Susceptibility of Fluconazole-Resistant Candida albicans to Thyme Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Characterization of Candida albicans
2.1.1. Identification of Candida sp. by CHROMagar
2.1.2. Germ Tube Test for the Identification of Candida albicans
2.1.3. API 20c Aux System for C. albicans Identification
2.1.4. Differentiation between C. albicans Isolates by RAPD-PCR Technique
2.1.5. Fluconazole Susceptibility Test
2.2. Plant Material Collection and Extraction of Thyme Essential Oil (TEO)
2.3. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis of Thyme Essential Oil (TEO)
2.4. Antifungal Activity of Thyme Essential Oil (TEO) against C. albicans Isolates
2.4.1. Inhibitory Effect of TEO on C. albicans Using Disc Diffusion Assay
2.4.2. Determination of Minimal Inhibitory (MIC) and Fungicidal Concentration (MFC)
2.4.3. Effect of TEO on Budding of C. albicans Isolates
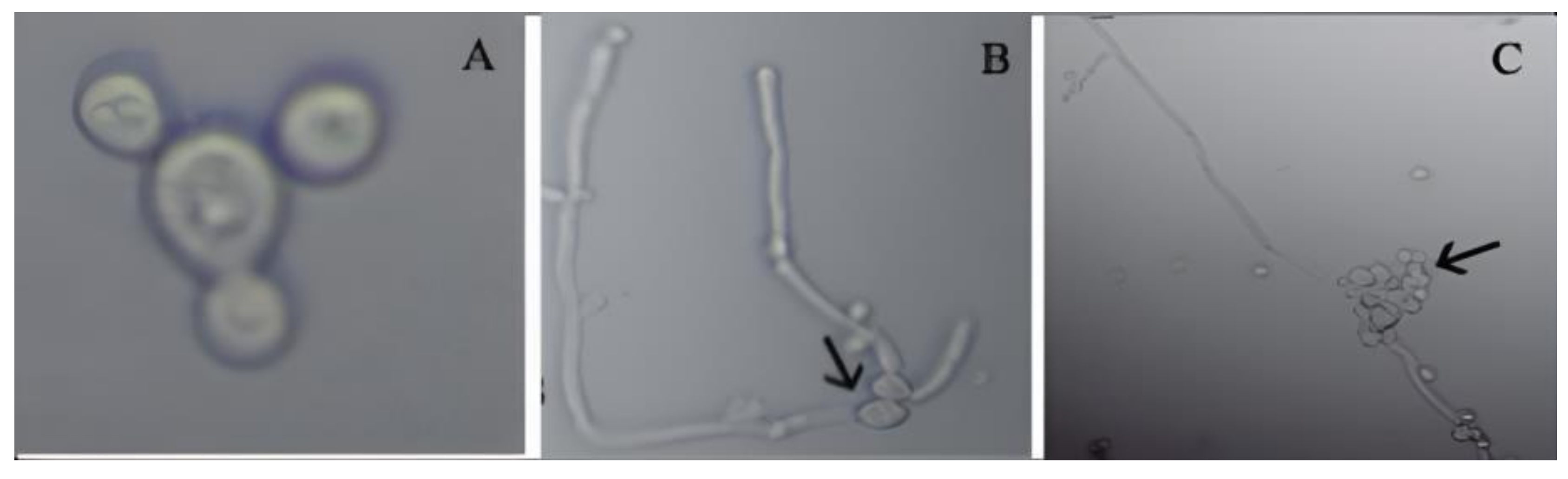
2.4.4. Effect of TEO on Germ Tubes Formation of C. albicans Isolates
2.4.5. Time Kill Curve Assay
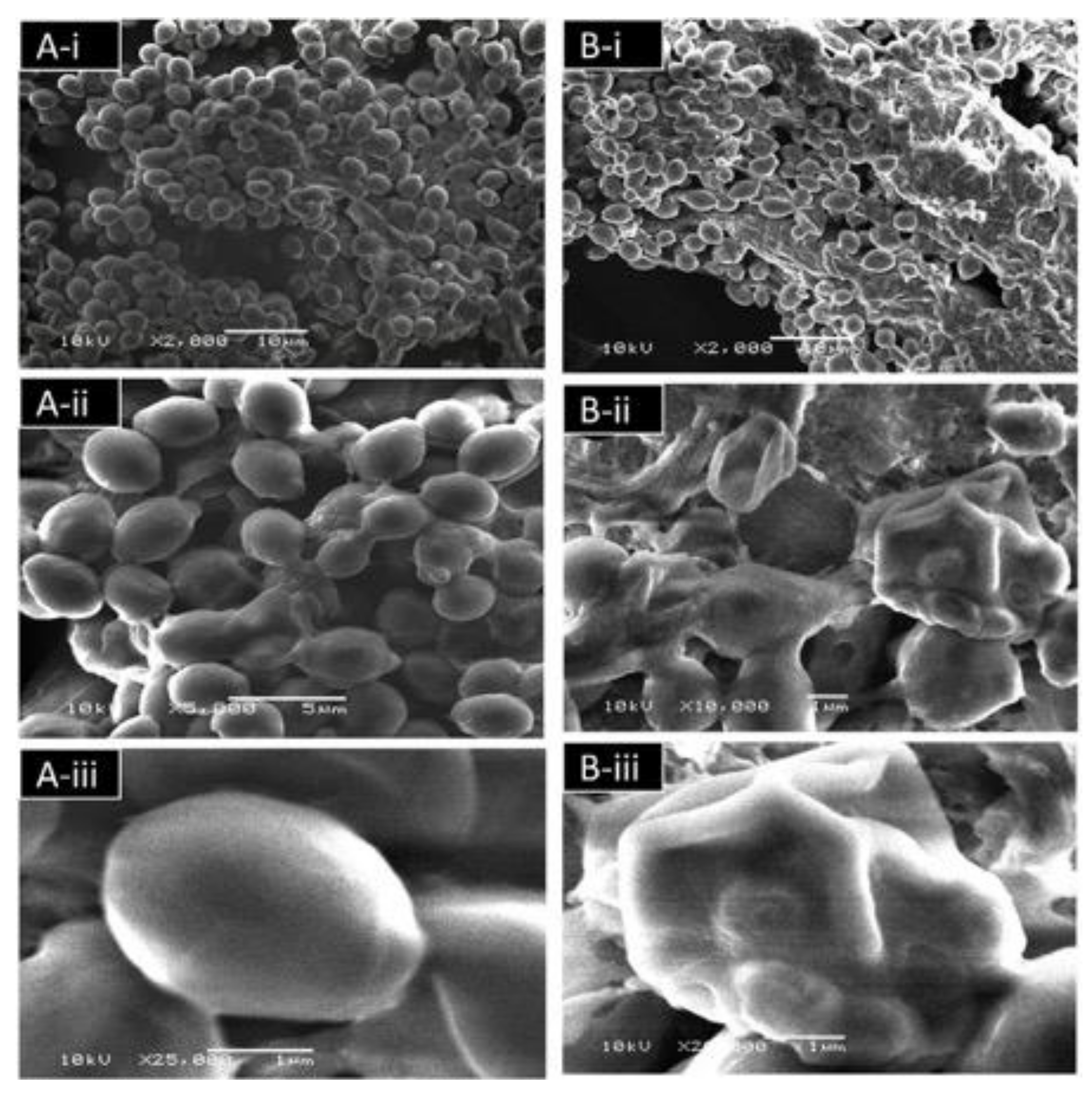
2.4.6. Effect of TEO on the C. albicans Ultrastructure by Scanning Electron Microscopic (SEM)
3. Results
3.1. Candida spp. Isolates
3.1.1. CHROMagar Based Identification of Candida spp.
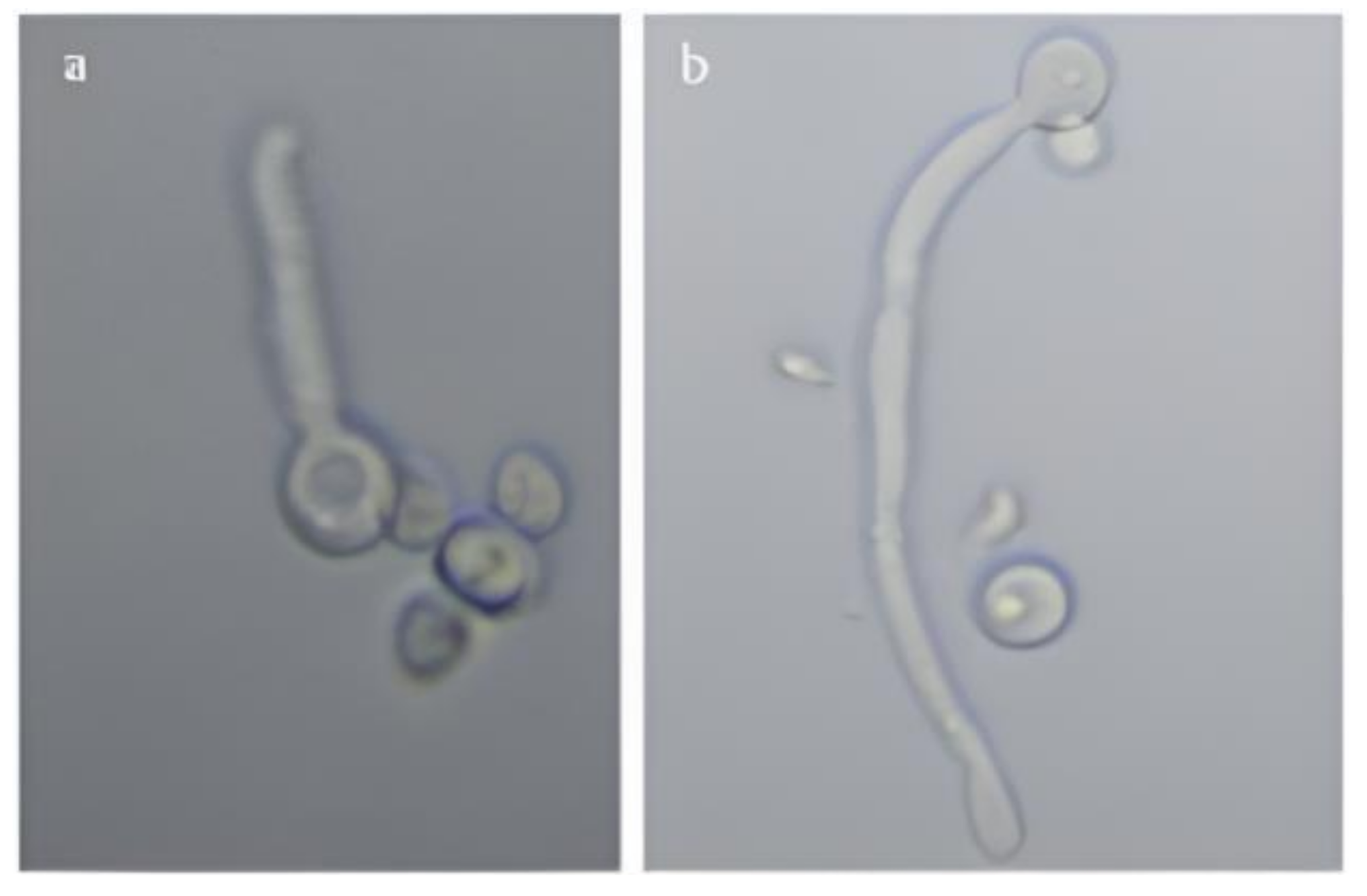
3.1.2. Determination of Germ Tube Formation of C. albicans
3.1.3. Morphological Examination Using Microscopy
3.1.4. API 20c Aux Based C. albicans Identification
3.1.5. Differentiation between C. albicans Isolates by RAPD-PCR Technique
3.2. Extraction of Thyme Essential Oil (TEO)
3.3. GC/MS Analysis of Thyme Essential Oil (TEO)
3.4. Antifungal Activity of Thyme Essential Oil (TEO) against C. albicans Isolates
3.4.1. Inhibitory Effect of TEO on C. albicans Isolates Using Disc Diffusion Assay
3.4.2. Determination of Minimal Inhibitory Concentration (MIC) and Fungicidal Concentration (MFC) of Thyme Essential Oil (TEO)
3.4.3. Effect of TEO on Budding of C. albicans
3.4.4. Effect of TEO on Germ Tubes Formation of C. albicans Isolates
3.4.5. Time-Kill Curves of C. albicans Isolates
3.4.6. Effect of TEO on the C. albicans Ultrastructure Detected by Scanning Electron Microscopy (SEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Jasser, A.M.; Elkhizzi, N.A. Distribution of Candida species among bloodstream isolates. Saudi Med. J. 2004, 25, 566–569. [Google Scholar]
- Badhman, H. Variation in Growth of Candida albicans in Different Media. Ph.D. Thesis, King Abdul-Aziz University, Jeddah, Saudi Arabia, 2006. [Google Scholar]
- Edwards, J. Candida species. In Principles and Practice of Infectious Diseases; Mandell, G.L., Douglas, R.G., Bennett, J.E., Eds.; Churchill Livingstone: New York, NY, USA, 1995; pp. 2289–2301. [Google Scholar]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Eggimann, P.; Garbino, J.; Pittet, D. Management of candidiasis Management of Candida species infections in critically ill patients. Lancet Infect. Dis. 2003, 3, 772–785. [Google Scholar] [CrossRef]
- Al-Hedaithy, S. Spectrum and proteinase production of yeasts causing vaginitis in Saudi Arabian women. Med. Sci. Monit. 2002, 8, CR498–CR501. [Google Scholar]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Manzoor, N.; Khan, L.A. Antifungal Activities of Ocimum sanctum Essential Oil and its Lead Molecules. Nat. Prod. Commun. 2010, 5, 1934578X1000500. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Khan, A.; Manzoor, N.; Khan, L.A. Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida. Microb. Pathog. 2010, 48, 35–41. [Google Scholar] [CrossRef]
- Pinto, E.; Pina-Vaz, C.; Salgueiro, L.; Gonçalves, M.J.; de-Oliveira, C.S.; Cavaleiro, C.; Palmeira, A.; Rodrigues, A.; de-Oliveira, M.J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2006, 55, 1367–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzouz, M.; Bullerman, L. Comparative Antimycotic Effects of Selected Herbs, Spices, Plant Components and Commercial Antifungal Agents1. J. Food Prot. 1982, 45, 1298–1301. [Google Scholar] [CrossRef]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and Antifungal Properties of Essential Oil Components. J. Essent. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Ahmad, I.; Qais, F.; Samreen; Abulreesh, H.; Ahmad, S.; Rumbaugh, K. Antibacterial Drug Discovery: Perspective Insights. In Antibacterial Drug Discovery to Combat MDR; Springer: Singapore, 2019; pp. 1–21. [Google Scholar]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Barreca, S.; La Bella, S.; Maggio, A.; Licata, M.; Buscemi, S.; Leto, C.; Pace, A.; Tuttolomondo, T. Flavouring Extra-Virgin Olive Oil with Aromatic and Medicinal Plants Essential Oils Stabilizes Oleic Acid Composition during Photo-Oxidative Stress. Agriculture 2021, 11, 266. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; del Contreras, M.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phyther. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phyther. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil—New insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Nadeem, S.G.; Hakim, S.T.; Kazmi, S.U. Use of CHROMagar Candida for the presumptive identification of Candida species directly from clinical specimens in resource-limited settings. Libyan J. Med. 2010, 5, 2144. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Locas, M.-C.; Restieri, C.; Laverdiere, M. Utility of the Germ Tube Test for Direct Identification of Candida albicans from Positive Blood Culture Bottles. J. Clin. Microbiol. 2008, 46, 3508–3509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gundes, S.; Gulenc, S.; Bingol, R. Comparative performance of Fungichrom I, Candifast and API 20C Aux systems in the identification of clinically significant yeasts. J. Med. Microbiol. 2001, 50, 1105–1110. [Google Scholar] [CrossRef]
- Scherer, S.; Stevens, D. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J. Clin. Microbiol. 1987, 25, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Abed, K.M.; Naife, T.M. Extraction of Essential Oil from Iraqi Eucalyptus Camadulensis Leaves by Water Distillation Methods. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012163. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Malik, A.; Ahmad, I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med. Mycol. 2012, 50, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafri, H.; Banerjee, G.; Khan, M.S.A.; Ahmad, I.; Abulreesh, H.H.; Althubiani, A.S. Synergistic interaction of eugenol and antimicrobial drugs in eradication of single and mixed biofilms of Candida albicans and Streptococcus mutans. AMB Express 2020, 10, 185. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, N.; Perveen, K. Anti-candidal Activity and Chemical Composition of Essential Oil of Clove (Syzygium aromaticum). J. Essent. Oil Bear. Plants 2017, 20, 951–958. [Google Scholar] [CrossRef]
- Odds, F.; Bernaerts, R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J. Clin. Microbiol. 1994, 32, 1923–1929. [Google Scholar] [CrossRef] [Green Version]
- Yucesoy, M.; Marol, S. Performance of CHROMAgar and BIGGY agar for identification of yeast species. Ann. Clin. Microbiol. Antimicrob. 2003, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakumar, V.G.; Shankar, P.; Nalina, K.; Menon, T. Use of CHROMagar in the Differentiation of Common Species of Candida. Mycopathologia 2009, 167, 47–49. [Google Scholar] [CrossRef]
- Yera, H.; Poulain, D.; Lefebvre, A.; Camus, D.; Sendid, B. Polymicrobial candidaemia revealed by peripheral blood smear and chromogenic medium. J. Clin. Pathol. 2004, 57, 196–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linares, M.J.; Charriel, G.; Solís, F.; Casal, M. CHROMAgar Candida with fluconazole: Comparison with microdilution techniques. Enferm. Infecc. Microbiol. Clin. 2003, 21, 493–497. [Google Scholar] [CrossRef] [PubMed]
- AlHedaithy, S. The yeast species causing fungemia at a university hospital in Riyadh, Saudi Arabia, during a 10-year period. Das Hefespektrum der Fungamien an einem Universitatshospital in Riad, Saudi-Arabien, wahrend einer Zehnjahresperiode. Mycoses 2003, 46, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Salkin, I.; Land, G.; Hurd, N.; Goldson, P.; McGinnis, M. Evaluation of Yeast- Ident and Uni-Yeast-tek yeast identification systems. J. Clin. Microbiol. 1987, 25, 624–627. [Google Scholar] [CrossRef] [Green Version]
- Makwana, G.; Gadhavi, H.; Sinha, M. Comparison of germ tube production by Candida albicans in various media. NJIRM 2012, 3, 6–8. [Google Scholar]
- Arora, D.R.; Saini, S.; Aparna; Gupta, N. Evaluation of germ tube test in various media. Indian J. Pathol. Microbiol. 2003, 46, 124–126. [Google Scholar]
- Deorukhkar, S.; Saini, S.; Jadhav, P. Evaluation of different media for germ tube production of Candida albicans and Candida dubliniensis. IJBAR 2012, 3, 704–707. [Google Scholar] [CrossRef]
- Schuffenecker, I.; Freydière, A.; Montclos, H.; Gille, Y. Evaluation of four commercial systems for identification of medically important yeasts. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 255–260. [Google Scholar] [CrossRef]
- Anwar, K.P.; Malik, A.; Subhan, K.H. Profile of candidiasis in HIV infected patients. Iran. J. Microbiol. 2012, 4, 204–209. [Google Scholar]
- Costa, C.; Silva, M.; Souza, L.; ElAssal, F.; Ataíde, F.; Paula, C. RAPD profile among Candida albicans isolates by using different primers. Rev. Patol. Trop. 2010, 39, 41–47. [Google Scholar]
- Dassanayake, R.; Samaranayake, L. Characterization of the genetic diversity in superficial and systemic human isolates of Candida parapsilosis by randomly amplified polymorphic DNA (RAPD). APMIS 2000, 108, 153–160. [Google Scholar] [CrossRef]
- Khan, A.A.; Amjad, M.S. Saboon GC-MS analysis and biological activities of Thymus vulgaris and Mentha arvensis essential oil. Turk. J. Biochem. 2019, 44, 388–396. [Google Scholar] [CrossRef]
- Sefidkon, F.; Jamzad, Z. Chemical composition of the essential oil of three Iranian Satureja species (S. mutica, S. macrantha and S. intermedia). Food Chem. 2005, 91, 1–4. [Google Scholar] [CrossRef]
- Móricz, Á.; Ott, P.; Böszörményi, A.; Lemberkovics, É.; Mincsovics, E.; Tyihák, E. Bioassay-Guided Isolation and Identification of Antimicrobial Compounds from Thyme Essential Oil by Means of Overpressured Layer Chromatography, Bioautography and GC–MS. Chromatographia 2012, 75, 991–999. [Google Scholar] [CrossRef] [Green Version]
- Moghtader, M. Antifungal effects of the essential oil from Thymus vulgaris L. and comparison with synthetic thymol on Aspergillus niger. J. Yeast Fungal Res. 2012, 3, 83–88. [Google Scholar] [CrossRef]
- Negahban, M.; Saeedfar, S. Essential Oil Composition of Thymus vulgaris L. Russ. J. Biol. Res. 2015, 3, 35–38. [Google Scholar] [CrossRef]
- Behbahani, M.; Ghasemi, Y.; Khoshnoud, M.; Faridi, P.; Moradli, G.; Najafabady, N. Volatile oil composition and antimicrobial activity of two Thymus species. Pharmacogn. J. 2013, 5, 77–79. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Lech, K.; Szumny, A.; Carbonell-Barrachina, Á.A. Effects of Drying Methods on the Composition of Thyme (Thymus vulgaris L.) Essential Oil. Dry. Technol. 2013, 31, 224–235. [Google Scholar] [CrossRef]
- Omidbeygi, M.; Barzegar, M.; Hamidi, Z.; Naghdibadi, H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control 2007, 18, 1518–1523. [Google Scholar] [CrossRef]
- Gad, H.A.; Ayoub, I.M.; Wink, M. Phytochemical profiling and seasonal variation of essential oils of three Callistemon species cultivated in Egypt. PLoS ONE 2019, 14, e0219571. [Google Scholar] [CrossRef] [Green Version]
- Pavel, M.; Alecu, F. Antifungal activity of Thymus serpyllum essential oil against Candida albicans and Candida non-albicans clinical isolates: R2458. Clin. Microbiol. Infect. 2008, 14, 19–22. [Google Scholar]
- Naeini, A.; Khosravi, A.R.; Chitsaz, M.; Shokri, H.; Kamlnejad, M. Anti- Candida albicans activity of some Iranian plants used in traditional medicine. J. Mycol. Med. 2009, 19, 168–172. [Google Scholar] [CrossRef]
- Omran, S.; Esmailzadeh, S. Comparison of anti-Candida activity of thyme, pennyroyal, and lemon essential oils versus antifungal drugs against Candida species. Jundishapur J. Microbiol. 2009, 2, 53–60. [Google Scholar]
- Ownagh, A.; Majdani, R.; Yaghobzadeh, N.; Nemati, Z. Antifungal effects of Thyme oil on Candida albicans and Aspergillus fumigates. Iran. J. Vet. Med. 2008, 22, 1–7. [Google Scholar]
- Dalirsani, Z.; Adibpour, M.; Aghazadeh, M.; Amirchaghmaghi, M.; Mozafari, P.; Hamzei, F. In vitro comparison of inhibitory activity of 10 plant extracts against Candida albicans. Aust. J. Basic Appl. Sci. 2011, 5, 930–935. [Google Scholar]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and Cytotoxic Activities of Origanum Essential Oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Rota, C.; Carraminana, J.; Burillo, J.; Herrera, A. In Vitro Antimicrobial Activity of Essential Oils from Aromatic Plants against Selected Foodborne Pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [CrossRef]
- Omran, S.; Esmaeilzadeh, S.; Rahmani, Z. Laboratory study of anticandidal activity of thyme, pennyroyal and lemon essential oils by micro dilution method. Jundishapur J. Microbiol. 2010, 3, 161–167. [Google Scholar]
- Adam, K.; Sivropoulou, A.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antifungal Activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa Essential Oils against Human Pathogenic Fungi. J. Agric. Food Chem. 1998, 46, 1739–1745. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and Antimicrobial Activity of the Essential Oils of Two Origanum Species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Roccaro, S.A.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Nguefack, J.; Leth, V.; Zollo, A.P.H.; Mathur, S.B. Evaluation of five essential oils from aromatic plants of Cameroon for controlling food spoilage and mycotoxin producing fungi. Int. J. Food Microbiol. 2004, 94, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Pina-Vaz, C.; Rodrigues, G.A.; Pinto, E.; de-Oliveira, C.S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Goncalves, M.; de-Oliveira, M.J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ueda-Nakamura, T.; Mendonça-Filho, R.R.; Morgado-Díaz, J.A.; Maza, K.P.; Filho, B.P.D.; Cortez, A.G.D.; Alviano, D.S.; do Rosa, M.S.S.; Lopes, A.H.C.S.; Alviano, C.S.; et al. Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum. Parasitol. Int. 2006, 55, 99–105. [Google Scholar] [CrossRef]
- Makki, S.; Olama, Z.; Holail, H. Anti-fungal activity of plant oils against oral clinical isolates of Candida albicans in Lebanese community. Topclass J. Microbiol. 2012, 1, 42–54. [Google Scholar]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Ricci, D. Thymol-Induced Alterations in Candida albicans Imaged by Atomic Force Microscopy. In Atomic Force Microscopy in Biomedical Research; Springer: Berlin/Heidelberg, Germany, 2011; pp. 401–410. [Google Scholar]
- Braga, P.; Sasso, M.; Culici, M.; Alfieri, M. Eugenol and thymol, alone or in combination, induce morphological alterations in the envelope of Candida albicans. Fitoterapia 2007, 78, 396–400. [Google Scholar] [CrossRef]





| Isolate No. | Carbohydrate 1 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLU | GLY | 2KG | ARA | XYL | ADO | XLT | GAL | INO | SOR | MDG | NAG | CEL | LAC | MAL | SAC | TRE | MLZ | RAF | |
| 1 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 2 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 3 | + | - | + | - | + | - | - | + | - | + | + | + | - | - | + | + | + | - | - |
| 4 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 5 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 6 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 7 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 8 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 9 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 10 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 11 | + | - | + | - | + | - | - | + | - | - | + | + | - | - | + | + | + | - | - |
| 12 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 13 | + | - | + | - | + | - | - | + | - | - | + | + | - | - | + | + | + | - | - |
| 14 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 15 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 16 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 17 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 18 | + | - | + | - | + | - | + | + | - | + | + | + | - | - | + | + | + | - | - |
| 19 | + | - | + | - | + | - | - | + | - | + | + | + | - | - | + | + | + | - | - |
| 20 | + | - | + | - | + | - | - | + | - | + | + | + | - | - | + | + | + | - | - |
| No. | Compound Name | RI 1 | % |
|---|---|---|---|
| 1 | α-Pinene | 938 | 0.49 |
| 2 | 1-octen-3-ol | 981 | 1.1 |
| 3 | β-myrcene | 995 | 0.4 |
| 4 | 3-octanol | 998 | 0.3 |
| 5 | α-Phellandrene | 1008 | 0.2 |
| 6 | α-Terpinene | 1019 | 0.98 |
| 7 | Cymol | 1026 | 7.7 |
| 8 | Limonene | 1034 | 0.1 |
| 9 | 1,8-Cineole | 1035 | 0.48 |
| 10 | γ-Terpinene | 1065 | 8.9 |
| 11 | Terpinolene | 1090 | 2.9 |
| 12 | Linalool | 1105 | 1.4 |
| 13 | Borneol | 1167 | 1 |
| 14 | (-)4-trpineol | 1177 | 0.6 |
| 15 | γ-Terpineiol | 1210 | 0.2 |
| 16 | Thymol | 1301 | 68.1 |
| 17 | Carvacrol | 1315 | 1.5 |
| 18 | Isobornyl propionate | 1379 | 0.27 |
| 19 | Caryophyllene | 1423 | 1.1 |
| 20 | α-Humulene | 1455 | 0.15 |
| 21 | Germacrene-D | 1482 | 0.12 |
| 22 | γ-Cadinene | 1520 | 0.18 |
| 23 | Caryophyllene oxide | 1584 | 0.9 |
| 24 | α-Cadinol | 1655 | 0.13 |
| Total | 99.2 | ||
| Oil yield (%) | 0.85 |
| TEO Concentration % | Isolates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean | |
| 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 3 | 0.77 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 |
| 5 | 0.90 | 0.00 | 0.87 | 1.03 | 0.83 | 0.00 | 0.00 | 0.73 | 0.70 | 1.20 | 0.38 |
| 10 | 1.03 | 1.93 | 2.10 | 2.13 | 1.07 | 0.87 | 0.87 | 1.10 | 1.03 | 1.77 | 0.80 |
| 15 | 2.30 | 2.10 | 2.30 | 2.27 | 1.17 | 1.07 | 0.90 | 2.13 | 1.63 | 2.00 | 1.14 |
| 20 | 2.50 | 2.47 | 2.40 | 2.77 | 1.17 | 1.60 | 2.17 | 2.37 | 2.00 | 2.47 | 1.69 |
| 25 | 3.50 | 2.80 | 3.30 | 3.63 | 2.50 | 2.03 | 2.57 | 3.53 | 2.17 | 2.80 | 2.45 |
| 30 | 3.90 | 3.00 | 3.77 | 3.80 | 4.00 | 3.30 | 2.80 | 4.03 | 3.20 | 3.83 | 3.27 |
| 100 | 5.27 | 5.13 | 5.17 | 5.37 | 5.43 | 5.07 | 4.83 | 5.27 | 5.30 | 5.07 | 4.93 |
| Fluco | 2.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.40 | 0.00 | 3.00 | 0.00 | 0.38 |
| mean | 2.0 | 1.7 | 1.6 | 2.0 | 2.0 | 1.4 | 1.4 | 1.1 | 1.3 | 2.0 | |
| LSD 5% | |||||||||||
| Concentration | 0.201 | ||||||||||
| Isolates | 0.181 | ||||||||||
| Conc × isolates | 0.132 | ||||||||||
| TEO Concentration % | Isolates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | Mean | |
| 2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.00 |
| 3 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 | 0.08 |
| 5 | 1.6 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | 0.63 |
| 10 | 1.3 | 0.8 | 0.0 | 1.2 | 1.6 | 0.8 | 1.0 | 0.0 | 0.0 | 1.3 | 1.39 |
| 15 | 1.2 | 1.2 | 0.9 | 1.2 | 1.9 | 0.8 | 1.0 | 0.9 | 0.8 | 1.4 | 1.79 |
| 20 | 2.0 | 1.9 | 1.7 | 2.1 | 2.3 | 1.2 | 1.2 | 1.0 | 1.4 | 2.0 | 2.19 |
| 25 | 2.4 | 3.1 | 3.1 | 3.3 | 3.5 | 1.7 | 2.1 | 1.1 | 1.6 | 2.6 | 2.88 |
| 30 | 3.5 | 3.6 | 3.3 | 3.9 | 4.1 | 3.6 | 2.8 | 2.0 | 2.5 | 3.4 | 3.56 |
| 100% | 4.9 | 5.1 | 5.1 | 5.2 | 4.8 | 4.8 | 4.7 | 4.6 | 5.1 | 5.1 | 5.19 |
| Fluco | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.8 | 0.0 | 2.0 | 0.76 |
| mean | 2.24 | 1.94 | 2.21 | 2.33 | 1.80 | 1.55 | 1.57 | 2.13 | 1.78 | 2.13 | |
| LSD 5% | |||||||||||
| Concentration | 0.0403 | ||||||||||
| Isolates | 0.0419 | ||||||||||
| Conc × isolates | 0.0967 | ||||||||||
| Isolates | MIC | MFC | ||
|---|---|---|---|---|
| Thyme | Fluconazole | Thyme | Fluconazole | |
| 1 | 0.6 | 0 | 1.25 | 0 |
| 2 | 0.6 | 0 | 1.25 | 0 |
| 3 | 0.6 | 0 | 1.25 | 0 |
| 4 | 1.25 | 0 | 2.5 | 0 |
| 5 | 0.6 | 0 | 1.25 | 0 |
| 6 | 0.6 | 0 | 1.25 | 0 |
| 7 | 0.6 | 0 | 1.25 | 0 |
| 8 | 1.25 | 64 | 2.5 | 128 |
| 9 | 1.25 | 0 | 2.5 | 0 |
| 10 | 0.6 | 64 | 1.25 | 128 |
| 11 | 0.6 | 64 | 1.25 | 128 |
| 12 | 0.6 | 0 | 1.25 | 0 |
| 13 | 0.6 | 0 | 1.25 | 0 |
| 14 | 0.6 | 0 | 1.25 | 0 |
| 15 | 0.6 | 0 | 1.25 | 0 |
| 16 | 0.6 | 0 | 1.25 | 0 |
| 17 | 0.6 | 32 | 1.25 | 128 |
| 18 | 0.6 | 0 | 1.25 | 0 |
| 19 | 0.6 | 32 | 1.25 | 64 |
| 20 | 0.6 | 0 | 1.25 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaikh, N.A.; Perveen, K. Susceptibility of Fluconazole-Resistant Candida albicans to Thyme Essential Oil. Microorganisms 2021, 9, 2454. https://doi.org/10.3390/microorganisms9122454
Alshaikh NA, Perveen K. Susceptibility of Fluconazole-Resistant Candida albicans to Thyme Essential Oil. Microorganisms. 2021; 9(12):2454. https://doi.org/10.3390/microorganisms9122454
Chicago/Turabian StyleAlshaikh, Najla A, and Kahkashan Perveen. 2021. "Susceptibility of Fluconazole-Resistant Candida albicans to Thyme Essential Oil" Microorganisms 9, no. 12: 2454. https://doi.org/10.3390/microorganisms9122454






