Isolation of Bacterial and Fungal Microbiota Associated with Hermetia illucens Larvae Reveals Novel Insights into Entomopathogenicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rearing of Hermetia illucens
2.2. Cultivation of Bacterial and Fungal Isolates
2.3. DNA Extraction
2.4. 16S rRNA and ITS PCR
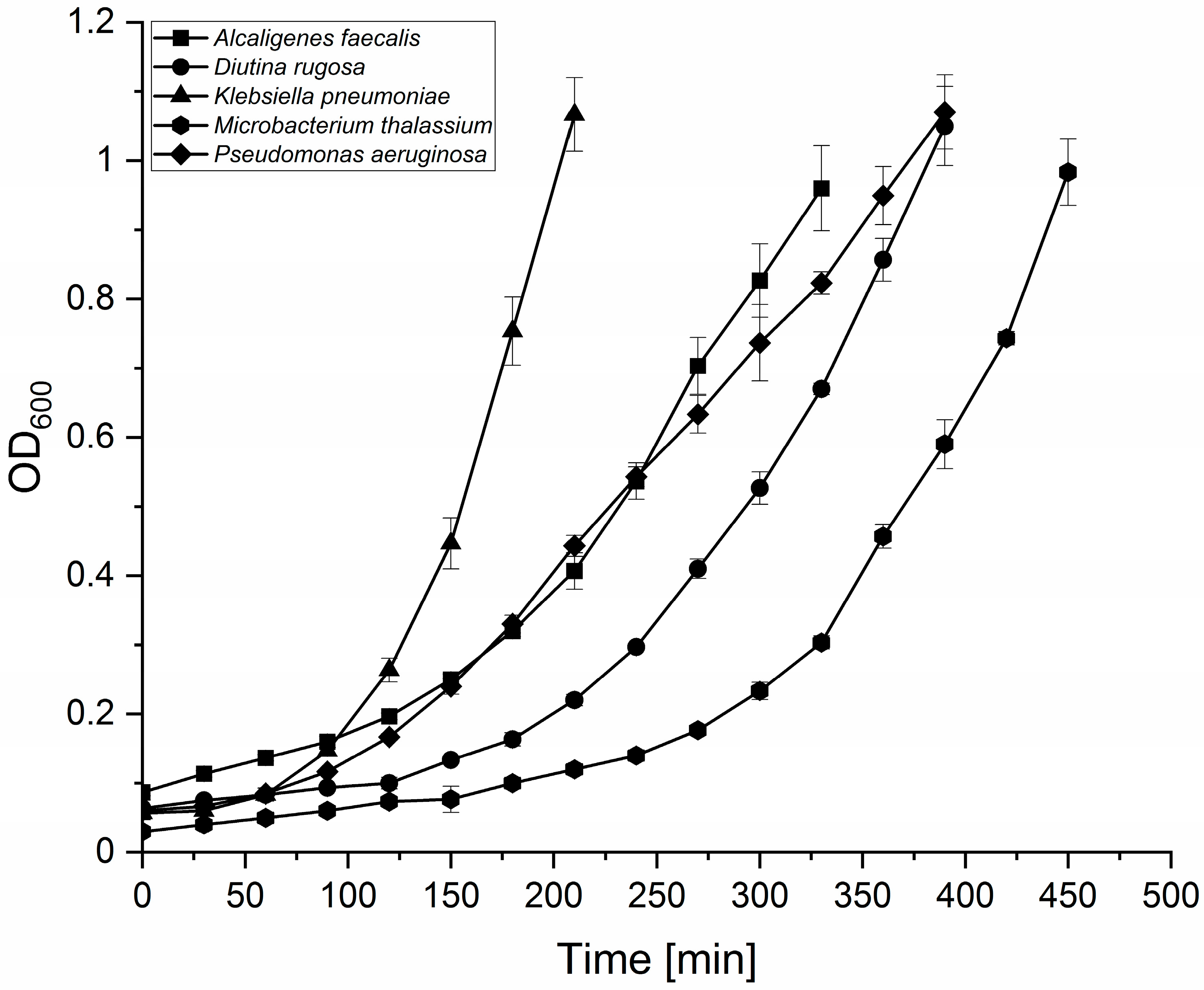
2.5. Growth Curves, OD600/CFU Relationship, and Antibiotic Susceptibility Tests of Putative Entomopathogens
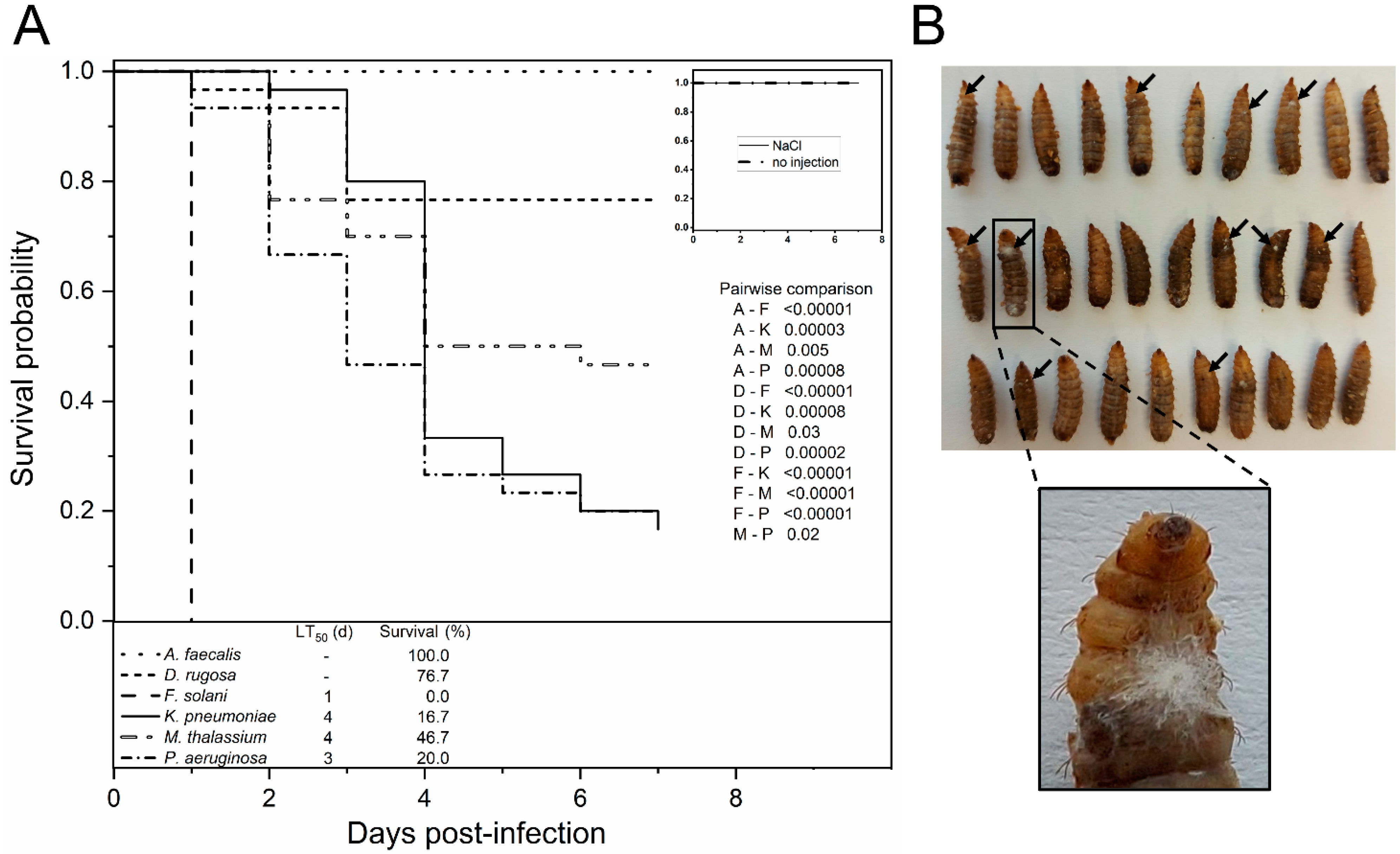
2.6. Injection Assay
2.7. Data Processing
3. Results
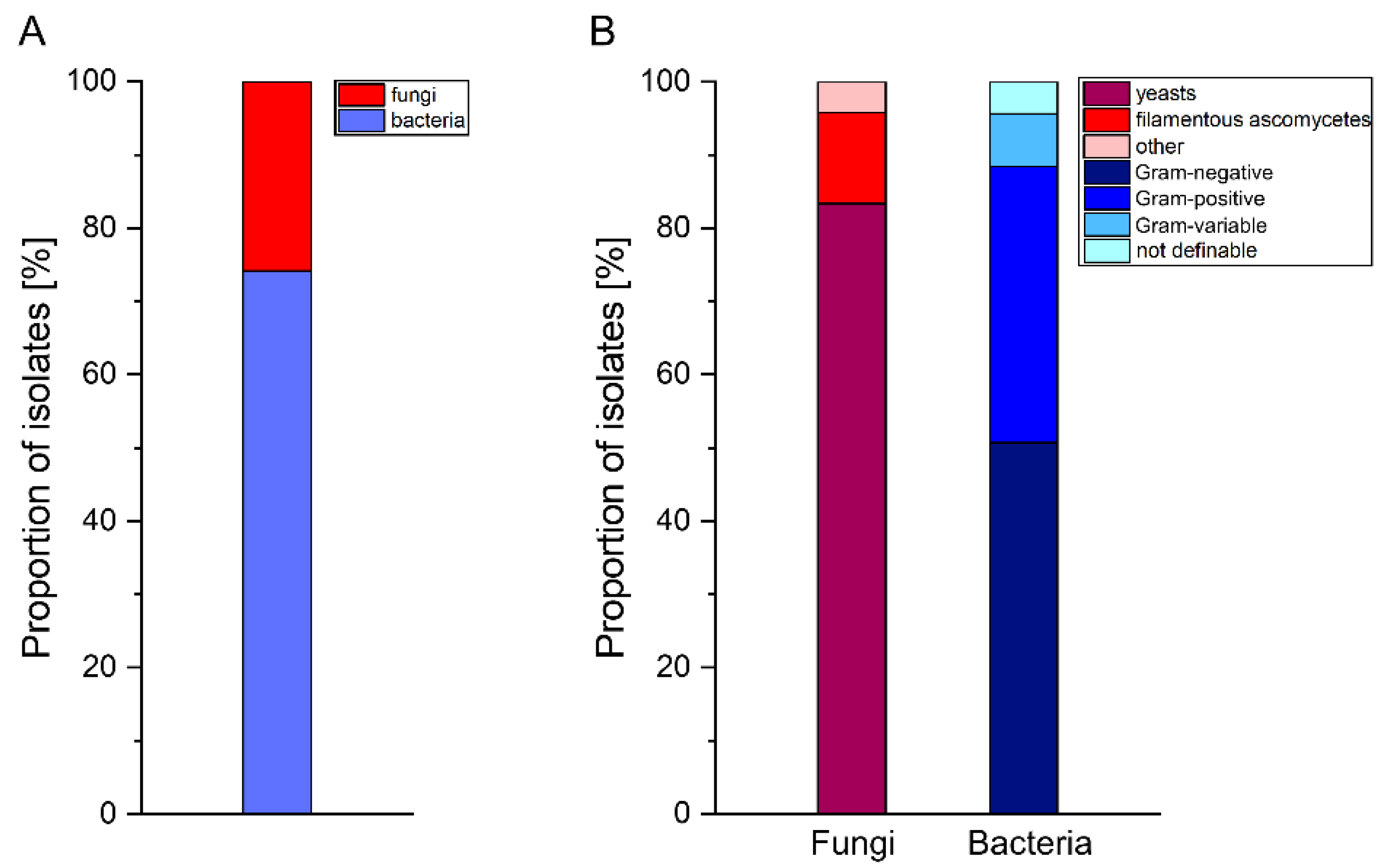
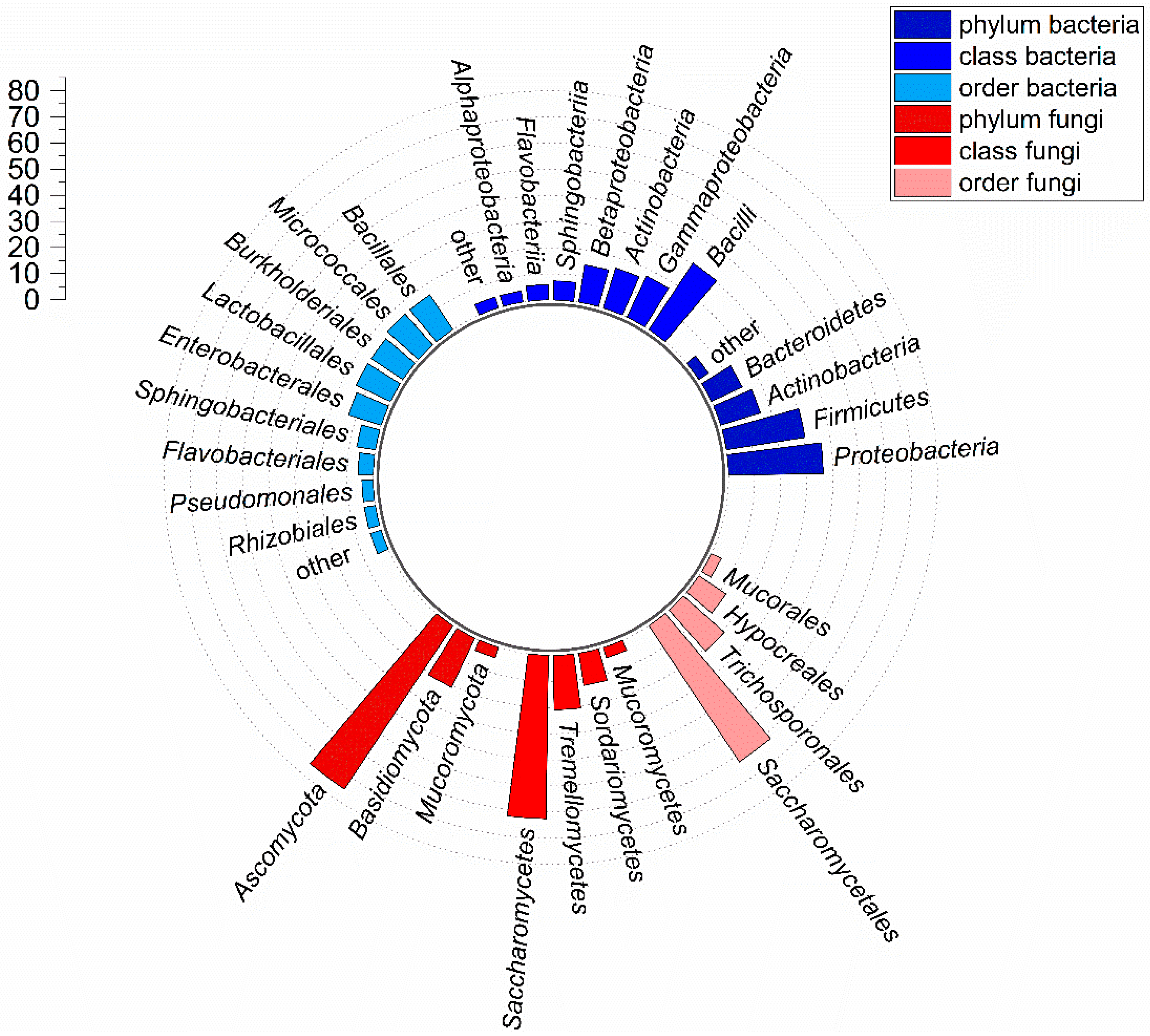
3.1. Taxonomic Composition of the Culturable Gut Microbiota in BSF Larvae Grown on PKM
3.2. Characterization of Putative Entomopathogenic Candidates from BSF Guts
3.3. In Vivo Evaluation of Putative Entomopathogens in BSF Larvae
4. Discussion
4.1. Analysis of the Cultivable Bacterial and Fungal Gut Microbiota in BSF Larvae Grown on PKM
4.2. Investigation of Putative Entomopathogenic Isolates
4.3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- de Smet, J.; Wynants, E.; Cos, P.; van Campenhout, L. Microbial community dynamics during rearing of black soldier fly larvae (Hermetia illucens) and impact on exploitation potential. Appl. Environ. Microbiol. 2018, 84, e02722-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samayoa, A.C.; Chen, W.T.; Hwang, S.Y. Survival and development of Hermetia illucens (Diptera: Stratiomyidae): A biodegradation agent of organic waste. J. Econ. Entomol. 2016, 109, 2580–2585. [Google Scholar] [CrossRef] [PubMed]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; van Huis, A.; Borgemeister, C. The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef] [PubMed]
- St-Hilaire, S.; Cranfill, K.; McGuire, M.A.; Mosley, E.E.; Tomberlin, J.K.; Newton, L.; Sealey, W.; Sheppard, C.; Irving, S. Fish offal recycling by the black soldier fly produces a foodstuff high in omega-3 fatty acids. J. World Aquacult. Soc. 2007, 38, 309–313. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Newton, G.L.; Thompson, S.A.; Savage, S. A value added manure management system using the black soldier fly. Bioresour. Technol. 1994, 50, 275–279. [Google Scholar] [CrossRef]
- Newton, G.L.; Sheppard, D.C.; Watson, D.W.; Burtle, G.; Dove, R. Using the Black Soldier Fly, Hermetia illucens, as a Value-Added Tool for the Management of Swine Manure; Report for Mike Williams, Director of the Animal and Poultry Waste Management Center, North Carolina State University: Raleigh, NC, USA, 2005. [Google Scholar]
- Wang, Y.S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, S.; Spampinato, G.; Macavei, L.I.; Lugli, L.; Candeliere, F.; Rossi, M.; Maistrello, L.; Amaretti, A. Effect of rearing temperature on growth and microbiota composition of Hermetia illucens. Microorganisms 2020, 8, 902. [Google Scholar] [CrossRef]
- Jeon, H.; Park, S.; Choi, J.; Jeong, G.; Lee, S.-B.; Choi, Y.; Lee, S.-J. The intestinal bacterial community in the food waste-reducing larvae of Hermetia illucens. Curr. Microbiol. 2011, 62, 1390–1399. [Google Scholar] [CrossRef]
- Varotto Boccazzi, I.; Ottoboni, M.; Martin, E.; Comandatore, F.; Vallone, L.; Spranghers, T.; Eeckhout, M.; Mereghetti, V.; Pinotti, L.; Epis, S. A survey of the mycobiota associated with larvae of the black soldier fly (Hermetia illucens) reared for feed production. PLoS ONE 2017, 12, e0182533. [Google Scholar] [CrossRef] [Green Version]
- Broderick, N.A.; Buchon, N.; Lemaitre, B. Microbiota-induced changes in Drosophila melanogaster host gene expression and gut morphology. mBio 2014, 5, e01117-14. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.F.; Scharf, M.E. Lower termite associations with microbes: Synergy, protection, and interplay. Front. Microbiol. 2016, 7, 422. [Google Scholar] [CrossRef] [PubMed]
- Sabree, Z.L.; Kambhampati, S.; Moran, N.A. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc. Natl. Acad. Sci. USA 2009, 106, 19521–19526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.C.; Islam, M.; Sheppard, C.; Liao, J.; Doyle, M.P. Reduction of Escherichia coli O157:H7 and Salmonella enterica serovar enteritidis in chicken manure by larvae of the black soldier fly. J. Food Prot. 2004, 67, 685–690. [Google Scholar] [CrossRef] [Green Version]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrügg, C.; Lindström, A.; Vinnerås, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—From a hygiene aspect. Sci. Total Environ. 2013, 458–460, 312–318. [Google Scholar] [CrossRef]
- Shelomi, M.; Wu, M.K.; Chen, S.M.; Huang, J.J.; Burke, C.G. Microbes associated with black soldier fly (Diptera: Stratiomiidae) degradation of food waste. Environ. Entomol. 2020, 49, 405–411. [Google Scholar] [CrossRef]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2018, 78, 141–148. [Google Scholar] [CrossRef]
- Yu, G.; Cheng, P.; Chen, Y.; Li, Y.; Yang, Z.; Chen, Y.; Tomberlin, J.K. Inoculating poultry manure with companion bacteria influences growth and development of black soldier fly (Diptera: Stratiomyidae) larvae. Environ. Entomol. 2011, 40, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Fritzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef]
- Corley, R.H.V.; Tinker, P.B. The Oil Palm; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- FAOSTAT Database Food and Agriculture Organization of the United Nations. 2020. Available online: http://faostat3.fao.org/home/E (accessed on 15 November 2020).
- Rachmawati, R.; Buchori, D.; Hidayat, P.; Hem, S.; Fahmi, M.R. Perkembangan dan kandungan nutrisi larva Hermetia illucens (Linnaeus) (Diptera: Stratiomyidae) pada bungkil kelapa sawit. J. Entomol. Indones. 2010, 7, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Sulabo, R.C.; Ju, S.W.; Stein, H.H. Amino acid digestability and concentration of digestable and metabolizable energy in copra meal, palm kernel expellers, and palm kernel meal fed to growing pigs. J. Anim. Sci. 2013, 91, 1391–1399. [Google Scholar] [CrossRef] [Green Version]
- Yaophakdee, N.; Ruangpanit, Y.; Attamangkune, S. Effects of palm kernel meal level on live performance and gut morphology of broilers. Agric. Nat. Resour. 2018, 52, 75–78. [Google Scholar] [CrossRef]
- Nakamura, S.; Ichiki, R.T.; Shimoda, M.; Morioka, S. Small-scale rearing of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), in the laboratory: Low-cost and year-round rearing. Appl. Entomol. Zool. 2016, 51, 161–166. [Google Scholar] [CrossRef]
- Giunti, G.; Campolo, O.; Laudani, F.; Palmeri, V. Male courtship behaviour and potential for female mate choice in the black soldier fly Hermetia illucens L. (Diptera: Stratiomyidae). Entomol. Gen. 2018, 38, 29–46. [Google Scholar] [CrossRef]
- Klüber, P.; Bakonyi, D.; Zorn, H.; Rühl, M. Does light color temperature influence aspects of oviposition by the black soldier fly (Diptera: Stratiomyidae)? J. Econ. Entomol. 2020, 113, 2549–2552. [Google Scholar] [CrossRef]
- Barros, L.M.; Gutjahr, A.L.N.; Ferreira-Keppler, R.L.; Martins, R.T. Morphological description of the immature stages of Hermetia illucens (Linnaeus, 1758) (Diptera: Stratiomyidae). Microsc. Res. Tech. 2019, 82, 178–189. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria (CLSI Guideline M45); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; pp. 24–26. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 10.0. 2020. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 12 December 2020).
- Beekman, C.N.; Meckler, L.; Kim, E.; Bennett, R.J. Galleria mellonella as an insect model for P. destructans, the cause of white-nose syndrome in bats. PLoS ONE 2018, 13, e0201915. [Google Scholar] [CrossRef] [Green Version]
- Montoya, A.M.; Luna-Rodríguez, C.E.; Gracia-Robles, G.; Rojas, O.C.; Treviño-Rangel, R.J.; González, G.M. In vitro virulence determinants, comparative pathogenicity of Diutina (Candida) mesorugosa clinical isolates and literature review of the D. rugosa complex. Mycologia 2019, 111, 395–407. [Google Scholar] [CrossRef]
- Mannala, G.K.; Izar, B.; Rupp, O.; Schultze, T.; Goesmann, A.; Chakraborty, T.; Hain, T. Listeria monocytogenes induces a virulence-dependent microRNA signature that regulates the immune response in Galleria mellonella. Front. Microbiol. 2017, 8, 2463. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, A.C.; Thompson, M.G.; Black, C.C.; Kessler, J.L.; Clark, L.P.; McQueary, C.N.; Gancz, H.Y.; Corey, B.W.; Moon, J.K.; Si, Y.; et al. AB5075, a highly virulent isolate of Acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. mBio 2014, 5, e01076-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secil, E.; Sevim, A.; Demirbag, Z.; Demir, I. Isolation, characterization and virulence of bacteria from Ostrinia nubilaris (Lepidoptera: Pyralidae). Biologia 2012, 67, 767–776. [Google Scholar] [CrossRef]
- Insua, J.L.; Llobet, E.; Moranta, D.; Pérez-Gutiérrez, C.; Tomás, A.; Garmendia, J.; Bengoechea, J.A. Modeling Klebsiella pneumoniae pathogenesis by infection of the wax moth Galleria mellonella. Infect. Immun. 2013, 81, 3552–3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizerska-Dudka, M.; Andrejko, M. Galleria mellonella hemocytes destruction after infection with Pseudomonas aeruginosa. J. Basic Microbiol. 2014, 54, 232–246. [Google Scholar] [CrossRef]
- Quiroz-Castañeda, R.E.; Mendoza-Mejía, A.; Obregón-Barboza, V.; Martínez-Ocampo, F.; Hernández-Mendoza, A.; Martínez-Garduño, F.; Guillén-Solís, G.; Sánchez-Rodríguez, F.; Peña-Chora, G.; Ortíz-Hernández, L.; et al. Identification of a new Alcaligenes faecalis strain MOR02 and assessment of its toxicity and pathogenicity to insects. Biomed. Res. Int. 2015, 2015, 570243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, B.; Conway, H.E.; Kunta, M.; Vacek, D.; Vitek, C. Pathogenicity of Zygosaccharomyces bailii and other yeast species to mexican fruit fly (Diptera: Tephritidae) and mass rearing implications. J. Econ. Entomol. 2018, 111, 2081–2088. [Google Scholar] [CrossRef]
- Moore, G.E. Pathogenicity of three entomogenous fungi to the southern pine beetle at various temperatures and humidities. Environ. Entomol. 1973, 2, 54–57. [Google Scholar] [CrossRef]
- Chorost, M.S.; Smith, N.C.; Hutter, J.N.; Ong, A.C.; Stam, J.A.; McGann, P.T.; Hinkle, M.K.; Schaecher, K.E.; Kamau, E. Bacteraemia due to Microbacterium paraoxydans in a patient with chronic kidney disease, refractory hypertension and sarcoidosis. JMM Case Rep. 2018, 5, e005169. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Ormeño-Orrillo, E.; López-López, A.; Rogel, M.A.; Reyes-Hernández, B.J.; Martínez-Romero, J.C.; Reddy, P.M.; Martínez-Romero, E. Nitrogen fixation in cereals. Front. Microbiol. 2018, 9, 1794. [Google Scholar] [CrossRef] [Green Version]
- Segers, F.H.; Kešnerová, L.; Kosoy, M.; Engel, P. Genomic changes associated with the evolutionary transition of an insect gut symbiont into a blood-borne pathogen. ISME J. 2017, 11, 1232–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ao, Y.; Yang, C.; Wang, S.; Hu, Q.; Yi, L.; Zhang, J.; Yu, Z.; Cai, M.; Yu, C. Characteristics and nutrient function of intestinal bacterial communities in black soldier fly (Hermetia illucens L.) larvae in livestock manure conversion. Microb. Biotechnol. 2020, 14, 886–896. [Google Scholar] [CrossRef]
- Anand, A.A.P.; Vennison, S.J.; Sankar, S.G.; Prabhu, D.I.G.; Vasan, P.T.; Raghuraman, T.; Geoffrey, C.J.; Vendan, S.E. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J. Insect. Sci. 2010, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Li, Y.; Chen, Y.; Fu, C.; Long, W.; Xiao, X.; Liao, H.; Yang, Y. Bamboo lignocellulose degradation by gut symbiotic microbiota of the bamboo snout beetle Cyrtotrachelus burqueti. Biotechnol. Biofuels 2019, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Bonelli, M.; Filippis, F.; de Di Lelio, I.; Tettamanti, G.; Casartelli, M.; Ercolini, D.; Caccia, S. The intestinal microbiota of Hermetia illucens larvae is affected by diet and shows a diverse composition in the different midgut regions. Appl. Environ. Microbiol. 2019, 85, e01864-18. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.-L.; Jin, W.-Z.; Tao, X.-H.; Zhang, Q.; Zhu, J.; Feng, S.-Y.; Xu, X.-H.; Li, H.-Y.; Wang, Z.-H.; Zhang, Z.-J. Black soldier fly larvae (Hermetia illucens) strengthen the metabolic function of food waste biodegradation by gut microbiome. Microb. Biotechnol. 2019, 12, 528–543. [Google Scholar] [CrossRef] [Green Version]
- Wynants, E.; Frooninckx, L.; Crauwels, S.; Verreth, C.; Smet, J.; de Sandrock, C.; Wohlfahrt, J.; van Schelt, J.; Depraetere, S.; Lievens, B.; et al. Assessing the microbiota of black soldier fly larvae (Hermetia illucens) reared on organic waste streams on four different locations at laboratory and large scale. Microb. Ecol. 2019, 77, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Klammsteiner, T.; Walter, A.; Bogataj, T.; Heussler, C.D.; Stres, B.; Steiner, F.M.; Schlick-Steiner, B.C.; Arthofer, W.; Insam, H. The core gut microbiome of black soldier fly (Hermetia illucens) larvae raised on low-bioburden diets. Front. Microbiol. 2020, 11, 993. [Google Scholar] [CrossRef]
- Zheng, L.; Crippen, T.L.; Holmes, L.; Singh, B.; Pimsler, M.L.; Benbow, M.E.; Tarone, A.M.; Dowd, S.; Yu, Z.; Vanlaerhoven, S.L.; et al. Bacteria mediate oviposition by the black soldier fly, Hermetia illucens (L.), (Diptera: Stratiomyidae). Sci. Rep. 2013, 3, 2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, H.; Keesey, I.W.; Hansson, B.S.; Knaden, M. Gut microbiota affects development and olfactory behavior in Drosophila melanogaster. J. Exp. Biol. 2019, 222, jeb192500. [Google Scholar] [CrossRef] [Green Version]
- Stefanini, I. Yeast-insect associations: It takes guts. Yeast 2018, 35, 315–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatoum, R.; Labrie, S.; Fliss, I. Antimicrobial and probiotic properties of yeasts: From fundamental to novel applications. Front. Microbiol. 2012, 3, 421. [Google Scholar] [CrossRef] [Green Version]
- Amoikon, T.L.S.; Aké, M.D.F.; Djéni, N.T.; Grondin, C.; Casaregola, S.; Djè, K.M. Diversity and enzymatic profiles of indigenous yeasts isolated from three types of palm wines produced in Côte d’Ivoire. J. Appl. Microbiol. 2019, 126, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Elahi, A.; Rehman, A. Bioconversion of hemicellulosic materials into ethanol by yeast, Pichia kudriavzevii 2-KLP1, isolated from industrial waste. Rev. Argent. Microbiol. 2018, 50, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Vakhlu, J.; Kour, A. Yeast lipases: Enzyme purification, biochemical properties and gene cloning. Electron. J. Biotechnol. 2006, 9, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Tegtmeier, D.; Hurka, S.; Klüber, P.; Brinkrolf, K.; Heise, P.; Vilcinskas, A. Cottonseed press cake as a potential diet for industrially farmed black soldier fly larvae triggers adaptations of their bacterial and fungal gut microbiota. Front. Microbiol. 2021, 12, 634503. [Google Scholar] [CrossRef]
- Dashtban, M.; Schraft, H.; Syed, T.A.; Qin, W. Fungal biodegradation and enzymatic modification of lignin. Int. J. Mol. Biol. 2010, 1, 36–50. [Google Scholar]
- Sutherland, J.B.; Pometto, A.L., 3rd; Crawford, D.L. Lignocellulose degradation by Fusarium species. Can. J. Bot. 1983, 61, 1194–1198. [Google Scholar] [CrossRef]
- Assis Costa OY de Tupinambá, D.D.; Bergmann, J.C.; Barreto, C.C.; Quirino, B.F. Fungal diversity in oil palm leaves showing symptoms of fatal yellowing disease. PLoS ONE 2018, 13, e0191884. [Google Scholar] [CrossRef] [Green Version]
- Kukor, J.J.; Martin, M.M. Cellulose digestion in Monochamus marmorator Kby. (Coleoptera: Cerambycidae): Role of acquired fungal enzymes. J. Chem. Ecol. 1986, 12, 1057–1070. [Google Scholar] [CrossRef] [Green Version]
- Park, S.I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Joosten, L.; Lecocq, A.; Jensen, A.B.; Haenen, O.; Schmitt, E.; Eilenberg, J. Review of insect pathogen risk for the black soldier fly (Hermetia illucens) and guidelines for reliable production. Entomol. Exp. Appl. 2020, 168, 432–447. [Google Scholar] [CrossRef]
- Yalpani, N.; Altier, D.; Barry, J.; Kassa, A.; Nowatzki, T.M.; Sethi, A.; Zhao, J.-Z.; Diehn, S.; Crane, V.; Sandahl, G.; et al. An Alcaligenes strain emulates Bacillus thuringiensis producing a binary protein that kills corn rootworm through a mechanism similar to Cry34Ab1/Cry35Ab1. Sci. Rep. 2017, 7, 3063. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Schmidt, S.; Christensen, B.M. The antibacterial innate immune response by the mosquito Aedes aegypti is mediated by hemocytes and independent of Gram type and pathogenicity. Microbes. Infect. 2004, 6, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Panayidou, S.; Ioannidou, E.; Apidianakis, Y. Human pathogenic bacteria, fungi, and viruses in Drosophila: Disease modeling, lessons, and shortcomings. Virulence 2014, 5, 253–269. [Google Scholar] [CrossRef] [Green Version]
- Camprubí, S.; Merino, S.; Benedí, V.J.; Tomás, J.M. The role of the O-antigen lipopolysaccharide and capsule on an experimental Klebsiella pneumoniae infection of the rat urinary tract. FEMS Microbiol. Lett. 1993, 111, 9–13. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the offence with a strong defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Laing, S.; Unger, M.; Koch-Nolte, F.; Haag, F. ADP-ribosylation of arginine. Amino Acids 2011, 41, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Simon, N.C.; Aktories, K.; Barbieri, J.T. Novel bacterial ADP-ribosylating toxins: Structure and function. Nat. Rev. Microbiol. 2014, 12, 599–611. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Chu, K.B.; Kang, H.J.; Lee, S.H.; Quan, F.S. Peptides in the hemolymph of Hermetia illucens larvae completely inhibit the growth of Klebsiella pneumonia in vitro and in vivo. J. Asia Pac. Entomol. 2020, 23, 36–43. [Google Scholar] [CrossRef]
- Scholte, E.J. The Entomopathogenic Fungus Metarhizium anisopliae for Mosquito Control. Impact on the Adult Stage of the African Malaria Vector Anopheles gambiae and Filariasis Vector Culex quinquefasciatus. Ph.D. Thesis, University of Wageningen, Wageningen, The Netherlands, 2004. [Google Scholar]
- Sharma, L.; Marques, G. Fusarium, an entomopathogen—A myth or reality? Pathogens 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jestoi, M. Emerging Fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Dickel, F.; Freitak, D.; Mappes, J. Long-term prophylactic antibiotic treatment: Effects on survival, immunocompetence and reproduction success of Parasemia plantaginis (Lepidoptera: Erebidae). J. Insect. Sci. 2016, 16, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic resistance in the food chain: A developing country-perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef] [PubMed]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fèvre, E.M.; Woolhouse, M.E.J.; van Bunnik, B.A.D. Are food animals responsible for transfer of antimicrobial-resistant Escherichia coli or their resistance determinants to human populations? A systematic review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Bacteria | Yeasts | Filamentous Ascomycetes | ||||
|---|---|---|---|---|---|---|
| LB | TSA | YPD | M2 | YPD | M2 | |
| CFU/conidia gut−1 | 1.06 × 109 | 7.04 × 108 | 1.10 × 106 | 4.67 × 106 | 1.40 × 104 | 1.31 × 104 |
| (±SD) | (±9.81 × 108) | (±4.63 × 108) | (±3.22 × 105) | (±2.83 × 106) | (±7.87 × 103) | (±9.44 × 103) |
| CFU/conidia (mg gut)−1 | 1.35 × 107 | 8.52 × 106 | 1.47 × 104 | 6.56 × 104 | 1.90 × 102 | 1.63 × 102 |
| (±SD) | (±1.39 × 107) | (±5.48 × 106) | (±3.90 × 103) | (±4.34 × 104) | (±1.11 × 102) | (±1.03 × 102) |
| Drug | Gram-Negative | Gram-Positive | ||||||
|---|---|---|---|---|---|---|---|---|
| Alcaligenes faecalis | Klebsiella pneumoniae | Pseudomonas aeruginosa | Microbacterium thalassium | |||||
| MIC | Interpretation | MIC | Interpretation | MIC | Interpretation | MIC | Interpretation | |
| Amikacin | n | n | n | n | ≤2 | S | (-) | (-) |
| Ampicillin | >256 | R | ≥32 | R | (-) | (-) | >0.75 | S |
| Ampicillin/ Subactam | ≥64 | R | ≤2 | S | (-) | (-) | n | n |
| Aztreonam | n | n | n | n | 32 | R | (-) | (-) |
| Cefepime | >48 | R | n | n | 4 | I | >6 | R |
| Cefotaxime | >256 | R | ≤1 | S | (-) | (-) | ≥2 | I |
| Cefpodoxime | n | IE | ≤0.25 | S | (-) | (-) | n | IE |
| Ceftazidime | >256 | R | ≤1 | S | 4 | I | n | IE |
| Cefuroxime | n | n | ≤1 | I | (-) | (-) | n | IE |
| Cefuroxime axetil | n | n | ≤1 | S | (-) | (-) | n | IE |
| Ciprofloxacin | ≥0.19 | S | ≤0.25 | S | 0.5 | I | ≥0.5 | S |
| Clindamycin | (-) | (-) | (-) | (-) | (-) | (-) | 16 | R |
| Colistin | n | n | n | n | ≤0.5 | S | (-) | (-) |
| Ertapenem | ≥0.032 | S | ≤0.5 | S | (-) | (-) | n | IE |
| Erythromycin | n | IE | n | n | N | n | ≤0.5 | S |
| Fosfomycin | n | IE | n | n | (-) | (-) | (-) | (-) |
| Gentamicin | <1.5 | S | ≤1 | S | ≤1 | IE | ≤12 | I |
| Imipenem | ≥2 | I | ≤0.25 | S | 2 | I | ≤0.5 | S |
| Meropenem | ≥0.38 | S | ≤0.25 | S | ≤0.25 | S | ≥0.125 | S |
| Moxifloxacin | n | IE | ≤0.25 | S | (-) | (-) | n | IE |
| Penicillin | (-) | (-) | (-) | (-) | (-) | (-) | ≥0.5 | I |
| Piperacillin | n | n | 8 | R | 16 | I | n | IE |
| Piperacillin/ Tazobactam | >256 | R | ≤4 | S | 32 | R | n | IE |
| Rifampicin | ≥1.5 | I | >32 | R | 8 | R | ≥12 | R |
| Tetracyclin | n | IE | n | n | (-) | (-) | ≤12 | I |
| Tigecycline | ≥0.5 | S | n | n | (-) | (-) | n | IE |
| Tobramycin | n | n | n | n | ≤1 | S | (-) | (-) |
| Trimethoprim/ Sulfamethoxazole | ≥1.5 | S | ≤20 | S | (-) | (-) | >0.5 | S |
| Vancomycin | (-) | (-) | (-) | (-) | (-) | (-) | >2 | S/IE |
| Liquid Culture | n | Function | R² |
|---|---|---|---|
| Alcaligenes faecalis | 5 | y = 2.18x + 6.84 | 0.9954 |
| Diutina rugosa | 6 | y = 1.57x + 6.80 | 0.9901 |
| Klebsiella pneumoniae | 5 | y = 3.30x + 5.87 | 0.9985 |
| Microbacterium thalassium | 5 | y = 2.79x + 6.25 | 0.9827 |
| Pseudomonas aeruginosa | 7 | y = 1.76x + 6.87 | 0.9964 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klüber, P.; Müller, S.; Schmidt, J.; Zorn, H.; Rühl, M. Isolation of Bacterial and Fungal Microbiota Associated with Hermetia illucens Larvae Reveals Novel Insights into Entomopathogenicity. Microorganisms 2022, 10, 319. https://doi.org/10.3390/microorganisms10020319
Klüber P, Müller S, Schmidt J, Zorn H, Rühl M. Isolation of Bacterial and Fungal Microbiota Associated with Hermetia illucens Larvae Reveals Novel Insights into Entomopathogenicity. Microorganisms. 2022; 10(2):319. https://doi.org/10.3390/microorganisms10020319
Chicago/Turabian StyleKlüber, Patrick, Stephanie Müller, Jonas Schmidt, Holger Zorn, and Martin Rühl. 2022. "Isolation of Bacterial and Fungal Microbiota Associated with Hermetia illucens Larvae Reveals Novel Insights into Entomopathogenicity" Microorganisms 10, no. 2: 319. https://doi.org/10.3390/microorganisms10020319
APA StyleKlüber, P., Müller, S., Schmidt, J., Zorn, H., & Rühl, M. (2022). Isolation of Bacterial and Fungal Microbiota Associated with Hermetia illucens Larvae Reveals Novel Insights into Entomopathogenicity. Microorganisms, 10(2), 319. https://doi.org/10.3390/microorganisms10020319






