Anti-Mold Effectiveness of a Green Emulsion Based on Citrus aurantium Hydrolate and Cinnamomum zeylanicum Essential Oil for the Modern Paintings Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Canvas Samples
2.2. Headspace-Gas Chromatography and Mass Spectroscopy Analysis
2.3. Spray Formulation
2.4. In Situ Microbiological Test
2.5. Chemical–Physical Analysis
2.5.1. Ageing Conditions
2.5.2. Spray Treatment
2.5.3. pH Measurements
2.5.4. Colorimetric Measurements
2.6. Treatment of the Artwork
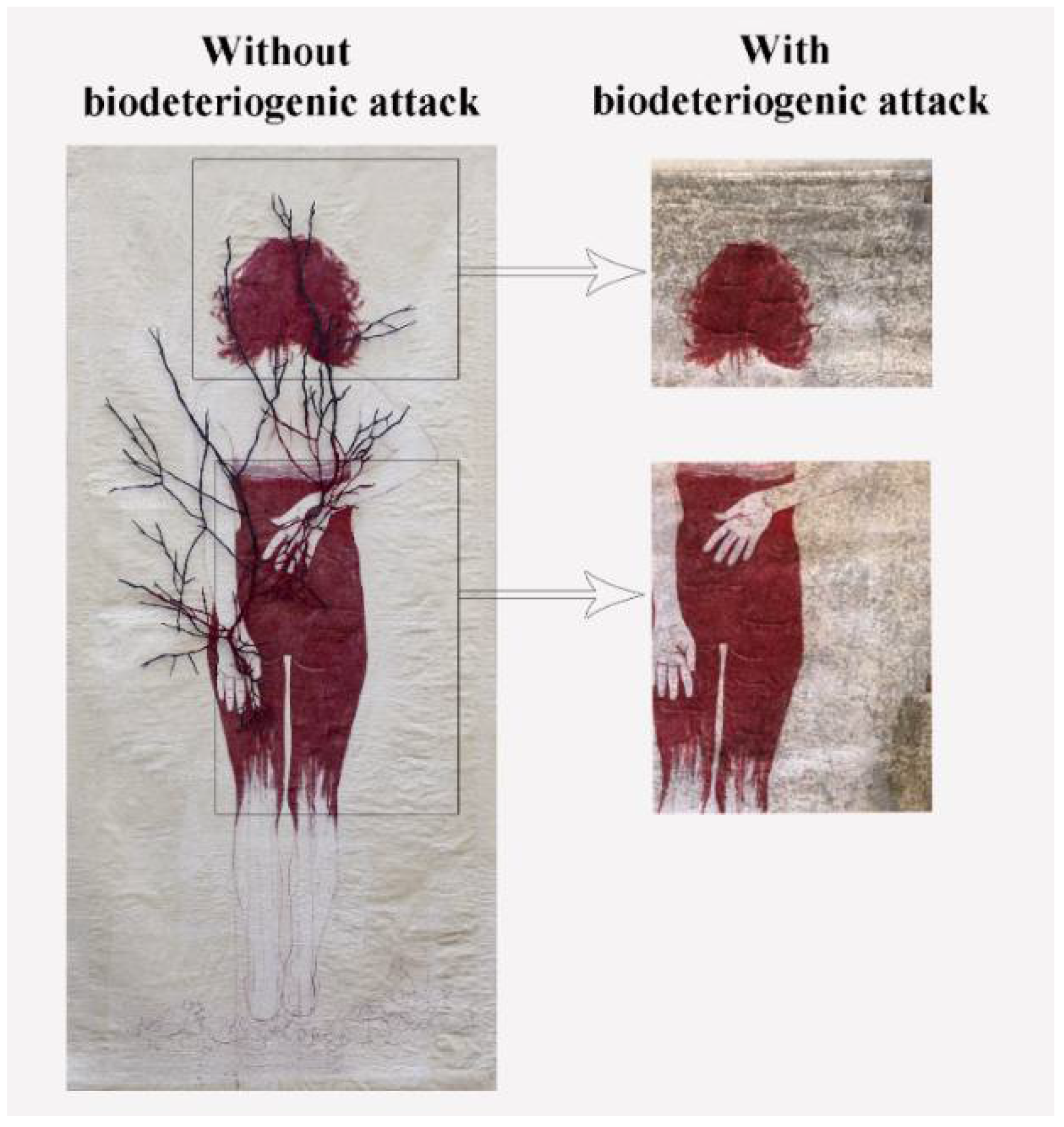
2.6.1. Sampling and Microbial Analysis of the Artwork
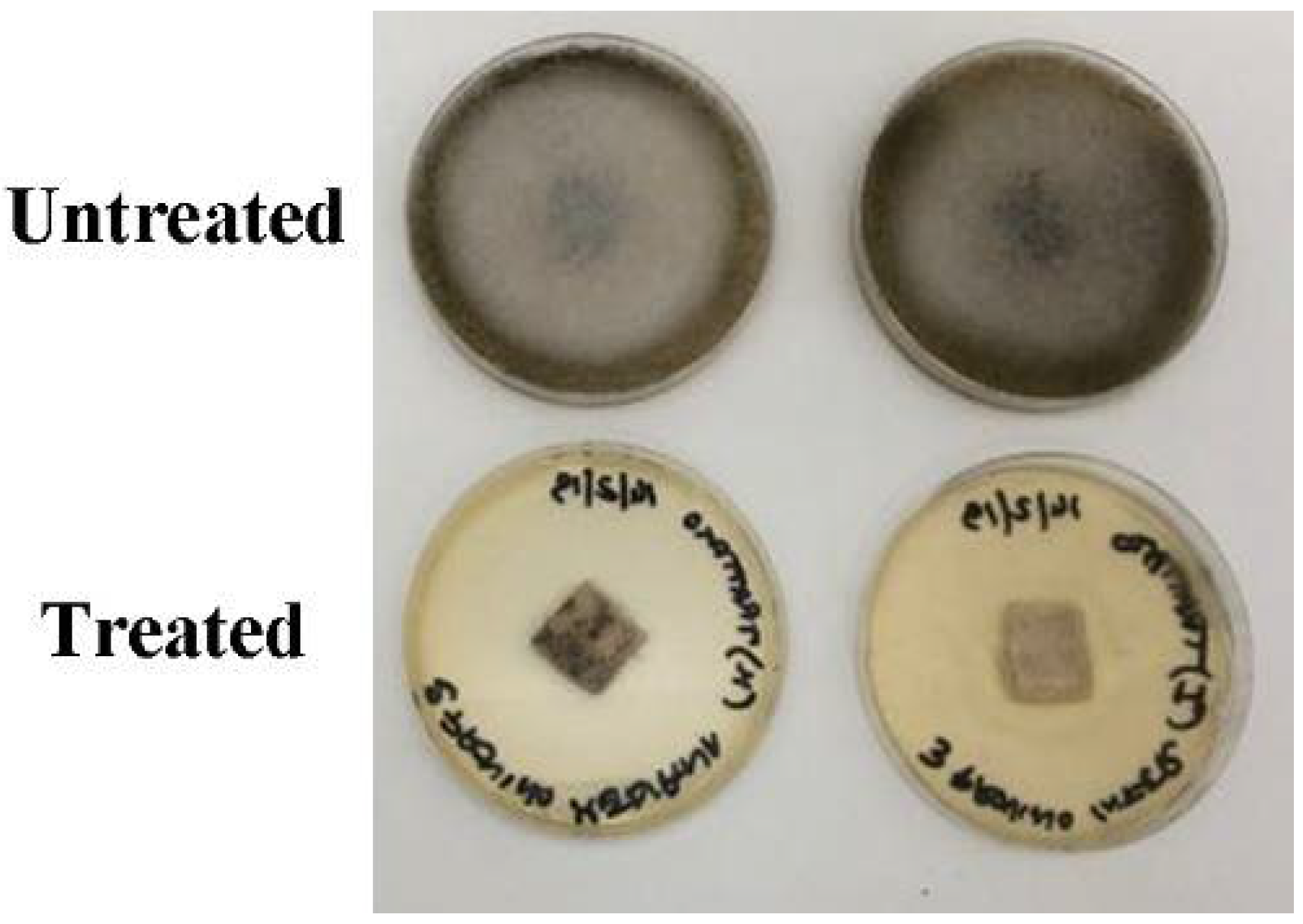
2.6.2. Spray Cytocidal Treatment
3. Results
3.1. Gas Chromatography and Mass Spectroscopy Analysis
3.2. In Situ Microbiological Test
3.3. Ageing Conditions
3.4. pH Measurements
3.5. Colorimetric Measurements
3.6. Sampling and Microbial Analysis of the Artwork
3.7. Spray Cytocidal Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odokuma, L.O.; Berebon, D.P.; Ogbonna, C.B. Potential Biodeteriogens of Indoor and Outdoor Surfaces (Coated with Gloss, Emulsion and Textcoat Paints). IOSR J. Pharm. Biol. Sci. 2013, 7, 12–19. [Google Scholar] [CrossRef]
- Poyatos, F.; Morales, F.; Nicholson, A.W.; Giordano, A. Physiology of biodeterioration on canvas paintings. J. Cell. Physiol. 2018, 233, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremonesi, P. L’ambiente Acquoso per Il Trattamento di Opere Policrome. Metodologie, Tecniche e Formazione Nel Mondo del Restauro, 2nd ed.; Il Prato Publishing House s.r.l.: Padova, Italy, 2012. [Google Scholar]
- Borgioli, L.; de Comelli, A.; Pressi, G. Indagini Microbiologiche per La Verifica Dell’efficacia Di Alcuni Biocidi Esenti Da Metalli Pesanti. Progett. Restauro 2006, 11, 24–29. [Google Scholar]
- Borgioli, L.; Pressi, G.; Secondin, S. Valutazione Dell’efficacia Di Prodotti Biocidei Attraverso Test Microbiologici Di Laboratorio e Saggi Applicativi in Cantiere. Progett. Restauro 2003, 9, 39–46. [Google Scholar]
- Hwang, J.-H.; Jeong, H.; Jung, Y.-O.; Nam, K.T.; Lim, K.-M. Skin irritation and inhalation toxicity of biocides evaluated with reconstructed human epidermis and airway models. Food Chem. Toxicol. 2021, 150, 112064. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.M.T. Novel Biocides for Cultural Heritage, Évora. Available online: https://dspace.uevora.pt/rdpc/bitstream/10174/21001/1/Tese%20doutoramento%20Mara%20Silva_vers%c3%a3o%20final.pdf (accessed on 13 January 2022).
- Schmitz-Felten, E.; Kuhl, K.; Graveling, R. Occupational Exposure to Biocides (Disinfectants and Metal Working Fluids)—OSHWiki. Available online: http://oshwiki.eu/wiki/Occupational_exposure_to_biocides_(disinfectants_and_metal_working_fluids) (accessed on 12 January 2022).
- Di Vito, M.; Bellardi, M.G.; Colaizzi, P.; Ruggiero, D.; Mazzuca, C.; Micheli, L.; Sotgiu, S.; Iannuccelli, S.; Michelozzi, M.; Mondello, F.; et al. Hydrolates and Gellan: An Eco-innovative Synergy for Safe Cleaning of Paper Artworks. Stud. Conserv. 2018, 63, 13–23. [Google Scholar] [CrossRef]
- Mecklenburg, M.F.; Charola, A.E.; Koestler, R.J. New Insights into the Cleaning of Paintings: Proceedings from the Cleaning 2010 International Conference, Universidad Politécnica de Valencia and Museum Conservation Institute. Smithson. Contrib. Mus. Conserv. 2013, 3, 1–243. [Google Scholar] [CrossRef]
- Cremonesi, P. Dipinti ad Olio Moderni. Pulitura Superficiale e Rimozione di Vernici; Il Prato: Padova, Italy, 2020. [Google Scholar]
- Bonino, V.E.S. Dall’olio All’acrilico, Dall’impressionismo All’arte Contemporanea; Il Prato: Padova, Italy, 2016. [Google Scholar]
- Stupar, M.; Grbić, M.L.; Simić, G.S.; Jelikić, A.; Vukojević, J.; Sabovljević, M. A sub-aerial biofilms investigation and new approach in biocide application in cultural heritage conservation: Holy Virgin Church (Gradac Monastery, Serbia). Indoor Built Environ. 2012, 23, 584–593. [Google Scholar] [CrossRef]
- Stupar, M.; Grbić, M.L.; Džamić, A.; Unković, N.; Ristić, M.; Jelikić, A.; Vukojevic, J. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. S. Afr. J. Bot. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential Oils as Natural Biocides in Conservation of Cultural Heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spada, M.; Cuzman, O.A.; Tosini, I.; Galeotti, M.; Sorella, F. Essential oils mixtures as an eco-friendly biocidal solution for a marble statue restoration. Int. Biodeterior. Biodegrad. 2021, 163, 105280. [Google Scholar] [CrossRef]
- Veneranda, M.; Blanco-Zubiaguirre, L.; Roselli, G.; Di Girolami, G.; Castro, K.; Madariaga, J.M. Evaluating the exploitability of several essential oils constituents as a novel biological treatment against cultural heritage biocolonization. Microchem. J. 2018, 138, 1–6. [Google Scholar] [CrossRef]
- Borrego, S.; Valdés, O.; Vivar, I.; Lavin, P.; Guiamet, P.; Battistoni, P.; de Saravia, S.G.; Borges, P. Essential Oils of Plants as Biocides against Microorganisms Isolated from Cuban and Argentine Documentary Heritage. ISRN Microbiol. 2012, 2012, 826786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsayed, Y.; Shabana, Y. The effect of some essential oils on aspergillus niger and alternaria alternata infestation in archaeological oil paintings. Mediterr. Archaeol. Archaeom. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Matusiak, K.; Machnowski, W.; Wrzosek, H.; Polak, J.; Rajkowska, K.; Śmigielski, K.B.; Kunicka-Styczyńska, A.; Gutarowska, B. Application of Cinnamomum zeylanicum essential oil in vapour phase for heritage textiles disinfection. Int. Biodeterior. Biodegrad. 2018, 131, 88–96. [Google Scholar] [CrossRef]
- Campanella, L.; Angeloni, R.; Cibin, F.; Dell’Aglio, E.; Grimaldi, F.; Reale, R.; Vitali, M. Capsulated essential oil in gel spheres for the protection of cellulosic cultural heritage. Nat. Prod. Res. 2021, 35, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad, M.; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 2016, 112, 116–131. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Ren, J.-N.; Li, X.; Fan, G.; Qu, S.-S.; Song, Y.; Li, Y.; Pan, S.-Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef] [PubMed]
| Sample | Technique | Preparation | Paint Layer |
|---|---|---|---|
| Ac | Acrylic | Industrial | Industrial colors: bright red, ultramarine blue, green, yellow ocher |
| V | Vinyl | Industrial | Industrial colors: ruby red, ultramarine blue, green, yellow ocher |
| Al | Alkyd | Industrial | Industrial colors: cadmium red, ultramarine blue, green earth, yellow ocher |
| N° | Component 1 | LRI 2 | LRI 3 | EO (%) 4 | EO (%) 5 |
|---|---|---|---|---|---|
| 1 | α-thujene | 920 | 923 | 0.1 | 8.3 |
| 2 | α-pinene | 938 | 943 | 2.7 | tr |
| 3 | camphene | 942 | 946 | 0.2 | tr |
| 4 | β-pinene | 985 | 986 | 0.2 | tr |
| 5 | α-phellandrene | 998 | 996 | 0.4 | 1.4 |
| 6 | p-cymene | 1020 | 1026 | 3.8 | 8.2 |
| 7 | 1,8-cineole | 1022 | 1027 | 4.4 | 17.5 |
| 8 | γ-terpinene | 1050 | 1054 | tr | tr |
| 9 | cis-linalool oxide | 1060 | 1058 | tr | tr |
| 10 | linalool | 1089 | 1092 | 4.9 | 17.2 |
| 11 | camphor | 1130 | 1126 | 0.1 | - |
| 12 | α-terpineol | 1019 | 1021 | 0.1 | tr |
| 13 | 4-terpinenyl acetate | 1283 | 1286 | 0.1 | tr |
| 14 | O-anisaldehyde | 1250 | 1242 * | 0.1 | - |
| 15 | cinnamaldheyde | 1269 | 1275 | 66.0 | 24.5 |
| 16 | eugenol | 1333 | 1331 | 4.1 | tr |
| 17 | β-caryophyllene | 1429 | 1426 | 5.8 | 19.2 |
| 18 | (E)-cinnamyl acetate | 1441 | 1439 | 5.5 | - |
| 19 | humulene | 1450 | 1454 | 0.4 | tr |
| 20 | eugenol acetate | 1480 | 1483 | 0.1 | - |
| 21 | O-methoxy cinnamaldheyde | 1507 | 1505 | 0.3 | - |
| 22 | δ-cadinene | 1529 | 1530 | 0.1 | tr |
| 23 | caryophyllene oxide | 1577 | 1583 | 0.3 | tr |
| 24 | benzyl benzoate | 1741 | 1739 | 0.3 | - |
| SUM | 100.0 | 96.3 | |||
| Monoterpene hydrocarbons | 7.4 | 17.9 | |||
| Oxygenated monoterpenes | 9.6 | 34.7 | |||
| Sesquiterpene hydrocarbons | 6.3 | 19.2 | |||
| Oxygenated sesquiterpene | 0.3 | - | |||
| Others | 76.4 | 24.5 |
| N. of Spray | Hours | ||
|---|---|---|---|
| 2 | 5 | 24 | |
| 5 | − | − | − |
| 4 | +/− | − | − |
| 3 | + | + | − |
| 2 | + | + | + |
| Color | Technique | Unaged | Aged | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A-BT 1 | A-AT 2 | AV 3 | SD 4 | A-BT | A-AT | AV | SD | ||
| Red | Ac 5 | 7.00 | 6.73 | −0.27 (n.s.) | 0.32 | 6.63 | 6.83 | 0.20 (+) | 0.00 |
| V 6 | 6.70 | 6.67 | −0.03 (n.s.) | 0.06 | 6.93 | 6.47 | −0.47 (**) | 0.06 | |
| Al 7 | 6.23 | 6.53 | 0.30 (n.s.) | 0.10 | 6.67 | 6.30 | −0.37 (+) | 0.12 | |
| Blue | Ac | 6.83 | 6.73 | −0.10 (n.s.) | 0.10 | 6.67 | 6.77 | 0.10 (n.s.) | 0.10 |
| V | 6.73 | 6.67 | −0.07 (n.s.) | 0.12 | 6.67 | 6.70 | 0.03 (n.s.) | 0.06 | |
| Al | 6.30 | 6.53 | 0.23 (n.s.) | 0.15 | 6.67 | 6.40 | −0.27 (*) | 0.06 | |
| Green | Ac | 7.27 | 6.83 | −0.43 (+) | 0.23 | 6.87 | 6.93 | 0.07 (n.s.) | 0.06 |
| V | 6.63 | 6.80 | 0.17 (*) | 0.06 | 6.80 | 6.70 | −0.10 (n.s.) | 0.10 | |
| Al | 6.17 | 6.27 | 0.10 (n.s.) | 0.10 | 6.27 | 6.17 | −0.10 (n.s.) | 0.20 | |
| Ocher | Ac | 7.03 | 6.70 | −0.33 (*) | 0.06 | 6.47 | 6.73 | 0.27 (+) | 0.15 |
| V | 6.47 | 6.57 | 0.10 (n.s.) | 0.10 | 6.67 | 6.70 | 0.03 (n.s.) | 0.06 | |
| Al | 6.43 | 6.40 | −0.03 (n.s.) | 0.23 | 6.50 | 6.40 | −0.10 (n.s.) | 0.10 | |
| Unaged Canvas (Mean Values) | Aged Canvas (Mean Values) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | Before Treatment | After Treatment | ||||||||||||||
| Color | Sample | L* | a* | b* | L* | a* | b* | ∆E | SD-DE 1 | L* | a* | b* | L* | a* | b* | ∆E | SD-DE |
| Red | A 2 | 48.9 | 57.5 | 46.5 | 49.5 | 58.0 | 46.5 | 1.15 | 0.44 | 47.5 | 55.2 | 40.8 | 46.9 | 54.8 | 40.4 | 0.82 | 0.09 |
| V 3 | 43.3 | 58.7 | 30.9 | 43.6 | 58.7 | 30.6 | 0.60 | 0.22 | 42.8 | 57.5 | 28.8 | 42.6 | 57.5 | 30.1 | 1.33 | 0.21 | |
| Al 4 | 50.8 | 54.7 | 37.4 | 50.8 | 55.4 | 38.2 | 1.06 | 0.17 | 48.0 | 53.2 | 35.3 | 49.1 | 53.7 | 35.8 | 1.35 | 0.17 | |
| Blue | A | 28.0 | 26.2 | −51.9 | 27.2 | 27.1 | −52.7 | 1.4 | 0.05 | 29.2 | 28.3 | −56.3 | 27.9 | 29.2 | −55.9 | 1.73 | 0.08 |
| V | 29.0 | 34.2 | −65.1 | 27.2 | 29.7 | −56.1 | 10.3 | 4.64 | 32.6 | 30.2 | −63.8 | 30.3 | 33.9 | −65.9 | 4.89 | 0.28 | |
| Al | 26.0 | 15.4 | −30.8 | 25.3 | 15.8 | −31.2 | 3.73 | 1.70 | 32.2 | 21.6 | −56.6 | 32.2 | 20.2 | −54.4 | 2.69 | 0.45 | |
| Green | A | 35.7 | −3.33 | 7.79 | 35.8 | −3.18 | 7.51 | 0.35 | 0.22 | 35.7 | −2.97 | 8.59 | 34.9 | −2.98 | 7.94 | 1.07 | 0.21 |
| V | 37.7 | −7.36 | 12.8 | 37.9 | −7.35 | 12.5 | 0.44 | 0.07 | 37.7 | −7.41 | 13.5 | 37.0 | −7.28 | 13.0 | 1.09 | 0.36 | |
| Al | 31.6 | −9.32 | 6.26 | 32.9 | −10.8 | 7.39 | 2.28 | 1.00 | 36.5 | −9.55 | 4.81 | 36.4 | −9.55 | 5.14 | 1.56 | 0.50 | |
| Ocher | A | 63.3 | 15.2 | 45.6 | 63.4 | 15.3 | 45.5 | 0.45 | 0.15 | 61.3 | 15.1 | 45.2 | 61.3 | 15.2 | 44.8 | 1.48 | 0.81 |
| V | 65.5 | 14.5 | 48.6 | 64.6 | 14.6 | 47.9 | 1.15 | 0.20 | 65.4 | 14.5 | 48.1 | 64.4 | 14.8 | 48.5 | 1.60 | 1.04 | |
| Al | 57.7 | 17.3 | 45.8 | 50.1 | 15.4 | 36.3 | 12.5 | 14.3 | 49.8 | 14.9 | 34.4 | 49.3 | 14.7 | 33.8 | 0.82 | 0.50 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Vito, M.; Vergari, L.; Mariotti, M.; Proto, M.R.; Barbanti, L.; Garzoli, S.; Sanguinetti, M.; Sabatini, L.; Peduzzi, A.; Bellardi, M.G.; et al. Anti-Mold Effectiveness of a Green Emulsion Based on Citrus aurantium Hydrolate and Cinnamomum zeylanicum Essential Oil for the Modern Paintings Restoration. Microorganisms 2022, 10, 205. https://doi.org/10.3390/microorganisms10020205
Di Vito M, Vergari L, Mariotti M, Proto MR, Barbanti L, Garzoli S, Sanguinetti M, Sabatini L, Peduzzi A, Bellardi MG, et al. Anti-Mold Effectiveness of a Green Emulsion Based on Citrus aurantium Hydrolate and Cinnamomum zeylanicum Essential Oil for the Modern Paintings Restoration. Microorganisms. 2022; 10(2):205. https://doi.org/10.3390/microorganisms10020205
Chicago/Turabian StyleDi Vito, Maura, Lara Vergari, Melinda Mariotti, Maria Rita Proto, Lorenzo Barbanti, Stefania Garzoli, Maurizio Sanguinetti, Luigia Sabatini, Alice Peduzzi, Maria Grazia Bellardi, and et al. 2022. "Anti-Mold Effectiveness of a Green Emulsion Based on Citrus aurantium Hydrolate and Cinnamomum zeylanicum Essential Oil for the Modern Paintings Restoration" Microorganisms 10, no. 2: 205. https://doi.org/10.3390/microorganisms10020205
APA StyleDi Vito, M., Vergari, L., Mariotti, M., Proto, M. R., Barbanti, L., Garzoli, S., Sanguinetti, M., Sabatini, L., Peduzzi, A., Bellardi, M. G., Mattarelli, P., Bugli, F., & De Luca, D. (2022). Anti-Mold Effectiveness of a Green Emulsion Based on Citrus aurantium Hydrolate and Cinnamomum zeylanicum Essential Oil for the Modern Paintings Restoration. Microorganisms, 10(2), 205. https://doi.org/10.3390/microorganisms10020205












