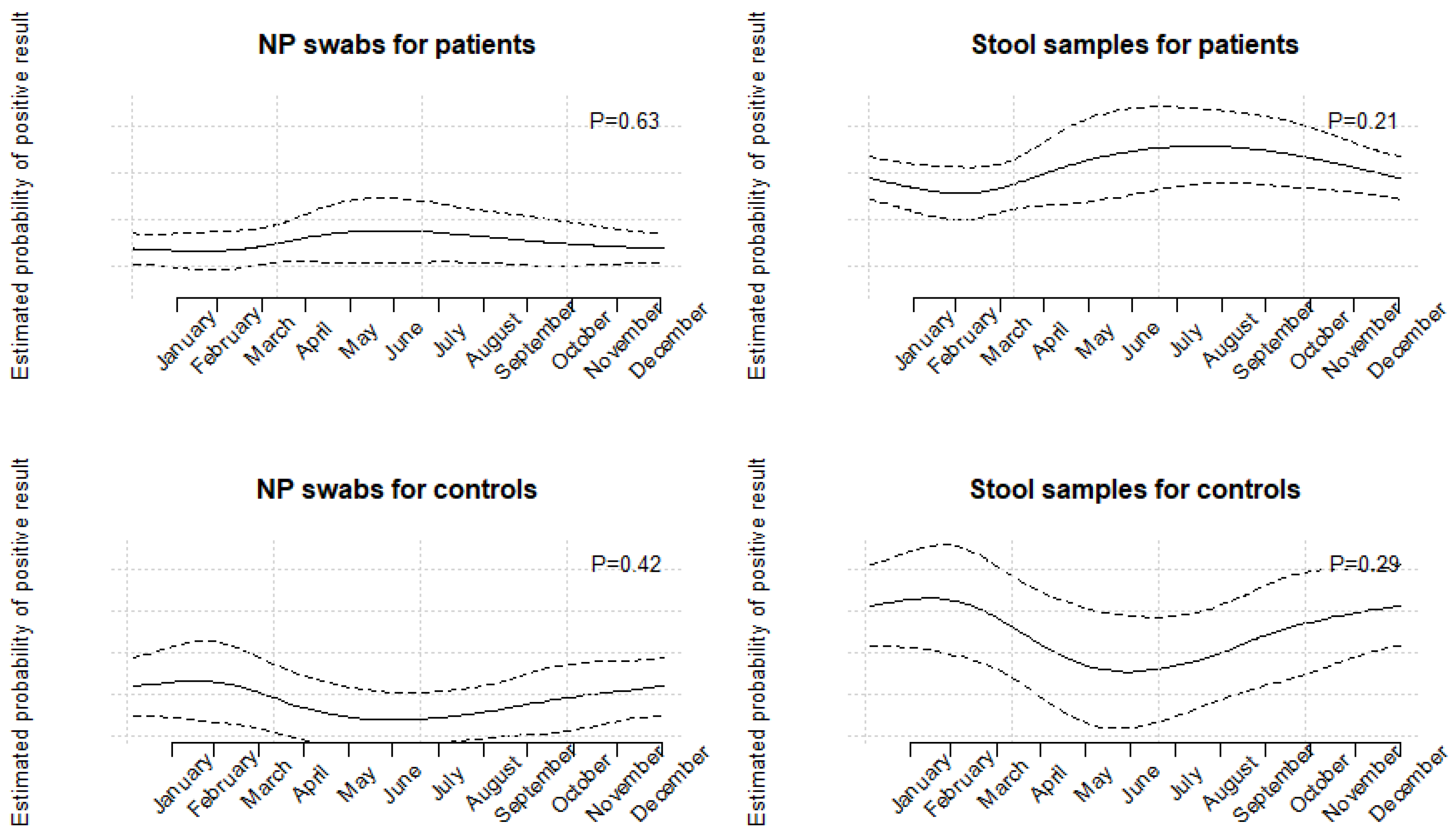
3.5. Follow-Up Testing
3.6. Molecular Characterization and Genotypes of HAdV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, X.; Erdman, D.D. Molecular typing of human adenoviruses by PCR and sequencing of a partial region of the hexon gene. Arch. Virol. 2006, 151, 1587–1602. [Google Scholar] [CrossRef] [PubMed]
- Kajon, A.E.; Echavarria, M.; de Jong, J.C. Designation of human adenovirus types based on sequence data: An unfinished debate. J. Clin. Virol. 2013, 58, 743–744. [Google Scholar] [CrossRef]
- Lynch, J.P., 3rd; Fishbein, M.; Echavarria, M. Adenovirus. Semin. Respir. Crit. Care Med. 2011, 32, 494–511. [Google Scholar] [CrossRef]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajon, A.E.; Lu, X.; Erdman, D.D.; Louie, J.; Schnurr, D.; George, K.S.; Koopmans, M.P.; Allibhai, T.; Metzgar, D. Molecular epidemiology and brief history of emerging adenovirus 14-associated respiratory disease in the United States. J. Infect. Dis. 2010, 202, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Houldcroft, C.J.; Roy, S.; Morfopoulou, S.; Margetts, B.K.; Depledge, D.P.; Cudini, J.; Shah, D.; Brown, J.R.; Romero, E.Y.; Williams, R.; et al. Use of Whole-Genome Sequencing of Adenovirus in Immunocompromised Pediatric Patients to Identify Nosocomial Transmission and Mixed-Genotype Infection. J. Infect. Dis. 2018, 218, 1261–1271. [Google Scholar] [CrossRef]
- Huang, X.; Yi, Y.; Chen, X.; Wang, B.; Long, Y.; Chen, J.; Rongkavilit, C. Clinical Characteristics of 204 Children with Human Adenovirus Type 7 Pneumonia Identified by Whole Genome Sequencing in Liuzhou, China. Pediatr. Infect. Dis. J. 2021, 40, 91–95. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, Y.; Zhang, J.; Ren, L.; Li, J.; Xie, Z.; Xu, B.; Yang, Y.; Qian, S.; Wang, J.; et al. Adenovirus infection in children with acute lower respiratory tract infections in Beijing, China, 2007 to 2012. BMC Infect. Dis. 2015, 15, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Choi, E.H.; Lee, H.J. Comprehensive serotyping and epidemiology of human adenovirus isolated from the respiratory tract of Korean children over 17 consecutive years (1991–2007). J. Med. Virol. 2010, 82, 624–631. [Google Scholar] [CrossRef]
- Edwards, K.M.; Thompson, J.; Paolini, J.; Wright, P.F. Adenovirus infections in young children. Pediatrics 1985, 76, 420–424. [Google Scholar] [CrossRef]
- Ison, M.G. Adenovirus Infections in Transplant Recipients. Clin. Infect. Dis. 2006, 43, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.L.; Chiou, S.S.; Hsiao, H.P.; Ke, G.M.; Lin, Y.C.; Lin, K.H.; Jong, Y.J. Respiratory adenoviral infections in children: A study of hospitalized cases in southern Taiwan in 2001–2002. J. Trop. Pediatr. 2004, 50, 279–284. [Google Scholar] [CrossRef]
- Sriwanna, P.; Chieochansin, T.; Vuthitanachot, C.; Vuthitanachot, V.; Theamboonlers, A.; Poovorawan, Y. Molecular characterization of human adenovirus infection in Thailand, 2009–2012. Virol. J. 2013, 10, 193. [Google Scholar] [CrossRef] [Green Version]
- Osborne, C.M.; Montano, A.C.; Robinson, C.C.; Schultz-Cherry, S.; Dominguez, S.R. Viral gastroenteritis in children in Colorado 2006–2009. J. Med. Virol. 2015, 87, 931–939. [Google Scholar] [CrossRef]
- Liu, L.; Qian, Y.; Zhang, Y.; Zhao, L.; Jia, L.; Dong, H. Epidemiological aspects of rotavirus and adenovirus in hospitalized children with diarrhea: A 5-year survey in Beijing. BMC Infect. Dis. 2016, 16, 508. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, P.; Payne, D.C.; Szilagyi, P.G.; Edwards, K.M.; Staat, M.A.; Shirley, S.H.; Wikswo, M.; Nix, W.A.; Lu, X.; Parashar, U.D.; et al. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008–2009. J. Infect. Dis. 2013, 208, 790–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colomba, C.; De Grazia, S.; Giammanco, G.M.; Saporito, L.; Scarlata, F.; Titone, L.; Arista, S. Viral gastroenteritis in children hospitalised in Sicily, Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 570–575. [Google Scholar] [CrossRef]
- Wilhelmi, I.; Roman, E.; Sanchez-Fauquier, A. Viruses causing gastroenteritis. Clin. Microbiol. Infect. 2003, 9, 247–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, M.S.; van Well, G.T.J.; van Loo, I.H.M. Diagnosis of viral gastroenteritis in children: Interpretation of real-time PCR results and relation to clinical symptoms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, T.H.; Wang, J.; Dong, C.; Pan, J.; Moe, C.; Chen, W.; Yang, L.; Wang, X.; Tang, H.; et al. Symptomatic and asymptomatic infections of rotavirus, norovirus, and adenovirus among hospitalized children in Xi’an, China. J. Med. Virol. 2011, 83, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Uhnoo, I.; Wadell, G.; Svensson, L.; Johansson, M.E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J. Clin. Microbiol. 1984, 20, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morfin, F.; Dupuis-Girod, S.; Mundweiler, S.; Falcon, D.; Carrington, D.; Sedlacek, P.; Bierings, M.; Cetkovsky, P.; Kroes, A.C.; van Tol, M.J.; et al. In vitro susceptibility of adenovirus to antiviral drugs is species-dependent. Antivir. Ther. 2005, 10, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; McDonough, M.C.; Erdman, D.D. Species-specific identification of human adenoviruses by a multiplex PCR assay. J. Clin. Microbiol. 2000, 38, 4114–4120. [Google Scholar] [CrossRef] [Green Version]
- Pring-Akerblom, P.; Trijssenaar, F.E.; Adrian, T.; Hoyer, H. Multiplex polymerase chain reaction for subgenus-specific detection of human adenoviruses in clinical samples. J. Med. Virol. 1999, 58, 87–92. [Google Scholar] [CrossRef]
- Jevsnik, M.; Steyer, A.; Pokorn, M.; Mrvic, T.; Grosek, S.; Strle, F.; Lusa, L.; Petrovec, M. The Role of Human Coronaviruses in Children Hospitalized for Acute Bronchiolitis, Acute Gastroenteritis, and Febrile Seizures: A 2-Year Prospective Study. PLoS ONE 2016, 11, e0155555. [Google Scholar] [CrossRef] [Green Version]
- De Vos, N.; Vankeerberghen, A.; Vaeyens, F.; Van Vaerenbergh, K.; Boel, A.; De Beenhouwer, H. Simultaneous detection of human bocavirus and adenovirus by multiplex real-time PCR in a Belgian paediatric population. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1305–1310. [Google Scholar] [CrossRef]
- Kuypers, J.; Wright, N.; Morrow, R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J. Clin. Virol. 2004, 31, 123–129. [Google Scholar] [CrossRef]
- Kuypers, J.; Wright, N.; Ferrenberg, J.; Huang, M.L.; Cent, A.; Corey, L.; Morrow, R. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J. Clin. Microbiol. 2006, 44, 2382–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daum, L.T.; Canas, L.C.; Arulanandam, B.P.; Niemeyer, D.; Valdes, J.J.; Chambers, J.P. Real-time RT-PCR assays for type and subtype detection of influenza A and B viruses. Influenza Other Respir. Viruses 2007, 1, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Maertzdorf, J.; Wang, C.K.; Brown, J.B.; Quinto, J.D.; Chu, M.; de Graaf, M.; van den Hoogen, B.G.; Spaete, R.; Osterhaus, A.D.; Fouchier, R.A. Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J. Clin. Microbiol. 2004, 42, 981–986. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Chittaganpitch, M.; Olsen, S.J.; Mackay, I.M.; Sloots, T.P.; Fry, A.M.; Erdman, D.D. Real-time PCR assays for detection of bocavirus in human specimens. J. Clin. Microbiol. 2006, 44, 3231–3235. [Google Scholar] [CrossRef] [Green Version]
- Scheltinga, S.A.; Templeton, K.E.; Beersma, M.F.; Claas, E.C. Diagnosis of human metapneumovirus and rhinovirus in patients with respiratory tract infections by an internally controlled multiplex real-time RNA PCR. J. Clin. Virol. 2005, 33, 306–311. [Google Scholar] [CrossRef]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef] [Green Version]
- Svraka, S.; van der Veer, B.; Duizer, E.; Dekkers, J.; Koopmans, M.; Vennema, H. Novel approach for detection of enteric viruses to enable syndrome surveillance of acute viral gastroenteritis. J. Clin. Microbiol. 2009, 47, 1674–1679. [Google Scholar] [CrossRef] [Green Version]
- Lusa, L.; Ahlin, C. Restricted cubic splines for modelling periodic data. PLoS ONE 2020, 15, e0241364. [Google Scholar] [CrossRef] [PubMed]
- Functions for Generating Periodic Curves 2018. Available online: https://CRAN.R-project.org/package=peRiodiCS (accessed on 5 September 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 5 September 2022).
- Tabain, I.; Ljubin-Sternak, S.; Cepin-Bogovic, J.; Markovinovic, L.; Knezovic, I.; Mlinaric-Galinovic, G. Adenovirus respiratory infections in hospitalized children: Clinical findings in relation to species and serotypes. Pediatr. Infect. Dis. J. 2012, 31, 680–684. [Google Scholar] [CrossRef]
- Demian, P.N.; Horton, K.C.; Kajon, A.; Siam, R.; Hasanin, A.M.; Elgohary Sheta, A.; Cornelius, C.; Gaynor, A.M. Molecular identification of adenoviruses associated with respiratory infection in Egypt from 2003 to 2010. BMC Infect. Dis. 2014, 14, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, S.; Zampiero, A.; Bianchini, S.; Mori, A.; Scala, A.; Tagliabue, C.; Sciarrabba, C.S.; Fossali, E.; Piralla, A.; Principi, N. Epidemiology and Clinical Characteristics of Respiratory Infections Due to Adenovirus in Children Living in Milan, Italy, during 2013 and 2014. PLoS ONE 2016, 11, e0152375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmielewicz, B.; Nitsche, A.; Schweiger, B.; Ellerbrok, H. Development of a PCR-based assay for detection, quantification, and genotyping of human adenoviruses. Clin. Chem. 2005, 51, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Larranaga, C.; Kajon, A.; Villagra, E.; Avendano, L.F. Adenovirus surveillance on children hospitalized for acute lower respiratory infections in Chile (1988–1996). J. Med. Virol. 2000, 60, 342–346. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lee, H.J.; Piedra, P.A.; Choi, E.H.; Park, K.H.; Koh, Y.Y.; Kim, W.S. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: Epidemiology, clinical features, and prognosis. Clin. Infect. Dis. 2001, 32, 1423–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Lee, S.K.; Ko, D.H.; Hyun, J.; Kim, H.S.; Song, W.; Kim, H.S. Associations of Adenovirus Genotypes in Korean Acute Gastroenteritis Patients with Respiratory Symptoms and Intussusception. BioMed Res. Int. 2017, 2017, 1602054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rosa, G.; Della Libera, S.; Petricca, S.; Iaconelli, M.; Donia, D.; Saccucci, P.; Cenko, F.; Xhelilaj, G.; Divizia, M. Genetic Diversity of Human Adenovirus in Children with Acute Gastroenteritis, Albania, 2013–2015. BioMed Res. Int. 2015, 2015, 142912. [Google Scholar] [CrossRef] [Green Version]
- Moyo, S.J.; Hanevik, K.; Blomberg, B.; Kommedal, O.; Nordbo, S.A.; Maselle, S.; Langeland, N. Prevalence and molecular characterisation of human adenovirus in diarrhoeic children in Tanzania; a case control study. BMC Infect. Dis. 2014, 14, 666. [Google Scholar] [CrossRef]
- Ye, S.; Whiley, D.M.; Ware, R.S.; Sloots, T.P.; Kirkwood, C.D.; Grimwood, K.; Lambert, S.B. Detection of viruses in weekly stool specimens collected during the first 2 years of life: A pilot study of five healthy Australian infants in the rotavirus vaccine era. J. Med. Virol. 2017, 89, 917–921. [Google Scholar] [CrossRef]
- Lyons, A.; Longfield, J.; Kuschner, R.; Straight, T.; Binn, L.; Seriwatana, J.; Reitstetter, R.; Froh, I.B.; Craft, D.; McNabb, K.; et al. A double-blind, placebo-controlled study of the safety and immunogenicity of live, oral type 4 and type 7 adenovirus vaccines in adults. Vaccine 2008, 26, 2890–2898. [Google Scholar] [CrossRef] [PubMed]
- Akihara, S.; Phan, T.G.; Nguyen, T.A.; Hansman, G.; Okitsu, S.; Ushijima, H. Existence of multiple outbreaks of viral gastroenteritis among infants in a day care center in Japan. Arch. Virol. 2005, 150, 2061–2075. [Google Scholar] [CrossRef]
- Probst, V.; Spieker, A.J.; Stopczynski, T.; Stewart, L.S.; Haddadin, Z.; Selvarangan, R.; Harrison, C.J.; Schuster, J.E.; Staat, M.A.; McNeal, M.; et al. Clinical Presentation and Severity of Adenovirus Detection Alone vs Adenovirus Co-detection With Other Respiratory Viruses in US Children with Acute Respiratory Illness from 2016 to 2018. J. Pediatr. Infect. Dis. Soc. 2022, 11, 430–439. [Google Scholar] [CrossRef]
- Miyaji, Y.; Kobayashi, M.; Sugai, K.; Tsukagoshi, H.; Niwa, S.; Fujitsuka-Nozawa, A.; Noda, M.; Kozawa, K.; Yamazaki, F.; Mori, M.; et al. Severity of respiratory signs and symptoms and virus profiles in Japanese children with acute respiratory illness. Microbiol. Immunol. 2013, 57, 811–821. [Google Scholar] [CrossRef]
- Proenca-Modena, J.L.; de Souza Cardoso, R.; Criado, M.F.; Milanez, G.P.; de Souza, W.M.; Parise, P.L.; Bertol, J.W.; de Jesus, B.L.S.; Prates, M.C.M.; Silva, M.L.; et al. Human adenovirus replication and persistence in hypertrophic adenoids and palatine tonsils in children. J. Med. Virol. 2019, 91, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
- Wishaupt, J.O.; Ploeg, T.V.; Smeets, L.C.; Groot, R.; Versteegh, F.G.; Hartwig, N.G. Pitfalls in interpretation of CT-values of RT-PCR in children with acute respiratory tract infections. J. Clin. Virol. 2017, 90, 1–6. [Google Scholar] [CrossRef]
- Schjelderup Nilsen, H.J.; Nordbo, S.A.; Krokstad, S.; Dollner, H.; Christensen, A. Human adenovirus in nasopharyngeal and blood samples from children with and without respiratory tract infections. J. Clin. Virol. 2019, 111, 19–23. [Google Scholar] [CrossRef]
- Li, L.; Phan, T.G.; Nguyen, T.A.; Kim, K.S.; Seo, J.K.; Shimizu, H.; Suzuki, E.; Okitsu, S.; Ushijima, H. Molecular epidemiology of adenovirus infection among pediatric population with diarrhea in Asia. Microbiol. Immunol. 2005, 49, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Portes, S.A.; Volotao Ede, M.; Rocha, M.S.; Rebelo, M.C.; Xavier Mda, P.; Assis, R.M.; Rose, T.L.; Miagostovich, M.P.; Leite, J.P.; Carvalho-Costa, F.A. A non-enteric adenovirus A12 gastroenteritis outbreak in Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Primo, D.; Pacheco, G.T.; Timenetsky, M.; Luchs, A. Surveillance and molecular characterization of human adenovirus in patients with acute gastroenteritis in the era of rotavirus vaccine, Brazil, 2012–2017. J. Clin. Virol. 2018, 109, 35–40. [Google Scholar] [CrossRef] [PubMed]


| Primer/Probe | Sequence, 5′–3′ | Detection Technology |
|---|---|---|
| AdV-F | GCC ACG GTG GGG TTT CTA AAC TT | RT-PCR |
| AdV-R | GCC CCA GTG GTC TTA CAT GCA CAT C | RT-PCR |
| AdV-P-FAM | FAM-TGC ACC AGA CCC GGG CTC AGG TAC TCC GA-TAMRA | RT-PCR |
| AdV-F2 | TTY CCC ATG GCN CAC AAC AC | PCR, sequencing |
| AdV-R2 | GYY TCR ATG AYG CCG CGG TG | PCR, sequencing |
| Group | AB | AGE | FS | Patients Total | Control Group | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NP | Stool | NP | Stool | NP | Stool | NP | Stool | NP | Stool | |
| Sample (n) | 307 | 245 | 218 | 218 | 192 | 165 | 717 | 628 | 150 | 150 |
| HAdV positivity (n) % (95% CI) | 32 10.4 (7.2–14.4) | 66 26.9 (21.5–32.9) | 44 20.2 (15.0–26.1) | 70 32.1 (25.9–38.7) | 30 15.6 (10.8–21.5) | 52 31.5 (24.5–39.2) | 106 14.8 (12.3–17.6) | 188 29.9 (26.4–33.7) | 14 9.3 (5.2–15.2) | 40 26.7 (19.8–34.5) |
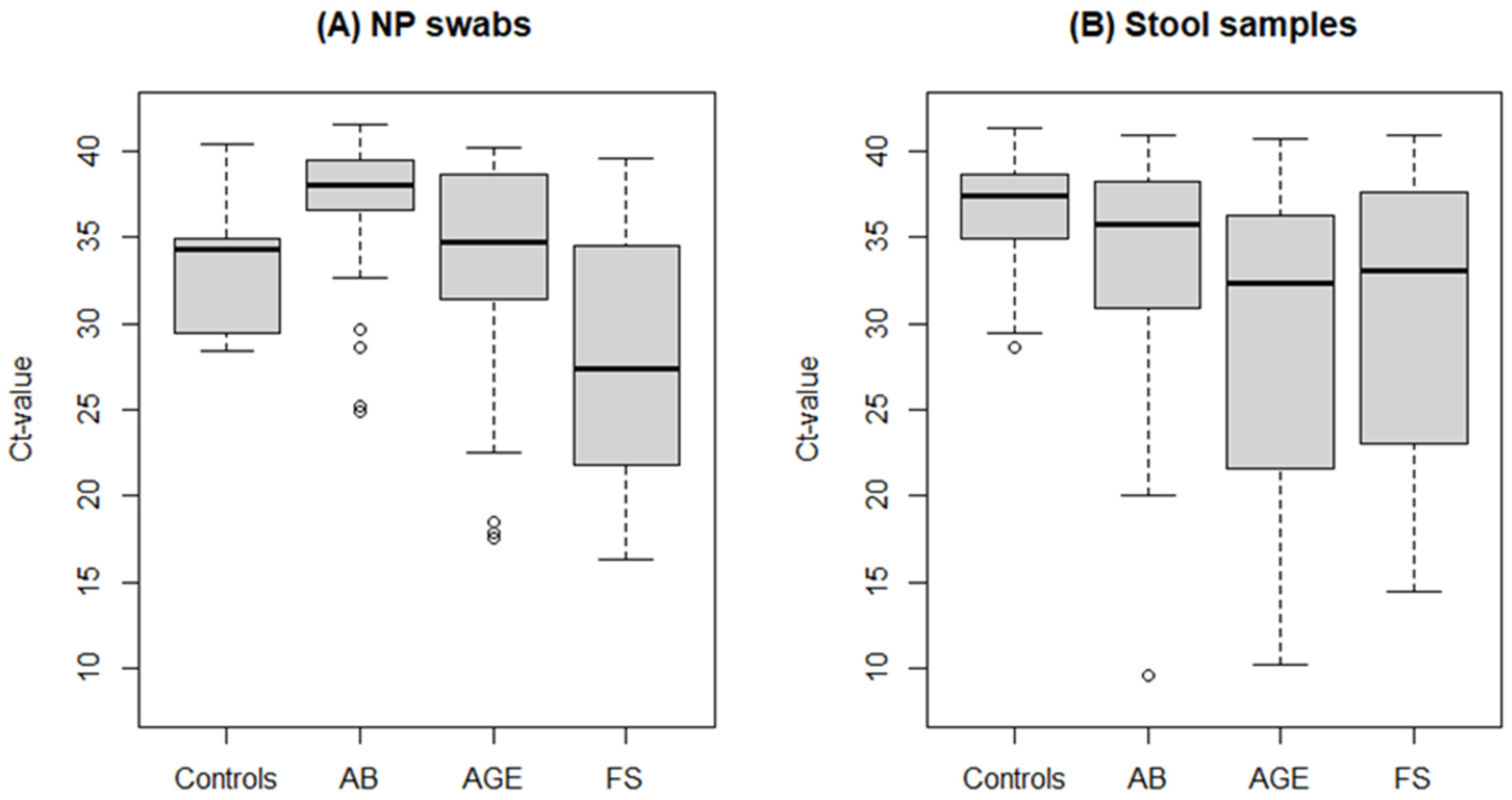
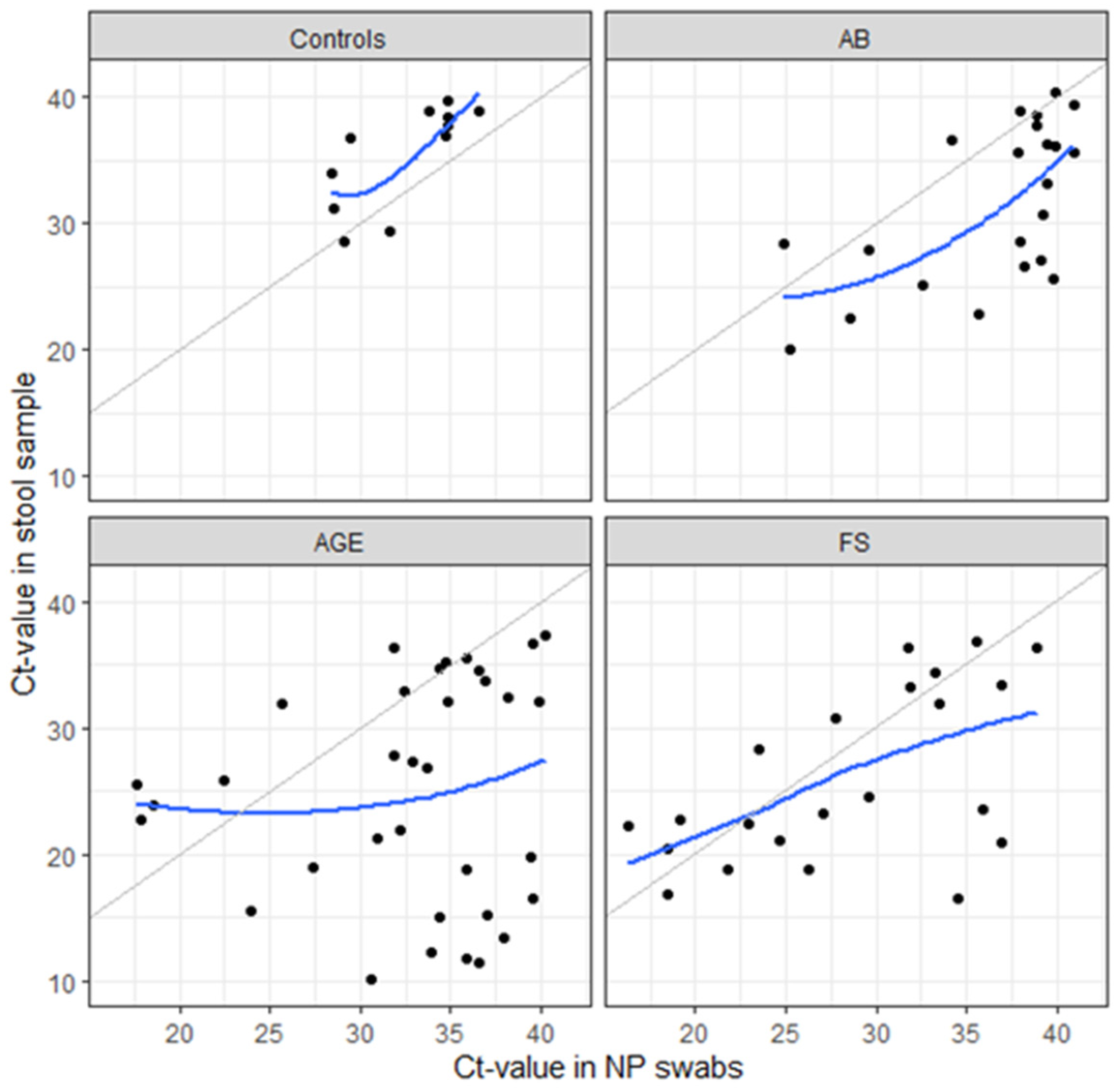
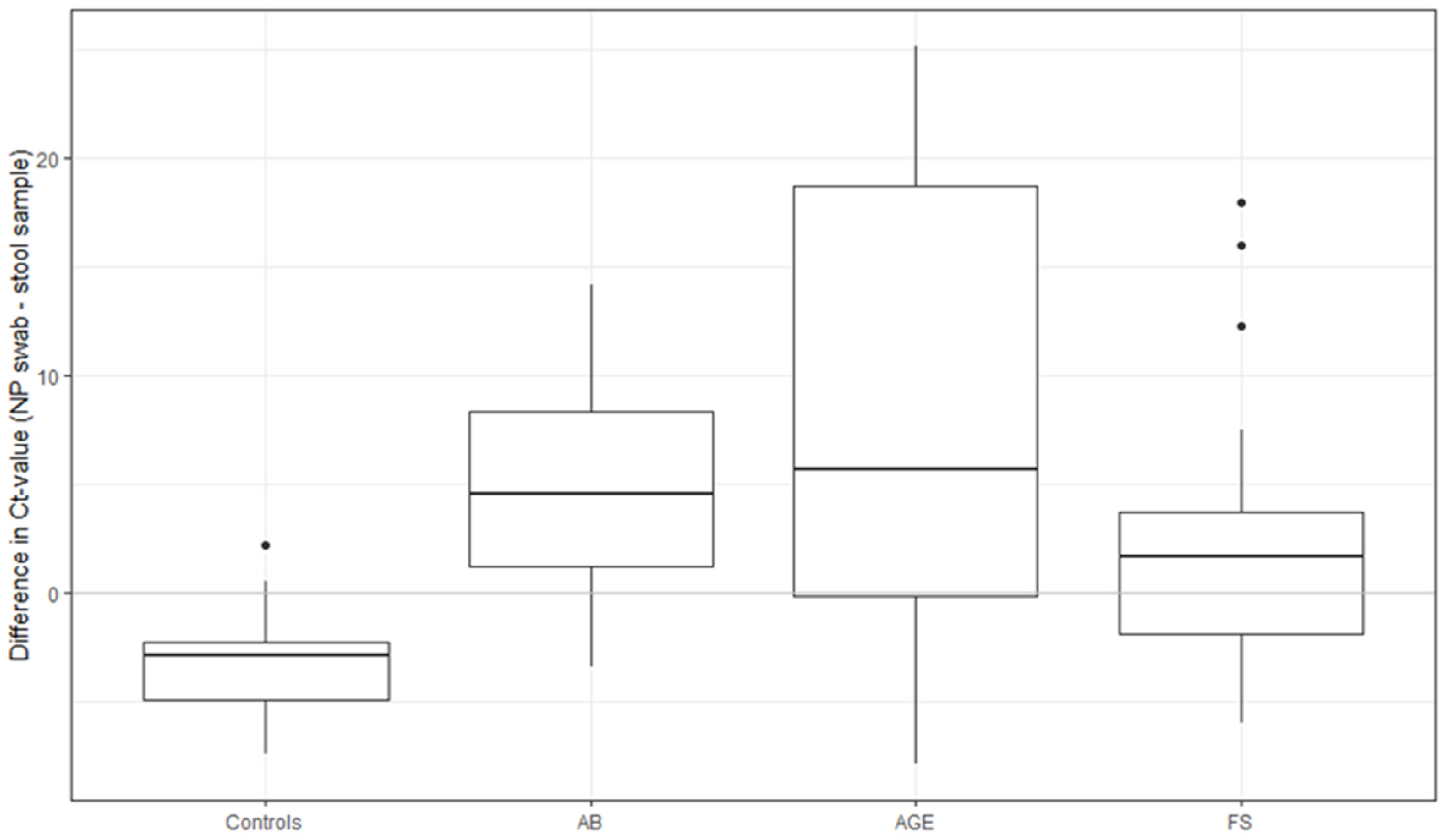
| Ct-value Median (IQR) | 38.1 (36.7–39.5) | 35.8 (31.1–38.2) | 34.8 (31.7–38.6) | 32.3 (21.7–36.3) | 27.4 (22.0–34.3) | 33 (23.1–37.4) | 35.6 (28.3–38.9) | 34.4 (25.8–37.1) | 34.2 (29.9–34.9) | 37.4 (35.0–38.6) |
| n of Positive/N Total (%, 95% CI) | |||
|---|---|---|---|
| AB | AGE | FS | |
| NP swabs | |||
| HAdV | 32/307 (10.4, 7.2–14.4) | 44/218 (20.2, 15.1–26.1) | 30/192 (15.6, 10.8–21.5) |
| HAdV only * | 5/32 (15.6, 5.3–32.8) | 17/44 (38.6, 24.4–54.5) | 13/30 (43.3, 25.5–62.6) |
| HAdV + additional ** | 27/32 (84.4, 67.2–94.7) | 27/44 (61.4, 45.5–75.6) | 17/30 (56.7, 37.4–74.5) |
| HAdV + 1 ** | 20/27 (74.1, 53.7–88.9) | 20/27 (74.1, 53.7–88.9) | 13/17 (76.5, 50.1–93.2) |
| HAdV + 2 ** | 6/27 (22.2, 8.6–42.3) | 6/27 (22.2, 8.6–42.3) | 1/17 (5.9, 0.1–28.7) |
| HAdV + 3 ** | 1/27 (3.7, 0.1–19) | 1/27 (3.7, 0.1–19) | 2/17 (11.8, 1.5–36.4) |
| HAdV + 4 ** | 0/27 (0, 0–12.8) | 0/27 (0, 0–12.8) | 1/17 (5.9, 0.1–28.7) |
| INFA ** | 0/27 (0, 0–12.8) | 0/27 (0, 0–12.8) | 2/17 (11.8, 1.5–36.4) |
| INFB ** | 0/27 (0, 0–12.8) | 0/27 (0, 0–12.8) | 1/17 (5.9, 0.1–28.7) |
| RSV ** | 15/27 (55.5, 35.3–74.5) | 7/27 (25.9, 11.1–46.3) | 2/17 (11.8, 1.5–36.4) |
| HCoV ** | 4/27 (14.8, 4.2–33.7) | 3/27 (11.1, 2.4–29.2) | 5/17 (29.4, 10.3–56.0) |
| HMPV ** | 2/27 (7.4. 0.9–24.3) | 0/27 (0, 0–12.8) | 0/17 (0, 0–19.5) |
| HBoV ** | 4/27 (14.8, 4.2–33.7) | 7/27 (25.9, 11.1–46.3) | 4/17 (23.5, 6.8–49.9) |
| HRV ** | 10/27 (37, 19.4–57.6) | 14/27 (51.8, 31.9–71.3) | 5/17 (29.4, 10.3–56.0) |
| PIV1–3 ** | 0/27 (0, 0–12.8) | 4/27 (14.8, 4.2–33.7) | 6/17 (35.3, 14.2–61.7) |
| Stool samples | |||
| HAdV | 70/218 (32.1, 26.0–38.7) | ||
| HAdV only * | 23/70 (32.9, 22.1–45.1) | ||
| HAdV + additional ** | 47/70 (67.1, 54.9–77.9) | ||
| HAdV + 1 ** | 41/47 (87.2, 74.3–95.2) | ||
| HAdV + 2 ** | 6/47 (12.8, 4.8–25.7) | ||
| HAdV + >2 ** | 0/47 (0, 0–7.5) | ||
| Astro ** | 1/47 (2.1, 0.1–11.3) | ||
| Noro ** | 16/47 (34.0, 20.9–49.3) | ||
| Rota ** | 31/47 (65.9, 50.7–79.1) | ||
| HBoV ** | 4/47 (8.5, 2.4–20.4) | ||
| HCoVs ** | 1/47 (2.1, 0.1–11.3) | ||
| Genotypes | |||
|---|---|---|---|
| Group | n | NP | Stool |
| AB | 1 | B3 | B3 |
| 1 | C5 | C5 | |
| AGE | 1 | C5 | F41 |
| 3 | C2 | C2 | |
| 1 | C5 | C5 | |
| 1 | C6 | C6 | |
| FS | 1 | B3 | B3 |
| 3 | C1 | C1 | |
| 4 | C2 | C2 | |
| 1 | C6 | C6 | |
| All patients | 2 | B3 | B3 |
| 3 | C1 | C1 | |
| 7 | C2 | C2 | |
| 2 | C5 | C5 | |
| 2 | C6 | C6 | |
| 1 | C5 | F41 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biškup, U.G.; Steyer, A.; Lusa, L.; Strle, F.; Pokorn, M.; Mrvič, T.; Grosek, Š.; Petrovec, M.; Jevšnik Virant, M. Molecular Typing of Mastadenoviruses in Simultaneously Collected Nasopharyngeal Swabs and Stool Samples from Children Hospitalized for Acute Bronchiolitis, Acute Gastroenteritis, and Febrile Seizures. Microorganisms 2023, 11, 780. https://doi.org/10.3390/microorganisms11030780
Biškup UG, Steyer A, Lusa L, Strle F, Pokorn M, Mrvič T, Grosek Š, Petrovec M, Jevšnik Virant M. Molecular Typing of Mastadenoviruses in Simultaneously Collected Nasopharyngeal Swabs and Stool Samples from Children Hospitalized for Acute Bronchiolitis, Acute Gastroenteritis, and Febrile Seizures. Microorganisms. 2023; 11(3):780. https://doi.org/10.3390/microorganisms11030780
Chicago/Turabian StyleBiškup, Urška Glinšek, Andrej Steyer, Lara Lusa, Franc Strle, Marko Pokorn, Tatjana Mrvič, Štefan Grosek, Miroslav Petrovec, and Monika Jevšnik Virant. 2023. "Molecular Typing of Mastadenoviruses in Simultaneously Collected Nasopharyngeal Swabs and Stool Samples from Children Hospitalized for Acute Bronchiolitis, Acute Gastroenteritis, and Febrile Seizures" Microorganisms 11, no. 3: 780. https://doi.org/10.3390/microorganisms11030780





