The Historical Development of Cultivation Techniques for Methanogens and Other Strict Anaerobes and Their Application in Modern Microbiology
Abstract
:1. Introduction
2. Milestones in the Historical Development of Anaerobic Microbiology
2.1. The Origin of Anaerobic Cultivation
2.2. First Attempts of Methanogens Isolation and the Discovery of Archaea
3. Anaerobic Cultivation Techniques
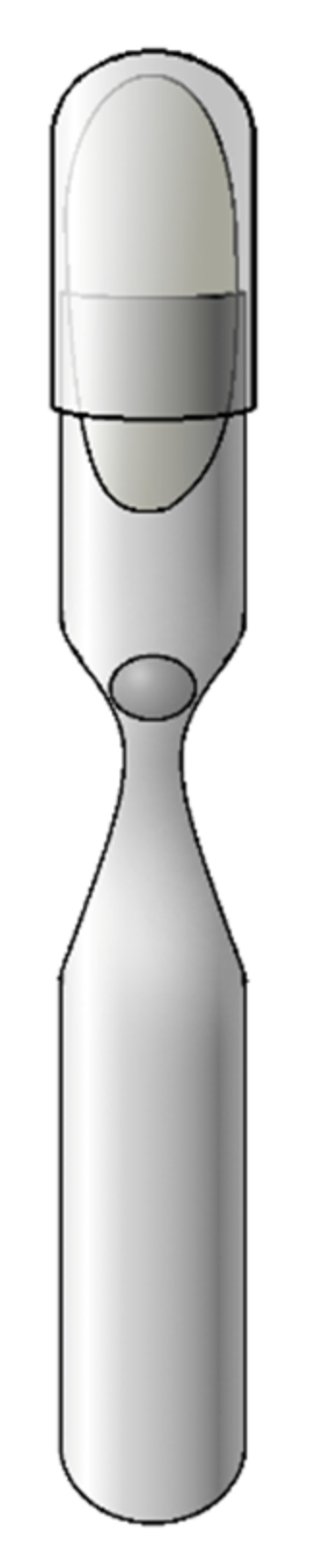
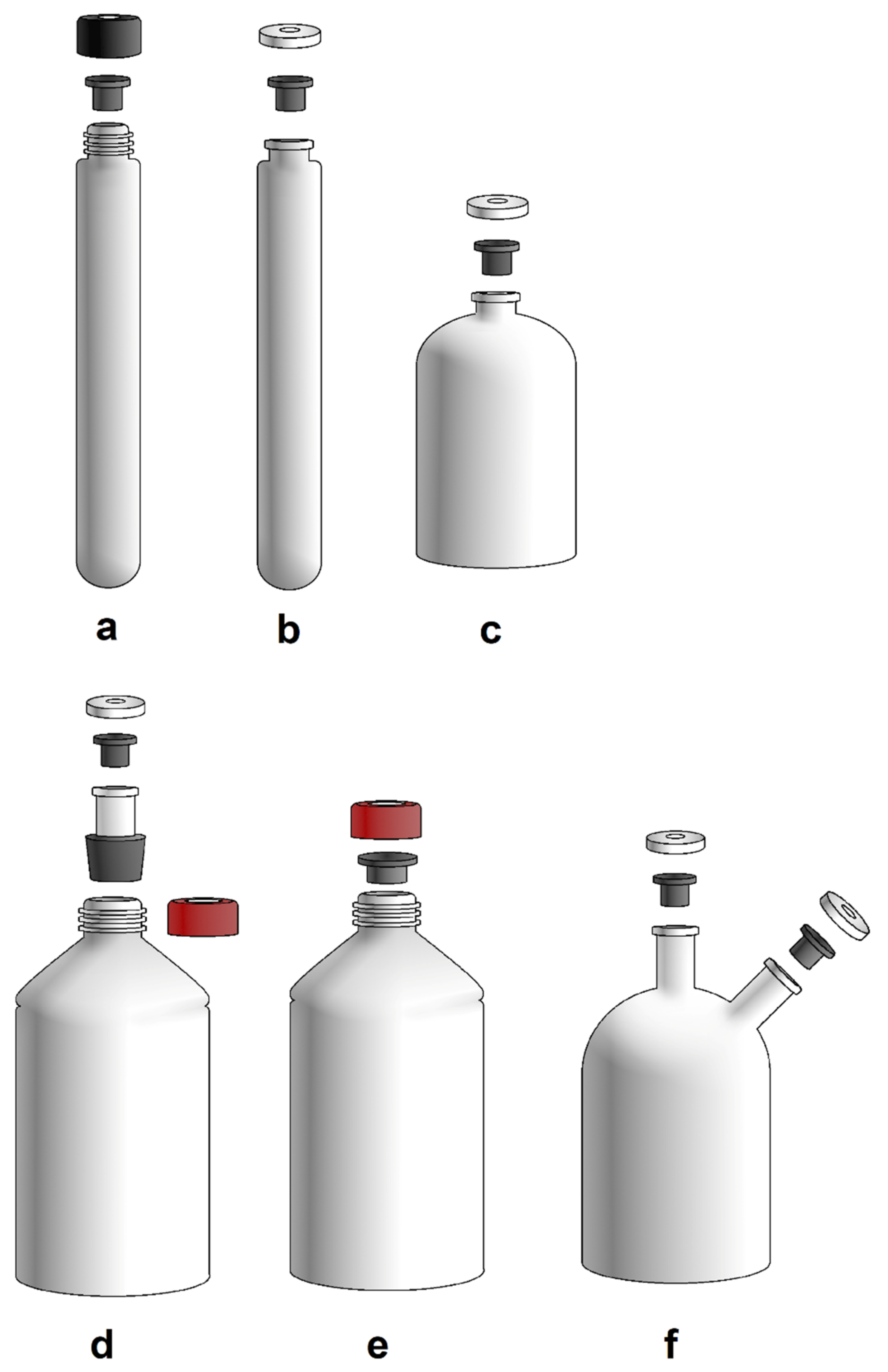
3.1. Laboratory Equipment for Cultivation of Methanogens
3.2. Preparation of Anoxic Media for Methanogens
3.2.1. Composition of Medium for Methanogens
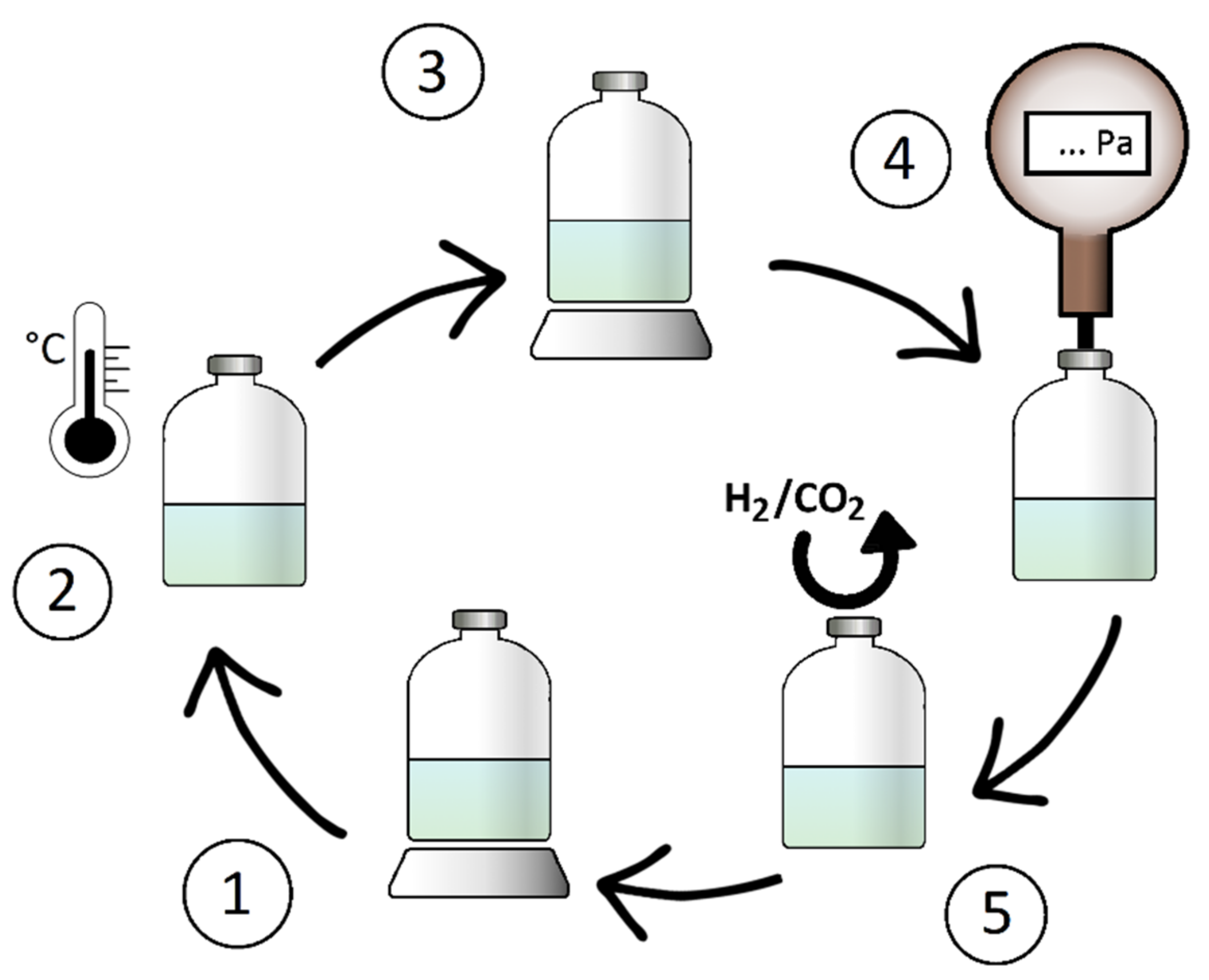
3.2.2. Process of Anaerobization of the Medium
3.3. Cultivation and Pure Cultures Isolation Techniques
3.3.1. Petri Dishes Cultivation in Anaerobic Jar
3.3.2. Hungate’s Roll Tube Technique
3.3.3. Agar Shake Dilution Tube Method
3.3.4. Lee Tube Method
3.3.5. Hermann’s Flat Flask Method
3.3.6. Single Cell Isolation Methods
3.3.7. Dilution to Extinction Method
3.4. Novel Insights in Cultivation Techniques
3.4.1. The Six-Well Method
3.4.2. Growth in Syntrophic Communities
3.4.3. Microplate Reader Technique
3.4.4. Microfluidic Techniques
4. Quantification Techniques
4.1. Optical Density Measurement Technique
4.2. ATP Determination Method
4.3. Methods Requiring the Cultivation of Anaerobic Microorganism
4.3.1. Methane Production Measurement Techniques
4.3.2. Manometric OxiTop Measurement
4.3.3. Indirect Quantification of Produced Methane via Weight Gain
5. Up-Scaling Process during Anaerobes Cultivation
Batch, Fed-Batch and Continuous Cultivation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Year | Technique | Description | Reference |
|---|---|---|---|
| 1898 | Agar shake tubes | Agar in test tube mixed with culture and cultivated after solidification of the agar | [4] |
| 1900 | Pyrogallic acid (Buchner’s method) | The use of pyrogallic acid on the cotton plug together with alkaline solution to absorb oxygen in the tube | [5] |
| 1921 | Hall’s marble seal | Special constricted tube with concave marble seal that is overpoured with sterile medium | [23] |
| 1929 | Paraffin oil seal | Layer of paraffin oil on top of culture medium in test tube | [13] |
| 1969 | Roll tube method | Layer of agar-culture mixture on walls of Hungate tube made by rolling the tube with liquid mixture until solidification occurs | [14] |
| 1969 | Copper column | Application of copper column for expelling O2 from used gas | [14] |
| 1969 | The use of reducing solutions | Reducing the redox potential in the medium for creating a more suitable environment | [14] |
| 1972 | Syringes and needles | Preservation of anaerobic environment by application of needles and syringes without the oxygen exposure during manipulation | [15] |
| 1974 | Serum bottle modification of Hungate technique | Application of serum bottles for cultivation of anaerobic microorganisms in liquid and solidified medium | [16] |
| 1976 | Pressurization of culture vessels | Increasing partial pressure in the cultivation vessel leads to lessening of the gassing frequency and an increase in the methanogenesis rate | [59] |
| 1979 | Gassing manifold | Special manifold for parallel gassing of culture vessels | [60] |
| 1979 | Lee’s tube | Layer of agar mixed with culture between two glass walls of special tube | [104] |
| 1981 | Widdel flask | Special conical flask for preparation media containing thermolabile solutions | [69,80] |
| 1986 | Modified Lee’s tube | Layer of agar mixed with culture between two walls of two tubes inserted in each other | [105] |
| 1986 | Hermann´s flat flask method | The usage of closed flat flask for cultivation of anaerobic microorganisms on agar | [108] |
| 1992 | Modified Hermann´s flat flask | Addition of an opening for gassing the flask while picking up the colonies from medium | [109] |
| 1999 | Single cell isolation technique | First application of a single-cell isolation technique which enables the picking up of single cells from sample on methanogens | [110] |
| 2007 | Microfluidics application | The first usage of microfluidics in anaerobic microbiology cultivating pure culture of methanogenic Methanosaeta concilii under N2/CO2 (4:1 (v/v)) conditions | [135] |
| 2009 | Vacuum-gas method | The dispersed medium is put through cycles of gassing and gas exhaustion to set anaerobic conditions in the vessel | [86] |
| 2010 | Vacuum-vortex method | Vortexing the dispersed medium in vessel while applying cycle gassing and gas exhaustion to set anaerobic conditions in the vessel | [84] |
| 2011 | Six-well method | Anaerobic cultivation and isolation using a six-well plate and supporting anaerobiosis generating system | [88] |
| 2012 | Application of microplate technique on methanogens cultivation | The first usage of microplate reader technique to cultivate methanogens under H2/CO2 (4:1 (v/v)) atmosphere and to measure their optical density | [130] |
References
- Liu, C.-T.; Miyaki, T.; Aono, T.; Oyaizu, H. Evaluation of Methanogenic Strains and Their Ability to Endure Aeration and Water Stress. Curr. Microbiol. 2008, 56, 214–218. [Google Scholar] [CrossRef]
- Hall, I.C. Practical Methods in the Purification of Obligate Anaerobes. J. Infect. Dis. 1920, 27, 576–590. [Google Scholar] [CrossRef]
- Söhngen, N.L. Sur Le Rôle Du Méthane Dans La Vie Organique. Recl. Trav. Chim. Pays-Bas Belg. 1910, 29, 238–274. [Google Scholar] [CrossRef]
- Veillon, A.; Zuber, A. Recherches Sur Quelques Microbes Strictement Anaérobies et Leur Rôle En Pathologie. Arch. Méd. Exp. Anat. Pathol. 1898, 10, 517–545. [Google Scholar]
- Wright, J.H. A Simple Method Of Cultivating Anaerobic Bacteria. J. Boston Soc. Med. Sci. 1900, 5, 114–115. [Google Scholar] [PubMed]
- Ergal, İ.; Fuchs, W.; Hasibar, B.; Thallinger, B.; Bochmann, G.; Rittmann, S.K.-M.R. The Physiology and Biotechnology of Dark Fermentative Biohydrogen Production. Biotechnol. Adv. 2018, 36, 2165–2186. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Bosáková, V.; Vítězová, M.; Rittmann, S.K.-M.R. Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide. Antioxidants 2021, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Mauerhofer, L.-M.; Zwirtmayr, S.; Pappenreiter, P.; Bernacchi, S.; Seifert, A.H.; Reischl, B.; Schmider, T.; Taubner, R.-S.; Paulik, C.; Rittmann, S.K.-M.R. Hyperthermophilic Methanogenic Archaea Act as High-Pressure CH4 Cell Factories. Commun. Biol. 2021, 4, 289. [Google Scholar] [CrossRef]
- Rittmann, S.K.-M.R.; Lee, H.S.; Lim, J.K.; Kim, T.W.; Lee, J.-H.; Kang, S.G. One-Carbon Substrate-Based Biohydrogen Production: Microbes, Mechanism, and Productivity. Biotechnol. Adv. 2015, 33, 165–177. [Google Scholar] [CrossRef]
- Beigelman, P.M.; Rantz, L.A. Clinical Significance of Bacteroides. Arch. Intern. Med. (Chic). 1949, 84, 605–631. [Google Scholar] [CrossRef]
- Veillon, A.; Zuber, A. Sur Quelques Microbes Strictement Anaerobies et Leur Rôle Dans La Pathologie Humaine. Comptes Rendus Hebd. Séances Mém. Soc. Biol. 1897, 49, 253–255. [Google Scholar]
- Gest, H. The Discovery of Microorganisms by Robert Hooke and Antoni van Leeuwenhoek, Fellows of the Royal Society. Notes Rec. R. Soc. Lond. 2004, 58, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.C. A Review Of The Development And Application Of Physical And Chemical Principles In The Cultivation Of Obligately Anaerobic Bacteria. J. Bacteriol. 1929, 17, 255–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hungate, R.E. Chapter IV A Roll Tube Method for Cultivation of Strict Anaerobes. In Methods in Microbiology; Norris, J.R., Ribbons, D.W., Eds.; Academic Press: Cambridge, MA, USA, 1969; Volume 3, pp. 117–132. [Google Scholar]
- Macy, J.M.; Snellen, J.E.; Hungate, R.E. Use of Syringe Methods for Anaerobiosis. Am. J. Clin. Nutr. 1972, 25, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Wolin, M.J. A Serum Bottle Modification of the Hungate Technique for Cultivating Obligate Anaerobes. Appl. Microbiol. 1974, 27, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Hitchens, A.P.; Leikind, M.C. The Introduction of Agar-Agar into Bacteriology. J. Bacteriol. 1939, 37, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasteur, L.; Faulkner, F. Studies on Fermentation: The Diseases of Beer, Their Causes, and the Means of Preventing Them; Macmillan & Company, London, UK, 1879.
- Sebald, M.; Hauser, D. Pasteur, Oxygen and the Anaerobes Revisited. Anaerobe 1995, 1, 11–16. [Google Scholar] [CrossRef]
- Finegold, S.M. A Century of Anaerobes: A Look Backward and a Call to Arms. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1993, 16 (Suppl. 4), S453–S457. [Google Scholar] [CrossRef] [PubMed]
- Pasteur, L. The Germ Theory And Its Applications To Medicine And Surgery. In Scientific Papers: Physiology, Medicine, Surgery, Geology: With Introductions, Notes and Illustrations; Harvard Classics; P.F. Collier & Son: New York, NY, USA, 1910; Volume 38, p. 440. ISBN 978-1-61640-122-1. [Google Scholar]
- Hall, I.C. Intestinal Flora in New-Born Infants. Am. J. Dis. Child. 1935, 49, 390–402. [Google Scholar] [CrossRef]
- Hall, I.C. A Constricted Tube with Mechanical Seal for Anaerobic Fermentation Tests. J. Infect. Dis. 1921, 29, 317–320. [Google Scholar] [CrossRef] [Green Version]
- Griffin, A.M. A Modification Of The Buchner Method Of Cultivating Anaerobic Bacteria. Science 1932, 75, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Mellon, R.R. A Modification of the Wright-Buchner Anaerobic Tube. J. Bacteriol. 1919, 4, 295–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwell, G.E. An Improved Method for Anaerobic Cultures. J. Infect. Dis. 1924, 35, 581–586. [Google Scholar] [CrossRef]
- Heller, H.H. Principles Concerning the Isolation of Anaerobes Studies in Pathogenic Anaerobes. Ii. J. Bacteriol. 1921, 6, 445–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazé, P. Ferment Formenique. Ferment Formenique de l’acetone. Procede de Culture Simple Du Ferment Formenique. C. R. Acad. Sci. 1915, 78, 398–405. [Google Scholar]
- Anderson, B.G. Gaseous Metabolism of Some Anaerobic Bacteria: XIX. Methods. J. Infect. Dis. 1924, 35, 213–243. [Google Scholar] [CrossRef]
- Lloyd, B.; Cranston, J.A. Studies in Gas Production by Bacteria: Denitrification and Bacterial Growth Phases. Biochem. J. 1930, 24, 529–548. [Google Scholar] [CrossRef] [PubMed]
- Sacks, L.E.; Barker, H.A. The Influence of Oxygen on Nitrate and Nitrite Reduction. J. Bacteriol. 1949, 58, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Bryant, M.P.; Burkey, L.A. Cultural Methods and Some Characteristics of Some of the More Numerous Groups of Bacteria in the Bovine Rumen. J. Dairy Sci. 1953, 36, 205–217. [Google Scholar] [CrossRef]
- Hungate, R.E. The Anaerobic Mesophilic Cellulolytic Bacteria. Bacteriol. Rev. 1950, 14, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Sowers, K.R. Methanogenesis. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 265–286. ISBN 978-0-12-373944-5. [Google Scholar]
- Hofmann, A.W.V.I. On the Action of Trichloride of Phosphorus on the Salts of the Aromatic Monamines. Proc. R. Soc. Lond. 1867, 15, 54–62. [Google Scholar] [CrossRef]
- Anaerobic Fermentations, 32nd ed.; Bulletin (Illinois State Water Survey); Buswell, A.M.; Hatfield, W.D. (Eds.) Department of Registration and Education, Division of the State Water Supply: Urbana, IL, USA, 1939.
- Omelianski, V.L. Sur La Fermentation Cellulosique. Comptes Rendus Hebd. Séances Mém. Soc. Biol. 1897, 125, 1131–1133. [Google Scholar]
- Barker, H.A. Studies upon the Methane-Producing Bacteria. Arch. Mikrobiol. 1936, 7, 420–438. [Google Scholar] [CrossRef]
- Beijer, W.H. Methane Fermentation in the Rumen of Cattle. Nature 1952, 170, 576–577. [Google Scholar] [CrossRef]
- Barker, H.A. Studies upon the Methane Fermentation. IV. The Isolation and Culture of Methanobacterium omelianskii. Antonie Leeuwenhoek 1939, 6, 201–220. [Google Scholar] [CrossRef]
- Bryant, M.P.; Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Methanobacillus omelianskii, a Symbiotic Association of Two Species of Bacteria. Arch. Mikrobiol. 1967, 59, 20–31. [Google Scholar] [CrossRef]
- Reddy, C.A.; Bryant, M.P.; Wolin, M.J. Characteristics of S Organism Isolated from Methanobacillus omelianskii. J. Bacteriol. 1972, 109, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadtman, T.C.; Barker, H.A. Studies on the Methane Fermentation. X. A New Formate-Decomposing Bacterium, Methanococcus vannielii. J. Bacteriol. 1951, 62, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.H.; Hungate, R.E. Isolation And Characterization Of Methanobacterium ruminantion n. SP1. J. Bacteriol. 1958, 75, 713–718. [Google Scholar] [CrossRef] [Green Version]
- Ferry, J.G.; Smith, P.H.; Wolfe, R.S. Methanospirillum, a New Genus of Methanogenic Bacteria, and Characterization of Methanospirillum hungatii Sp. Nov. Int. J. Syst. Bacteriol. 1974, 24, 465–469. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Henning, D.L. Methanobacterium arbophilicum Sp.Nov. An Obligate Anaerobe Isolated from Wetwood of Living Trees. Antonie Leeuwenhoek 1975, 41, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Zeikus, J.G.; Wolfe, R.S. Methanobacterium thermoautotrophicus Sp. n., an Anaerobic, Autotrophic, Extreme Thermophile. J. Bacteriol. 1972, 109, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woese, C.R.; Fox, G.E. Phylogenetic Structure of the Prokaryotic Domain: The Primary Kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef] [Green Version]
- Hammes, W.P.; Winter, J.; Kandler, O. The Sensitivity of the Pseudomurein-Containing Genus Methanobacterium to Inhibitors of Murein Synthesis. Arch. Microbiol. 1979, 123, 275–279. [Google Scholar] [CrossRef]
- Kandler, O.; König, H. Chemical Composition of the Peptidoglycan-Free Cell Walls of Methanogenic Bacteria. Arch. Microbiol. 1978, 118, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Kates, M.; Yengoyan, L.S.; Sastry, P.S. A Diether Analog of Phosphatidyl Glycerophosphate in Halobacterium cutirubrum. Biochim. Biophys. Acta BBA—Lipids Lipid Metab. 1965, 98, 252–268. [Google Scholar] [CrossRef]
- Kessel, M.; Klink, F. Archaebacterial Elongation Factor Is ADP-Ribosylated by Diphtheria Toxin. Nature 1980, 287, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Langworthy, T.A.; Smith, P.F.; Mayberry, W.R. Lipids of Thermoplasma acidophilum. J. Bacteriol. 1972, 112, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Godsy, E.M. Isolation of Methanobacterium bryantii from a Deep Aquifer by Using a Novel Broth-Antibiotic Disk Method. Appl. Environ. Microbiol. 1980, 39, 1074–1075. [Google Scholar] [CrossRef] [Green Version]
- König, H. Isolation and Characterization of Methanobacterium uliginosum Sp. Nov. from a Marshy Soil. Can. J. Microbiol. 1984, 30, 1477–1481. [Google Scholar] [CrossRef]
- Schönheit, P.; Moll, J.; Thauer, R.K. Growth Parameters (K s, Μmax, Y s) of Methanobacterium thermoautotrophicum. Arch. Microbiol. 1980, 127, 59–65. [Google Scholar] [CrossRef]
- Stetter, K.O. Archaeoglobus Fulgidus Gen. Nov., Sp. Nov.: A New Taxon of Extremely Thermophilic Archaebacteria. Syst. Appl. Microbiol. 1988, 10, 172–173. [Google Scholar] [CrossRef]
- Zabel, H.P.; König, H.; Winter, J. Isolation and Characterization of a New Coccoid Methanogen, Methanogenium tatii Spec. Nov. from a Solfataric Field on Mount Tatio. Arch. Microbiol. 1984, 137, 308–315. [Google Scholar] [CrossRef]
- Balch, W.E.; Wolfe, R.S. New Approach to the Cultivation of Methanogenic Bacteria: 2-Mercaptoethanesulfonic Acid (HS-CoM)-Dependent Growth of Methanobacterium ruminantium in a Pressureized Atmosphere. Appl. Environ. Microbiol. 1976, 32, 781–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R.S. Methanogens: Reevaluation of a Unique Biological Group. Microbiol. Rev. 1979, 43, 260–296. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.S. Chapter One—Techniques for Cultivating Methanogens. In Methods in Enzymology; Methods in Methane Metabolism, Part A.; Rosenzweig, A.C., Ragsdale, S.W., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 494, pp. 1–22. [Google Scholar]
- Ravichandran, M.; Munisamy, P.; Natarajan, S.D.; Varadharaju, C. Rare Detection And Identification of Methanogenic Bacteria from Diverse Ecological Niches in India for Carbon Balance and Management in Our Environment. Int. J. Adv. Res. 2016, 4, 1174–1186. [Google Scholar] [CrossRef] [Green Version]
- Sowers, K.R. Growth and Identification. In Archaea: A Laboratory Manual—Methanogens; Robb, F.T., Sowers, K.R., DasSarma, S., Place, A.R., Schreier, H.J., Fleischmann, E.M., Eds.; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 1995; pp. 15–59. [Google Scholar]
- Taubner, R.-S.; Rittmann, S.K.-M.R. Method for Indirect Quantification of CH4 Production via H2O Production Using Hydrogenotrophic Methanogens. Front. Microbiol. 2016, 7, 532. [Google Scholar] [CrossRef] [Green Version]
- Vítězová, M.; Kohoutová, A.; Vítěz, T.; Hanišáková, N.; Kushkevych, I. Methanogenic Microorganisms in Industrial Wastewater Anaerobic Treatment. Processes 2020, 8, 1546. [Google Scholar] [CrossRef]
- Kotelnikova, S.; Macario, A.J.L.; Pedersen, K. Methanobacterium subterraneum Sp. Nov., a New Alkaliphilic, Eurythermic and Halotolerant Methanogen Isolated from Deep Granitic Groundwater. Int. J. Syst. Evol. Microbiol. 1998, 48, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfennig, N. Rhodopseudomonas globiformis, Sp. n., a New Species of the Rhodospirillaceae. Arch. Microbiol. 1974, 100, 197–206. [Google Scholar] [CrossRef]
- Widdel, F.; Kohring, G.-W.; Mayer, F. Studies on Dissimilatory Sulfate-Reducing Bacteria That Decompose Fatty Acids—III. Characterization of the Filamentous Gliding Desulfonema limicola Gen. Nov. Sp. Nov., and Desulfonema magnum Sp. Nov. Arch. Microbiol. 1983, 134, 286–294. [Google Scholar] [CrossRef]
- Widdel, F.; Bak, F. Gram-Negative Mesophilic Sulfate-Reducing Bacteria. In The Prokaryotes; Balows, A., Trüper, H.G., Dworkin, M., Harder, W., Schleifer, K.-H., Eds.; Springer: New York, NY, USA, 1992; pp. 3352–3378. ISBN 978-1-4757-2193-5. [Google Scholar]
- Cheng, L.; Dai, L.; Li, X.; Zhang, H.; Lu, Y. Isolation and Characterization of Methanothermobacter crinale Sp. Nov., a Novel Hydrogenotrophic Methanogen from the Shengli Oil Field. Appl. Environ. Microbiol. 2011, 77, 5212–5219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, K. Methanothermococcus okinawensis Sp. Nov., a Thermophilic, Methane-Producing Archaeon Isolated from a Western Pacific Deep-Sea Hydrothermal Vent System. Int. J. Syst. Evol. Microbiol. 2002, 52, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Wolin, M.J. Methanosphaera stadtmaniae Gen. Nov., Sp. Nov.: A Species That Forms Methane by Reducing Methanol with Hydrogen. Arch. Microbiol. 1985, 141, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B.; Ankwanda, E.; Wolfe, R.S. Nutrition and Carbon Metabolism of Methanococcus voltae. J. Bacteriol. 1982, 149, 852–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morii, H.; Nishihara, M.; Koga, Y. Isolation, Characterization and Physiology of a New Formate-Assimilable Methanogenic Strain (A2) of Methanobrevibacter arboriphilus. Agric. Biol. Chem. 1983, 47, 2781–2789. [Google Scholar] [CrossRef] [Green Version]
- Sprenger, W.W.; van Belzen, M.C.; Rosenberg, J.; Hackstein, J.H.; Keltjens, J.T. Methanomicrococcus blatticola Gen. Nov., Sp. Nov., a Methanol- and Methylamine-Reducing Methanogen from the Hindgut of the Cockroach Periplaneta americana. Int. J. Syst. Evol. Microbiol. 2000, 50, 1989–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauerhofer, L.-M.; Pappenreiter, P.; Paulik, C.; Seifert, A.H.; Bernacchi, S.; Rittmann, S.K.-M.R. Methods for Quantification of Growth and Productivity in Anaerobic Microbiology and Biotechnology. Folia Microbiol. 2019, 64, 321–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, T.L.; Lin, C. Description of Methanobrevibacter gottschalkii Sp. Nov., Methanobrevibacter thaueri Sp. Nov., Methanobrevibacter woesei Sp. Nov. and Methanobrevibacter wolinii Sp. Nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 819–822. [Google Scholar] [CrossRef]
- Paynter, M.J.B.; Hungate, R.E. Characterization of Methanobacterium mobilis, Sp. n., Isolated from the Bovine Rumen. J. Bacteriol. 1968, 95, 1943–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, S.; Bowman, J.P.; Popovski, S.; Pimm, C.; Wright, A.-D.G. Methanobrevibacter millerae Sp. Nov. and Methanobrevibacter olleyae Sp. Nov., Methanogens from the Ovine and Bovine Rumen That Can Utilize Formate for Growth. Int. J. Syst. Evol. Microbiol. 2007, 57, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Widdel, F.; Pfennig, N. Studies on Dissimilatory Sulfate-Reducing Bacteria That Decompose Fatty Acids: I. Isolation of New Sulfate-Reducing Bacteria Enriched with Acetate from Saline Environments. Description of Desulfobacter postgatei Gen. Nov., Sp. Nov. Arch. Microbiol. 1981, 129, 395–400. [Google Scholar] [CrossRef]
- Bryant, M.P. Commentary on the Hungate Technique for Culture of Anaerobic Bacteria. Am. J. Clin. Nutr. 1972, 25, 1324–1328. [Google Scholar] [CrossRef]
- Laso-Pérez, R.; Krukenberg, V.; Musat, F.; Wegener, G. Establishing Anaerobic Hydrocarbon-Degrading Enrichment Cultures of Microorganisms under Strictly Anoxic Conditions. Nat. Protoc. 2018, 13, 1310–1330. [Google Scholar] [CrossRef] [Green Version]
- Shlimon, A.G. Methanobacterium aarhusense Sp. Nov., a Novel Methanogen Isolated from a Marine Sediment (Aarhus Bay, Denmark). Int. J. Syst. Evol. Microbiol. 2004, 54, 759–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, R.S.; Metcalf, W.W. A Vacuum-Vortex Technique for Preparation of Anoxic Solutions or Liquid Culture Media in Small Volumes for Cultivating Methanogens or Other Strict Anaerobes. Anaerobe 2010, 16, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Wang, L.; Lupa, B.; Whitman, W.B. A Flexible System for Cultivation of Methanococcus and Other Formate-Utilizing Methanogens. Archaea 2017, 2017, e7046026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stieglmeier, M.; Wirth, R.; Kminek, G.; Moissl-Eichinger, C. Cultivation of Anaerobic and Facultatively Anaerobic Bacteria from Spacecraft-Associated Clean Rooms. Appl. Environ. Microbiol. 2009, 75, 3484–3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowell, V.R.; Hawkins, T.M. Laboratory Methods in Anaerobic Bacteriology; CDC Laboratory Manual: Atlanta, GA, USA, 1974. [Google Scholar]
- Nakamura, K.; Tamaki, H.; Kang, M.S.; Mochimaru, H.; Lee, S.-T.; Nakamura, K.; Kamagata, Y. A Six-Well Plate Method: Less Laborious and Effective Method for Cultivation of Obligate Anaerobic Microorganisms. Microbes Environ. 2011, 26, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.J.; Whitman, W.B.; Fields, R.D.; Wolfe, R.S. Growth and Plating Efficiency of Methanococci on Agar Media. Appl. Environ. Microbiol. 1983, 46, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apolinario, E.A.; Sowers, K.R. Plate Colonization of Methanococcus maripaludis and Methanosarcina thermophila in a Modified Canning Jar. FEMS Microbiol. Lett. 1996, 145, 131–137. [Google Scholar] [CrossRef]
- Kiener, A.; Leisinger, T. Oxygen Sensitivity of Methanogenic Bacteria. Syst. Appl. Microbiol. 1983, 4, 305–312. [Google Scholar] [CrossRef]
- Sowers, K.R.; Boone, J.E.; Gunsalus, R.P. Disaggregation of Methanosarcina Spp. and Growth as Single Cells at Elevated Osmolarity. Appl. Environ. Microbiol. 1993, 59, 3832–3839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, W.J.; Leigh, J.A.; Mayer, F.; Woese, C.R.; Wolfe, R.S. Methanococcus jannaschii Sp. Nov., an Extremely Thermophilic Methanogen from a Submarine Hydrothermal Vent. Arch. Microbiol. 1983, 136, 254–261. [Google Scholar] [CrossRef]
- Huber, H.; Thomm, M.; König, H.; Thies, G.; Stetter, K.O. Methanococcus thermolithotrophicus, a Novel Thermophilic Lithotrophic Methanogen. Arch. Microbiol. 1982, 132, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Battumur, U.; Yoon, Y.; Bae, G.S.; Kim, C.-H. Isolation and Characterization of New Methanosarcina mazei Strains KOR-3, -4, -5, and -6 from an Anaerobic Digester Using Pig Slurry. Asian-Australas. J. Anim. Sci. 2017, 30, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuzin, N.; Labat, M.; Garcia, J.L.; Ouattara, A.S. Methanobacterium congolense Sp. Nov., from a Methanogenic Fermentation of Cassava Peel. Int. J. Syst. Evol. Microbiol. 2001, 51, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Liu, X.; Dong, X. Methanobacterium beijingense Sp. Nov., a Novel Methanogen Isolated from Anaerobic Digesters. Int. J. Syst. Evol. Microbiol. 2005, 55, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Dehority, B.A. Characterization of Several Bovine Rumen Bacteria Isolated with a Xylan Medium. J. Bacteriol. 1966, 91, 1724–1729. [Google Scholar] [CrossRef] [Green Version]
- Chong, S.C.; Liu, Y.; Cummins, M.; Valentine, D.L.; Boone, D.R. Methanogenium marinum Sp. Nov., a H2-Using Methanogen from Skan Bay, Alaska, and Kinetics of H2 Utilization. Antonie Van Leeuwenhoek 2002, 81, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Beckmann, S.; Engelen, B.; Thielemann, T.; Cramer, B.; Schippers, A.; Cypionka, H. Microbial Methane Formation from Hard Coal and Timber in an Abandoned Coal Mine. Geomicrobiol. J. 2008, 25, 315–321. [Google Scholar] [CrossRef]
- Miller, N.J.; Garrett, O.W.; Prickett, P.S. Anaerobic Technique–a Modified Deep Agar Shake. J. Food Sci. 1939, 4, 447–451. [Google Scholar] [CrossRef]
- Widdel, F.; Pfennig, N. A New Anaerobic, Sporing, Acetate-Oxidizing, Sulfate-Reducing Bacterium, Desulfotomaculum (Emend.) acetoxidans. Arch. Microbiol. 1977, 112, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.B.; Harrell, L.J. Agar Shake Tube Technique for Simultaneous Determination of Aerobic and Anaerobic Susceptibility to Antibioticst. Antimicrob. Agents Chemother. 1977, 12, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogg, J.E.; Lee, S.Y.; Ogg, B.J. A Modified Tube Method for the Cultivation and Enumeration of Anaerobic Bacteria. Can. J. Microbiol. 1979, 25, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Ababouch, L.; Busta, F.F. A Modified Lee Tube Technique for the Cultivation and Enumeration of Anaerobes. Int. J. Food Microbiol. 1986, 3, 211–216. [Google Scholar] [CrossRef]
- Lee, S.Y.; Moore, S.E.; Mabee, M.S. Selective-Differential Medium for Isolation and Differentiation of Pectinatus from Other Brewery Microorganisms. Appl. Environ. Microbiol. 1981, 41, 386–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, L.W.; Chandrasekaran, A.; Reuning, R.H.; Hui, J.; Rawal, B.D. Reduction of Digoxin to 20R-Dihydrodigoxin by Cultures of Eubacterium lentum. Appl. Environ. Microbiol. 1986, 51, 1300–1303. [Google Scholar] [CrossRef] [Green Version]
- Hermann, M.; Noll, K.M.; Wolfe, R.S. Improved Agar Bottle Plate for Isolation of Methanogens or Other Anaerobes in a Defined Gas Atmosphere. Appl. Environ. Microbiol. 1986, 51, 1124–1126. [Google Scholar] [CrossRef] [Green Version]
- Olson, K.D. Modified Bottle Plate for the Cultivation of Strict Anaerobes. J. Microbiol. Methods 1992, 14, 267–269. [Google Scholar] [CrossRef]
- Fröhlich, J.; König, H. Rapid Isolation of Single Microbial Cells from Mixed Natural and Laboratory Populations with the Aid of a Micromanipulator. Syst. Appl. Microbiol. 1999, 22, 249–257. [Google Scholar] [CrossRef]
- Huber, R.; Huber, H.; Stetter, K.O. Towards the Ecology of Hyperthermophiles: Biotopes, New Isolation Strategies and Novel Metabolic Properties. FEMS Microbiol. Rev. 2000, 24, 615–623. [Google Scholar] [CrossRef]
- Huser, B.A.; Wuhrmann, K.; Zehnder, A.J.B. Methanothrix soehngenii Gen. Nov. Sp. Nov., a New Acetotrophic Non-Hydrogen-Oxidizing Methane Bacterium. Arch. Microbiol. 1982, 132, 1–9. [Google Scholar] [CrossRef]
- Mochimaru, H.; Tamaki, H.; Katayama, T.; Imachi, H.; Sakata, S.; Kamagata, Y. Methanomicrobium antiquum Sp. Nov., a Hydrogenotrophic Methanogen Isolated from Deep Sedimentary Aquifers in a Natural Gas Field. Int. J. Syst. Evol. Microbiol. 2016, 66, 4873–4877. [Google Scholar] [CrossRef] [PubMed]
- Hohnadel, M.; Maumy, M.; Chollet, R. Development of a Micromanipulation Method for Single Cell Isolation of Prokaryotes and Its Application in Food Safety. PLoS ONE 2018, 13, e0198208. [Google Scholar] [CrossRef] [PubMed]
- Ishøy, T.; Kvist, T.; Westermann, P.; Ahring, B.K. An Improved Method for Single Cell Isolation of Prokaryotes from Meso-, Thermo- and Hyperthermophilic Environments Using Micromanipulation. Appl. Microbiol. Biotechnol. 2006, 69, 510–514. [Google Scholar] [CrossRef]
- Huber, R.; Burggraf, S.; Mayer, T.; Barns, S.M.; Rossnagel, P.; Stetter, K.O. Isolation of a Hyperthermophilic Archaeum Predicted by in Situ RNA Analysis. Nature 1995, 376, 57–58. [Google Scholar] [CrossRef]
- Kita, A.; Suehira, K.; Miura, T.; Okamura, Y.; Aki, T.; Matsumura, Y.; Tajima, T.; Nishio, N.; Nakashimada, Y. Characterization of a Halotolerant Acetoclastic Methanogen Highly Enriched from Marine Sediment and Its Application in Removal of Acetate. J. Biosci. Bioeng. 2016, 121, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Iino, T.; Suzuki, K.-I.; Yamaguchi, K.; Kamagata, Y. Aceticlastic and NaCl-Requiring Methanogen “Methanosaeta Pelagica” Sp. Nov., Isolated from Marine Tidal Flat Sediment. Appl. Environ. Microbiol. 2012, 78, 3416–3423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowers, K.R.; Ferry, J.G. Isolation and Characterization of a Methylotrophic Marine Methanogen, Methanococcoides methylutens Gen. Nov., Sp. Nov. Appl. Environ. Microbiol. 1983, 45, 684–690. [Google Scholar] [CrossRef] [Green Version]
- Dridi, B.; Fardeau, M.-L.; Ollivier, B.; Raoult, D.; Drancourt, M. The Antimicrobial Resistance Pattern of Cultured Human Methanogens Reflects the Unique Phylogenetic Position of Archaea. J. Antimicrob. Chemother. 2011, 66, 2038–2044. [Google Scholar] [CrossRef]
- Khelaifia, S.; Drancourt, M. Susceptibility of Archaea to Antimicrobial Agents: Applications to Clinical Microbiology. Clin. Microbiol. Infect. 2012, 18, 841–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, S.; Imachi, H.; Sekiguchi, Y.; Tseng, I.-C.; Ohashi, A.; Harada, H.; Kamagata, Y. Cultivation of Methanogens under Low-Hydrogen Conditions by Using the Coculture Method. Appl. Environ. Microbiol. 2009, 75, 4892–4896. [Google Scholar] [CrossRef] [Green Version]
- Imachi, H.; Sakai, S.; Sekiguchi, Y.; Hanada, S.; Kamagata, Y.; Ohashi, A.; Harada, H. Methanolinea tarda Gen. Nov., Sp. Nov., a Methane-Producing Archaeon Isolated from a Methanogenic Digester Sludge. Int. J. Syst. Evol. Microbiol. 2008, 58, 294–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, S.; Imachi, H.; Sekiguchi, Y.; Ohashi, A.; Harada, H.; Kamagata, Y. Isolation of Key Methanogens for Global Methane Emission from Rice Paddy Fields: A Novel Isolate Affiliated with the Clone Cluster Rice Cluster I. Appl. Environ. Microbiol. 2007, 73, 4326–4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, S.; Imachi, H.; Hanada, S.; Ohashi, A.; Harada, H.; Kamagata, Y. Methanocella paludicola Gen. Nov., Sp. Nov., a Methane-Producing Archaeon, the First Isolate of the Lineage “Rice Cluster I”, and Proposal of the New Archaeal Order Methanocellales Ord. Nov. Int. J. Syst. Evol. Microbiol. 2008, 58, 929–936. [Google Scholar] [CrossRef]
- Mytilinaios, I.; Salih, M.; Schofield, H.K.; Lambert, R.J.W. Growth Curve Prediction from Optical Density Data. Int. J. Food Microbiol. 2012, 154, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.-J.; Ahn, S.-J.; Browngardt, C.M.; Burne, R.A. Changes in Biochemical and Phenotypic Properties of Streptococcus Mutans during Growth with Aeration. Appl. Environ. Microbiol. 2009, 75, 2517–2527. [Google Scholar] [CrossRef] [Green Version]
- Candry, P.; Van Daele, T.; Denis, K.; Amerlinck, Y.; Andersen, S.J.; Ganigué, R.; Arends, J.B.A.; Nopens, I.; Rabaey, K. A Novel High-Throughput Method for Kinetic Characterisation of Anaerobic Bioproduction Strains, Applied to Clostridium kluyveri. Sci. Rep. 2018, 8, 9724. [Google Scholar] [CrossRef]
- Stringer, S.C.; Webb, M.D.; George, S.M.; Pin, C.; Peck, M.W. Heterogeneity of Times Required for Germination and Outgrowth from Single Spores of Nonproteolytic Clostridium botulinum. Appl. Environ. Microbiol. 2005, 71, 4998–5003. [Google Scholar] [CrossRef] [Green Version]
- Bang, C.; Schilhabel, A.; Weidenbach, K.; Kopp, A.; Goldmann, T.; Gutsmann, T.; Schmitz, R.A. Effects of Antimicrobial Peptides on Methanogenic Archaea. Antimicrob. Agents Chemother. 2012, 56, 4123–4130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weimar, M.R.; Cheung, J.; Dey, D.; McSweeney, C.; Morrison, M.; Kobayashi, Y.; Whitman, W.B.; Carbone, V.; Schofield, L.R.; Ronimus, R.S.; et al. Development of Multiwell-Plate Methods Using Pure Cultures of Methanogens To Identify New Inhibitors for Suppressing Ruminant Methane Emissions. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Kim, J.; Hatzenpichler, R.; Karymov, M.A.; Hubert, N.; Hanan, I.M.; Chang, E.B.; Ismagilov, R.F. Gene-Targeted Microfluidic Cultivation Validated by Isolation of a Gut Bacterium Listed in Human Microbiome Project’s Most Wanted Taxa. Proc. Natl. Acad. Sci. USA 2014, 111, 9768–9773. [Google Scholar] [CrossRef] [Green Version]
- Villa, M.M.; Bloom, R.J.; Silverman, J.D.; Durand, H.K.; Jiang, S.; Wu, A.; Dallow, E.P.; Huang, S.; You, L.; David, L.A. Interindividual Variation in Dietary Carbohydrate Metabolism by Gut Bacteria Revealed with Droplet Microfluidic Culture. mSystems 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Watterson, W.J.; Tanyeri, M.; Watson, A.R.; Cham, C.M.; Shan, Y.; Chang, E.B.; Eren, A.M.; Tay, S. Droplet-Based High-Throughput Cultivation for Accurate Screening of Antibiotic Resistant Gut Microbes. eLife 2020, 9, e56998. [Google Scholar] [CrossRef]
- Steinhaus, B.; Garcia, M.L.; Shen, A.Q.; Angenent, L.T. A Portable Anaerobic Microbioreactor Reveals Optimum Growth Conditions for the Methanogen Methanosaeta concilii. Appl. Environ. Microbiol. 2007, 73, 1653–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birgen, C.; Degnes, K.F.; Markussen, S.; Wentzel, A.; Sletta, H. Butanol Production from Lignocellulosic Sugars by Clostridium beijerinckii in Microbioreactors. Biotechnol. Bioeng. 2020, 14, 1–12. [Google Scholar] [CrossRef]
- Widdel, F. Theory and Measurement of Bacterial Growth. In Grundpraktikum Mikrobiologie, 4. sem. (B. Sc); Universität Bremen: Bremen, Germany, 2007; p. 11. [Google Scholar]
- Azim, A.A.; Pruckner, C.; Kolar, P.; Taubner, R.-S.; Fino, D.; Saracco, G.; Sousa, F.L.; Rittmann, S.K.-M.R. The Physiology of Trace Elements in Biological Methane Production. Bioresour. Technol. 2017, 241, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Floodgate, G.D. A Chemical Method for Estimating Methanogenic Biomass. Cont. Shelf Res. 1992, 12, 1187–1196. [Google Scholar] [CrossRef]
- Widdel, F.; Wolfe, R.S. Expression of Secondary Alcohol Dehydrogenase in Methanogenic Bacteria and Purification of the F420-Specific Enzyme from Methanogenium thermophilum Strain TCI. Arch. Microbiol. 1989, 152, 322–328. [Google Scholar] [CrossRef]
- Shimizu, S.; Ueno, A.; Tamamura, S.; Naganuma, T.; Kaneko, K. Methanoculleus horonobensis Sp. Nov., a Methanogenic Archaeon Isolated from a Deep Diatomaceous Shale Formation. Int. J. Syst. Evol. Microbiol. 2013, 63, 4320–4323. [Google Scholar] [CrossRef] [PubMed]
- Kaesler, B.; Schönheit, P. Methanogenesis and ATP Synthesis in Methanogenic Bacteria at Low Electrochemical Proton Potentials. Eur. J. Biochem. 1988, 174, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Schönheit, P.; Beimborn, D.B. ATP Synthesis in Methanobacterium thermoautotrophicum Coupled to CH4 Formation from H2 and CO2 in the Apparent Absence of an Electrochemical Proton Potential across the Cytoplasmic Membrane. Eur. J. Biochem. 1985, 148, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Fukuzaki, S.; Nishio, N.; Nagai, S. Kinetics of the Methanogenic Fermentation of Acetate. Appl. Environ. Microbiol. 1990, 56, 3158–3163. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.T.; Okos, M.R. Kinetic Study and Mathematical Modeling of Methanogenesis of Acetate Using Pure Cultures of Methanogens. Biotechnol. Bioeng. 1987, 30, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Pappenreiter, P.A.; Zwirtmayr, S.; Mauerhofer, L.-M.; Rittmann, S.K.-M.R.; Paulik, C. Development of a Simultaneous Bioreactor System for Characterization of Gas Production Kinetics of Methanogenic Archaea at High Pressure. Eng. Life Sci. 2019, 19, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Hanišáková, N. Methanogenic Archaea in Environmental Samples. Master’s Thesis, Masarykova Universita, Brno, Czechia, 2020. [Google Scholar]
- Taubner, R.-S.; Pappenreiter, P.; Zwicker, J.; Smrzka, D.; Pruckner, C.; Kolar, P.; Bernacchi, S.; Seifert, A.; Krajete, A.; Bach, W.; et al. Biological Methane Production under Putative Enceladus-like Conditions. Nat. Commun. 2018, 9, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.R.; Fornero, J.J.; Stark, R.; Mets, L.; Angenent, L.T. A Single-Culture Bioprocess of Methanothermobacter thermautotrophicus to Upgrade Digester Biogas by CO2-to-CH4 Conversion with H2. Archaea 2013, 2013, 157529. [Google Scholar] [CrossRef] [Green Version]
- Seifert, A.H.; Rittmann, S.; Herwig, C. Analysis of Process Related Factors to Increase Volumetric Productivity and Quality of Biomethane with Methanothermobacter marburgensis. Appl. Energy 2014, 132, 155–162. [Google Scholar] [CrossRef]
- Pappenreiter, P.A. Development of a Pressurised Biomethanation Reactor System Using CO2-Type Methanogenic Strains. Ph.D. Thesis, Johannes Kepler University Linz, Linz, Austria, 2020. [Google Scholar]
- Bryant, M.P.; McBride, B.C.; Wolfe, R.S. Hydrogen-Oxidizing Methane Bacteria I. Cultivation and Methanogenesis. J. Bacteriol. 1968, 95, 1118–1123. [Google Scholar] [CrossRef] [Green Version]
- Hoffarth, M.; Broeker, T.; Schneider, J. Effect of N2 on Biological Methanation in a Continuous Stirred-Tank Reactor with Methanothermobacter Marburgensis. Fermentation 2019, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Mauerhofer, L.-M.; Reischl, B.; Schmider, T.; Schupp, B.; Nagy, K.; Pappenreiter, P.; Zwirtmayr, S.; Schuster, B.; Bernacchi, S.; Seifert, A.H.; et al. Physiology and Methane Productivity of Methanobacterium thermaggregans. Appl. Microbiol. Biotechnol. 2018, 102, 7643–7656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittmann, S.; Seifert, A.; Herwig, C. Quantitative Analysis of Media Dilution Rate Effects on Methanothermobacter marburgensis Grown in Continuous Culture on H2 and CO2. Biomass Bioenergy 2012, 36, 293–301. [Google Scholar] [CrossRef]
- Schill, N.; van Gulik, W.M.; Voisard, D.; von Stockar, U. Continuous Cultures Limited by a Gaseous Substrate: Development of a Simple, Unstructured Mathematical Model and Experimental Verification with Methanobacterium thermoautotrophicum. Biotechnol. Bioeng. 1996, 51, 645–658. [Google Scholar] [CrossRef]
- Mukhopadhyay, B.; Johnson, E.F.; Wolfe, R.S. Reactor-Scale Cultivation of the Hyperthermophilic Methanarchaeon Methanococcus jannaschii to High Cell Densities. Appl. Environ. Microbiol. 1999, 65, 5059–5065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shieh, J.; Whitman, W.B. Autotrophic Acetyl Coenzyme A Biosynthesis in Methanococcus maripaludis. J. Bacteriol. 1988, 170, 3072–3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Wolfe’s Solution 1 (g/L) | SL10 2 (g/L) | SL6 (g/L) | |
|---|---|---|---|
| Nitrilotriacetic acid (NTA) | 1.5 | - | - |
| MgSO4.7H2O | 3 | - | - |
| MnSO4.H2O | 0.5 | - | - |
| MnCl2.4H2O | - | 0.1 | 0.003 |
| NaCl | 1 | - | - |
| NiCl2.6H2O | - | 0.024 | 0.002 |
| FeSO4.7H2O | 0.1 | - | - |
| FeCl2.4H2O | - | 1.5 | - |
| CoCl2.6H2O | 0.1 | 0.19 | 0.02 |
| CaCl2 | 0.1 | - | - |
| ZnSO4.7H2O | 0.1 | - | 0.01 |
| ZnCl2 | - | 0.07 | - |
| CuSO4.5H2O | 0.01 | - | - |
| CuCl2.2H2O | - | 0.002 | 0.001 |
| AlK(SO)4.12H2O | 0.01 | - | - |
| H3BO3 | 0.01 | 0.006 | 0.03 |
| Na2MoO4.2H2O | 0.01 | 0.036 | 0.003 |
| Reference | [60] | [68] | [67] |
| Wolfe’s Solution (mg/L) | Widdel’s 5 Vitamin Solution (mg/L) | |
|---|---|---|
| Pyridoxine-HCl | 10 | 15 |
| Thiamine-HCl | 5 | - |
| Riboflavin | 5 | - |
| Nicotinic acid | 5 | 10 |
| Calcium pantothenate | 5 | 5 |
| p-Aminobenzoic acid | 5 | 4 |
| α-Lipoic acid | 5 | - |
| Biotin | 2 | 1 |
| Folic acid | 2 | - |
| Cyanocobalamin | 0.1 | - |
| Reference | [60] | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanišáková, N.; Vítězová, M.; Rittmann, S.K.-M.R. The Historical Development of Cultivation Techniques for Methanogens and Other Strict Anaerobes and Their Application in Modern Microbiology. Microorganisms 2022, 10, 412. https://doi.org/10.3390/microorganisms10020412
Hanišáková N, Vítězová M, Rittmann SK-MR. The Historical Development of Cultivation Techniques for Methanogens and Other Strict Anaerobes and Their Application in Modern Microbiology. Microorganisms. 2022; 10(2):412. https://doi.org/10.3390/microorganisms10020412
Chicago/Turabian StyleHanišáková, Nikola, Monika Vítězová, and Simon K. -M. R. Rittmann. 2022. "The Historical Development of Cultivation Techniques for Methanogens and Other Strict Anaerobes and Their Application in Modern Microbiology" Microorganisms 10, no. 2: 412. https://doi.org/10.3390/microorganisms10020412






