Dietary Inclusion of a Saccharomyces cerevisiae-Derived Postbiotic Is Associated with Lower Salmonella enterica Burden in Broiler Chickens on a Commercial Farm in Honduras
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Litter Sampling
2.3. Ceca Sampling
2.4. Salmonella Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallen, E.; Hoekstra, R.M.; Tauxe, R.V.; Widdowson, M.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Interagency Food Safety Analytics Collaboration. Foodborne Illness Source Attribution Estimates for 2017 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter Using Multi-Year Outbreak Surveillance Data, United States. Available online: https://www.cdc.gov/foodsafety/ifsac/pdf/P19-2017-report-TriAgency-508-archived.pdf (accessed on 21 September 2021).
- Popa, G.L.; Papa, M.L. Salmonella spp. Infection—A continuous threat worldwide. Germs 2021, 11, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Wong, D.; Patrick, M.E.; Binsztein, N.; Cieslik, A.; Chalermchaikit, T.; Aidara-Kane, A.; Ellis, A.; Angulott, F.J.; Wegener, H.C. Web-based surveillance and global Salmonella distribution, 200–2002. Emerg. Infect. Dis. 2006, 12, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Parisi, A.; Stanaway, J.D.; Sarkar, K.; Crump, J.A. The global burden of non-typhoidal Salmonella invasive disease: A systemic analysis of the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Food Safety and Inspection Service. Final Report of a Remote Audit Conducted for Honduras: Evaluating the Food Safety Systems Governing Raw Beef and Raw Poultry Products Exported to the United States of America. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-09/honduras-foreign-audit-report.pdf (accessed on 21 September 2021).
- United States Department of Agriculture, Food Safety and Inspection Service. Microbiology Laboratory Guidebook 4.11: Isolation and Identification of Salmonella from Meat, Poultry, Pasteurized Egg, and Siluriformes (Fish) Products and Carcass and Environmental Sponges. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-08/MLG-4.11.pdf (accessed on 21 September 2021).
- United States Centers for Disease Control and Prevention, Foodborne Diseases Active Surveillance Network (FoodNet). Available online: https://www.cdc.gov/foodnet/index.html (accessed on 21 September 2021).
- United States Department of Agriculture, Food Safety and Inspection Service. Salmonella Verification Testing Program Monthly Posting. Available online: https://www.fsis.usda.gov/science-data/data-sets-visualizations/microbiology/microbiological-testing-program-rte-meat-and-0 (accessed on 21 September 2021).
- McEntire, J.; Acheson, D.; Siemens, A.; Eilert, S.; Robach, M. The public health value of reducing Salmonella levels in raw meat and poultry. Food Prot. Trends 2014, 34, 386–392. [Google Scholar]
- United States Department of Agriculture, Food Safety and Inspection Service. Roadmap to Reducing Salmonella: Driving Change through Science-Based Policy. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2020-12/FSISRoadmaptoReducingSalmonella.pdf (accessed on 21 September 2021).
- Sampedro, F.; Wells, S.J.; Bender, J.B.; Hedberg, C.W. Developing a risk management framework to improve public health outcomes by enumerating Salmonella in ground turkey. Epidemiol. Infect. 2019, 147, E69. [Google Scholar] [CrossRef] [Green Version]
- Oscar, T.P. Salmonella prevalence alone is not a good indicator of poultry food safety. Risk Anal. 2021, 41, 110–130. [Google Scholar] [CrossRef]
- Volkova, V.V.; Wills, R.W.; Hubbard, S.A.; Magee, D.L.; Byrd, J.A.; Bailey, R.H. risk factors associated with detection of Salmonella in broiler litter at the time of new flock placement. Zoonoses Public Health 2011, 58, 158–168. [Google Scholar] [CrossRef]
- Pulido-Landinez, M. Food safety—Salmonella update in broilers. Anim. Feed Sci. Technol. 2019, 250, 53–58. [Google Scholar] [CrossRef]
- Berghaus, R.D.; Mathis, D.L.; Bramwell, R.K.; Macklin, K.S.; Wilson, J.L.; Wineland, M.J.; Maurer, J.J.; Lee, M.D. Multilevel analysis of environmental Salmonella prevalences and management practices on 49 broiler breeder farms in four south-eastern states, USA. Zoonoses Public Health 2012, 59, 365–374. [Google Scholar] [CrossRef]
- Acevedo-Villanueva, K.; Renu, S.; Gourapura, R.; Selvaraj, R. Efficacy of a nanoparticle vaccine administered in-ovo against Salmonella in broilers. PLoS ONE 2021, 16, e0247938. [Google Scholar] [CrossRef] [PubMed]
- Dorea, F.C.; Cole, D.J.; Hofacre, C.L.; Zamperini, K.; Mathis, D.L.; Doyle, M.P.; Lee, M.D.; Maurer, J.J. Effect of Salmonella vaccination of breeder chickens on contamination of broiler chicken carcasses in integrated poultry operations. Appl. Environ. Microbiol. 2010, 76, 7820–7825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesse, M.; Weber, R.; Glunder, G. Antibody titers in turkeys increase after multiple booster vaccinations with an attenuated Salmonella live vaccine. BMC Res. Notes 2018, 11, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; McWhorter, A.R.; Andrews, D.M.; Underwood, G.J.; Chousaalkar, K.K. Challenges in vaccinating layer hens against Salmonella typhimurium. Vaccines 2020, 8, 696. [Google Scholar] [CrossRef]
- Tellez, G.; Pixley, C.; Wolfenden, R.E.; Layton, S.L.; Hargis, B.M. Probiotics/direct fed microbials for Salmonella control in poultry. Food Res. Int. 2012, 45, 628–633. [Google Scholar] [CrossRef]
- Johny, A.K.; Venkitanarayanan, K. Chatper 17—Preharvest Food Safety—Potential Use of Plant-Derived Compounds in Layer Chickens in Producing Safe Eggs; Ricke, S.C., Gast, R.K., Eds.; Elsevier: London, UK, 2016; pp. 347–372. [Google Scholar]
- Adhikari, P.; Lee, C.H.; Cosby, D.E.; Cox, N.A.; Kim, W.K. Effect of probiotics on fecal excretion, colonization in internal organs and immune gene expression in the ileum of laying hens challenged with Salmonella Enteritidis. Poult. Sci. 2019, 98, 1235–1242. [Google Scholar] [CrossRef]
- Micciche, A.C.; Foley, S.L.; Pavlidis, H.O.; McIntyre, D.R.; Ricke, S.C. A Review of prebiotics against Salmonella in poultry: Current and future potential for microbiome research applications. Front. Vet. Sci. 2018, 5, 191. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.I.; Sadekuzzaman, M.; Sang-Do, H. Probiotics as potential alternative biocontrol agents in the agriculture and food industries: A review. Food Res. Int. 2017, 100, 63–73. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott KStanton, C.; Swanson, K.S.; Cani, P.D.; Verbeke, K.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Toala, J.E.; Garcia-Varela, G.; Garcia, H.S.; Mata-Haro, V.; Gonsalez-Cordova, A.F.; Vallejo-Cordoba, B.; Hernandez-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; M.E., S.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.R.; McIntyre, D.R.; Pavlidis, H.O.; Archer, G.S. Reducing stress susceptibility of broiler chickens by supplementing a yeast fermentation product in the feed or drinking water. Animals 2018, 8, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lensing, M.; van der Klis, J.D.; Yoon, I.; Moore, D.T. Efficacy of Saccharomyces cerevisiae fermentation product on intestinal health and productivity of coccidian-challenged laying hens. Poult. Sci. 2012, 91, 1590–1597. [Google Scholar] [CrossRef]
- Labib, Z.M.; Elsamadony, H.A.; El Gabaly, L.S.; Zoghbi, A.F. Immunopathological studies on ducks experimentally infected with duck virus enteritis and Salmonella enteritidis with special references to the effect of XPC prebiotic. Zagazig Vet. J. 2014, 42, 41–62. [Google Scholar] [CrossRef] [Green Version]
- Firman, J.D.; Moore, D.; McIntyre, D. Effects of dietary inclusion of a Saccharomyces cerevisiae fermentation product on performance and gut characteristics of male turkeys to market weight. Int. J. Poult. Sci. 2013, 12, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Price, P.T.; Byrd, J.A.; Alvarado, C.Z.; Pavlidis, H.O.; McIntyre, D.R.; Archer, G.S. Utilizing original XPCTM in feed to reduce stress susceptibility of broilers. Poult. Sci. 2018, 97, 855–859. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.J.; Wu, S.G.; Yu, S.H.; Yoon, I.; Moore, D.; Gao, Y.P.; Yan, H.J.; Qi, G.H. Effect of Saccharomyces cerevisiae fermentation product on immune functions of broilers challenged with Eimeria tenella. Poult. Sci. 2009, 88, 2141–2151. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.J.; Wu, S.G.; Yu, S.H.; Yoon, I.; Quigley, J.; Gao, Y.P.; Qi, G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008, 87, 1377–1384. [Google Scholar] [CrossRef]
- Park, S.; Roto, S.; Pavlidis, H.; McIntyre, D.; Striplin, K.; Brammer, L.; Ricke, S. Effects of feeding original XPC to broilers with a live coccidiosis vaccine under industrial conditions: Part 2. Cecal microbiota analysis. Poult. Sci. 2017, 96, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Roto, S.; Park, S.; Lee, S.; Kaldhone, P.; Pavlidis, H.; Frankenbach, S.; McIntyre, D.; Striplin, K.; Brammer, L.; Ricke, S. Effects of feeding Original XPCTM to broilers with a live coccidiosis-vaccine under industry conditions: Part 1. Growth performance and Salmonella inhibition. Poult. Sci. 2017, 96, 1831–1837. [Google Scholar] [CrossRef]
- Feye, K.M.; Rubinelli, P.M.; Chaney, W.E.; Pavlidis, H.O.; Kogut, M.H.; Ricke, S.C. The preliminary development of an in vitro poultry cecal culture model to evaluate the effects of original XPCTM for the reduction of Campylobacter jejuni and its potential effects on the microbiota. Front. Microbiol. 2020, 10, 3062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong Park, S.; Ae Kim, S.; In Lee, S.; Rubinelli, P.; Roto, S.; Pavlidis, H.; McIntyre, D.; Ricke, S. Original XPCTM Effect on Salmonella typhimurium and cecal microbiota from three different ages of broiler chickens when incubated in an anaerobic in vitro culture system. Front. Microbiol. 2017, 8, 1070. [Google Scholar] [CrossRef]
- Rubinelli, P.; Roto, S.; Ae Kim, S.; Hong Park, S.; Pavlidis, H.; McIntyre, D.; Ricke, S. Reduction of Salmonella typhimurium by fermentation metabolites of diamond V original XPC in an in vitro anaerobic mixed chicken cecal culture. Front. Vet. Sci. 2016, 3, 83. [Google Scholar] [CrossRef] [Green Version]
- Pavic, A.; Goves, P.J.; Bailey, G.; Cox, J.M. A validated miniaturized MPN method, based on ISO 6579:2002, for the enumeration of Salmonella from poultry matrices. J. Appl. Microbiol. 2010, 109, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.7.0. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 9 December 2021).
- Moran, E.T.; Bilgili, S.F. Influence of feeding and fasting broilers prior to marketing on cecal access of orally administered Salmonella. J. Food Prot. 1990, 53, 205–207. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Sarlin, L.L.; Caldwell, D.J.; Yezak, C.R., Jr.; Hume, M.E.; Corrier, D.E.; Deloach, J.R.; Hargis, B.M. Effect of feed withdrawal on the incidence of Salmonella in the crops and ceca of market age broiler chickens. Poult. Sci. 1997, 76, 654–656. [Google Scholar] [CrossRef]
- Corrier, D.E.; Byrd, J.A.; Hargis, B.M.; Hume, M.E.; Bailey, R.H.; Stanker, L.H. Survival of Salmonella in the crop contents of market-age broilers during feed withdrawal. Avian Dis. 1999, 43, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Valeris-Chacin, R.; Pieters, M.; Hwang, H.; Johnson, T.J.; Singer, R.S. Association of Broiler Litter Microbiome Composition and Campylobacter Isolation. Front. Vet. Sci. 2021, 8, 4927. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M.G.; Zwirzitz, B.; Oladeinde, A.; Cook, K.L.; Plymel, C.; Zock, G.; Lakin, S.; Aggrey, S.E.; Ritz, C.; Looft, T.; et al. Reused poultry litter microbiome with competitive exclusion potential against Salmonella Heidelberg. J. Environ. Qual. 2019, 49, 869–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Machado Junior, P.C.; Chung, C.; Hagerman, A. Modeling Salmonella spread in broiler production: Identifying determinants and control strategies. Front. Vet. Sci. 2020, 7, 564. [Google Scholar] [CrossRef]
- Roll, V.F.B.; Dai Pra, M.A.; Roll, A.P. Research on Salmonella in broiler litter reused for up to 14 consecutive flocks. Poult. Sci. 2011, 2257–2262. [Google Scholar] [CrossRef]
- Volkova, V.V.; Bailey, R.H.; Wills, R.W. Salmonella in broiler litter and properties of soil at farm location. PLoS ONE 2009, 4, e6403. [Google Scholar] [CrossRef]
- Cressman, M.D.; Zhongtang, Y.; Nelson, M.C.; Moeller, S.J.; Lilburn, M.S.; Zerby, H.N. Interreltations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl. Environ. Microbiol. 2010, 76, 6572–6582. [Google Scholar] [CrossRef] [Green Version]
- Buhr, R.J.; Bourassa, D.V.; Hinton, A., Jr.; Fairchild, B.D.; Ritz, C.W. Impact of litter Salmonella status during feed withdrawal on Salmonella recovery from the broiler crop and ceca. Poult. Sci. 2017, 96, 4361–4369. [Google Scholar] [CrossRef]
- Corrier, D.E.; Byrd, J.A.; Hargis, B.M.; Hume, M.E.; Bailey, R.H.; Stanker, L.H. Presence of Salmonella in the crop and ceca of broiler chickens before and after preslaughter feed withdrawal. Poult. Sci. 1999, 78, 45–49. [Google Scholar] [CrossRef]
- Berghaus, R.D.; Thayer, S.G.; Law, B.F.; Mild, R.M.; Hofacre, C.L.; Singer, R.S. Enumeration of Salmonella and Campylobacter spp. in environmental farm samples and processing plant carcass rinses from commercial broiler chicken flocks. Appl. Environ. Microbiol. 2013, 79, 4106–4114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, A.W.L.; Tsolis, R.M.; Baumler, A.J. Salmonella versus the microbiome. Microbiol. Molec. Biol. Rev. 2020, 85, e00027-19. [Google Scholar] [CrossRef]
- Feye, K.M.; Dittoe, D.K.; Rubinelli, P.M.; Olson, E.G.; Ricke, S.C. Yeast fermentate-mediated reduction of Salmonella reading and typhimurium in an in vitro turkey cecal culture model. Front. Microbiol. 2021, 12, 645301. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, E.; Frana, T.; Logue, C.M.; Smith, D.P.; Pavlidis, H.; Chaney, W.E. Effect of feeding a postbiotic derived from Saccharomyces cerevisiae fermentation as a preharvest food safety hurdle for reducing Salmonella Enteritidis in the ceca of layer pullets. J. Food Protection 2021, 84, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Price, P.T.; Gaydos, T.A.; Berghaus, R.D.; Baxter, V.; Hofacre, C.L.; Sims, M.D. Salmonella Enteritidis reduction in layer ceca with a Bacillus probiotic. Vet. World 2020, 13, 184–187. [Google Scholar] [CrossRef]
- Wideman, N.; Bailey, M.; Bilgili, S.F.; Thippareddi, H.; Wang, L.; Bratcher, C.; Sanchez-Plata, M.; Singh, M. Evaluating best practices for Campylobacter and Salmonella reduction in poultry processing plants. Poult. Sci. 2016, 95, 306–315. [Google Scholar] [CrossRef]
- Loretz, M.; Stephan, R.; Zweifel, C. Antimicrobial activity of decontamination treatments for poultry carcasses: A literature survey. Food Control 2010, 21, 791–804. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.S.; Conte-Junior, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [Green Version]
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Lo Fo Wong, D.M.A.; Jensen, A.B.; Wgener, H.C.; Aarestrup, F.M. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infection Network Country Data Bank: Results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.; Pangloli, P.; Richards, H.A.; Mount, J.R.; Draughon, F.A. Prevalence of Salmonella in diverse environmental farm samples. J. Food Prot. 2006, 69, 2576–2580. [Google Scholar] [CrossRef]
- Velasquez, C.G.; Macklin, K.S.; Kumar, S.; Bailey, M.; Ebner, P.E.; Oliver, H.F.; Martin-Gonzalez, F.S.; Singh, M. Prevalence and antimicrobial resistance patterns of Salmonella isolated from poultry farms in southeastern United States. Poult. Sci. 2018, 97, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
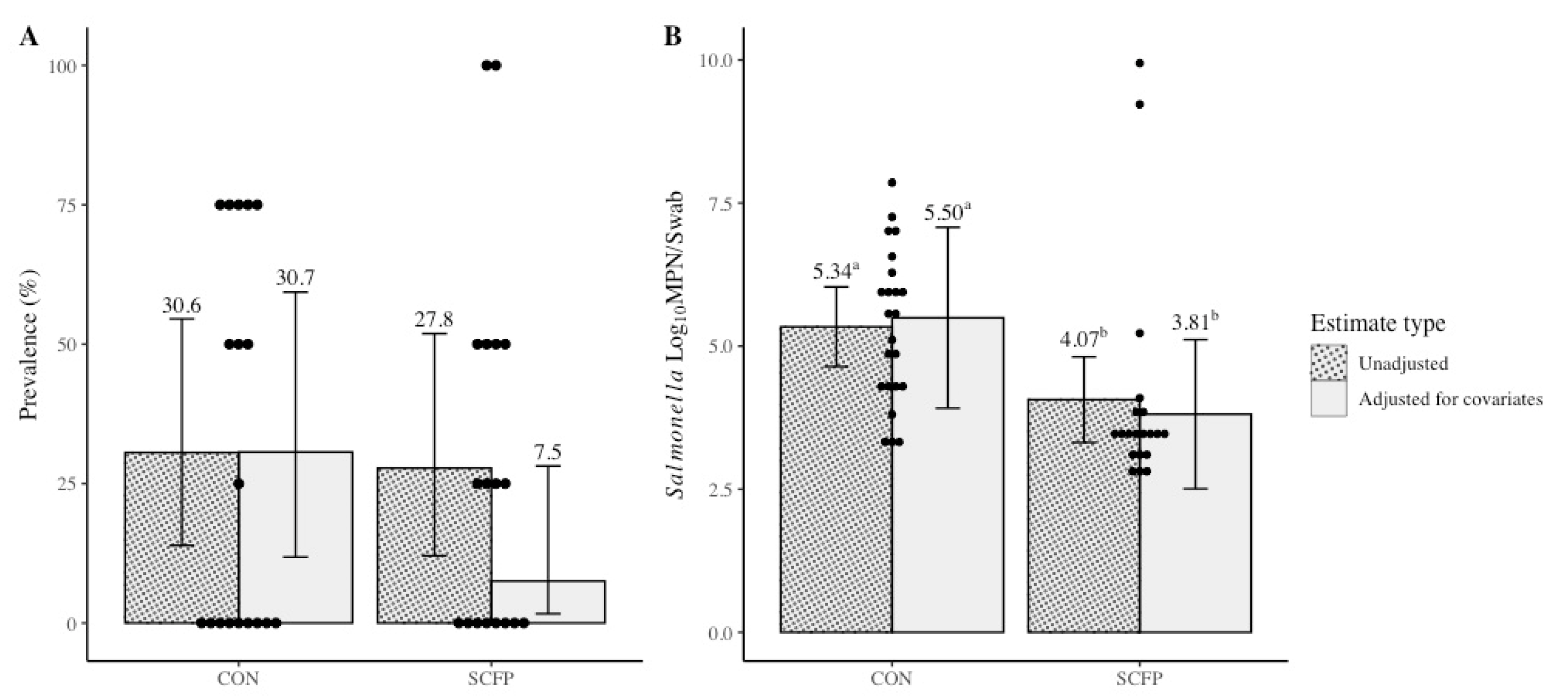
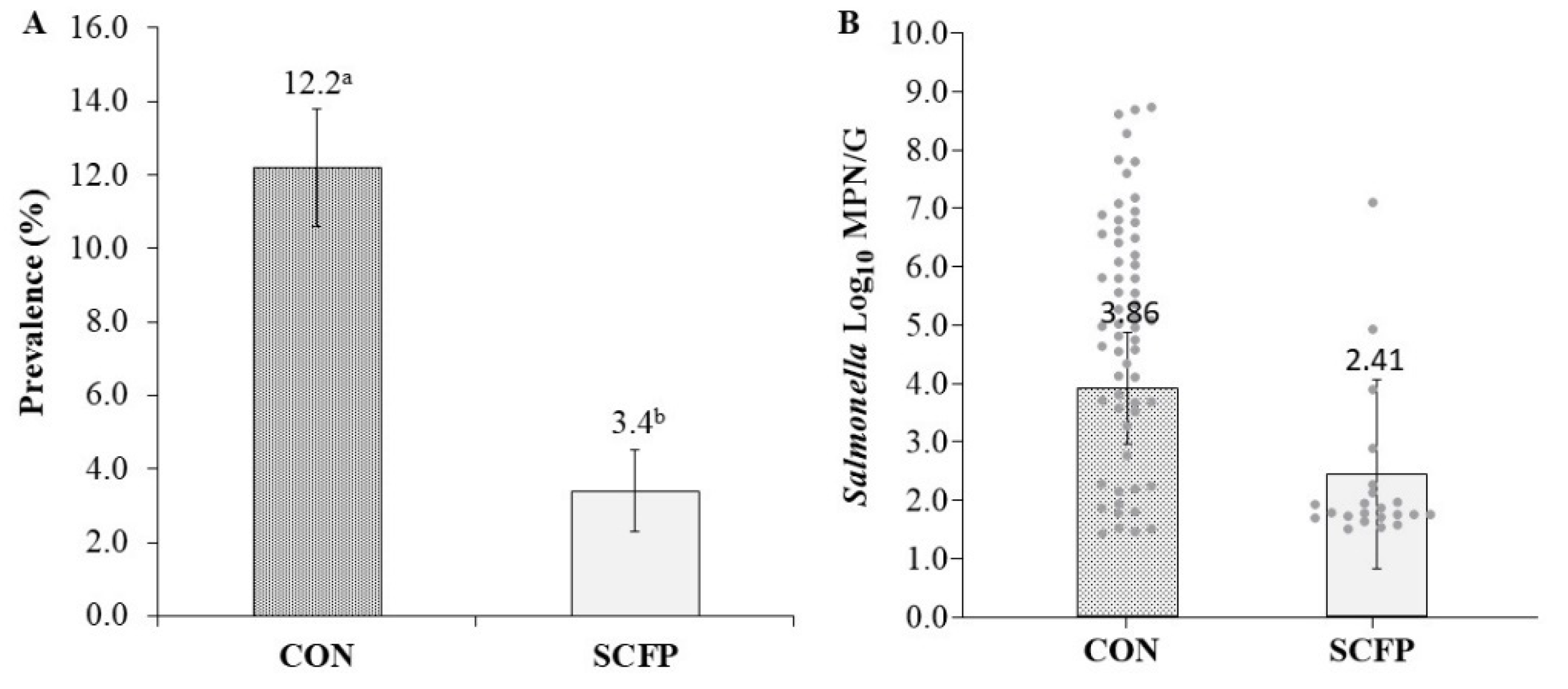
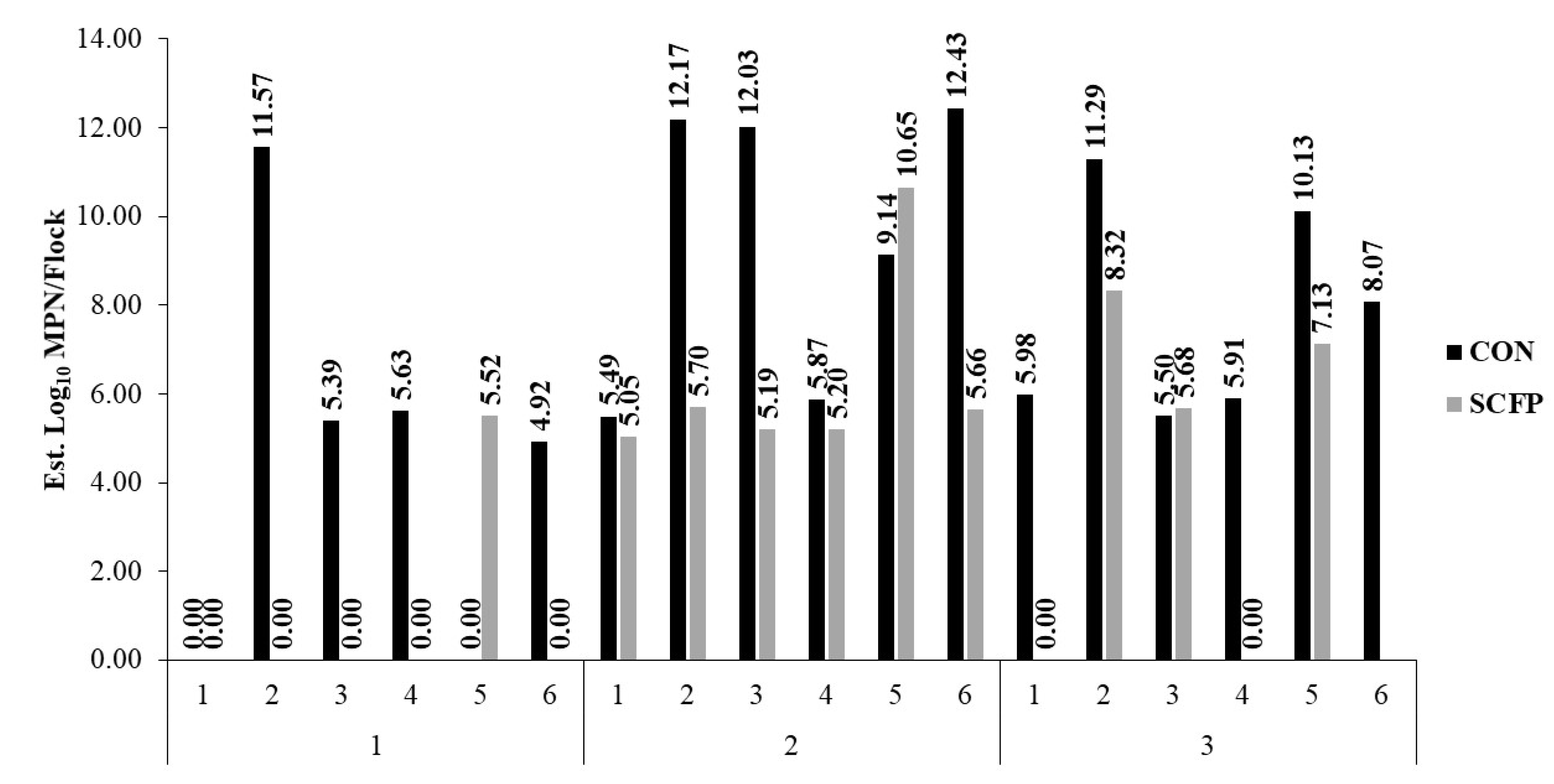

| Variable | Treatment | Preplacement | Cycle 1 | Cycle 2 | Cycle 3 |
|---|---|---|---|---|---|
| Salmonella Prevalence (%; no. positive/total no. samples) | CON | 25.0 (6/24) | 12.5 (3/24) | 50.0 (12/24) | 33.3 (8/24) |
| SCFP | 33.3 (8/24) | 20.8 (5/24) | 20.8 (5/24) | 41.7 (10/24) | |
| aSalmonella Concentration (log10 MPN/swab of culture-positive samples) | CON | 3.35 (0.66) | 5.77 (1.85) | 5.47 (1.48) | 4.98 (0.94) |
| SCFP | 6.17 (0.99) | 3.01 (0.32) | 3.54 (0.45) | 4.85 (2.56) |
| Flock-Rearing Cycle | ||||
|---|---|---|---|---|
| Variable | Treatment | Cycle 1 | Cycle 2 | Cycle 3 |
| Salmonella Prevalence (%; no. positive/total no. samples) | CON | 7.3 (11/150) | 22.0 (33/150) | 10.7 (16/150) |
| SCFP | 1.3 (2/150) | 12.0 (18/150) | 2.4 (3/125) | |
| aSalmonella Concentration (log10 MPN/g of culture-positive samples) | CON | 4.12 (2.58) | 5.44 (1.84) | 4.33 (1.94) |
| SCFP | 1.67 (0.04) | 2.26 (1.34) | 3.62 (1.33) | |
| Flock-Rearing Cycle | ||||
|---|---|---|---|---|
| Variable | Treatment | Cycle 1 | Cycle 2 | Cycle 3 |
| * Salmonella Prevalence (%; no. positive/total no. samples) | CON | 7.3 ± 2.1 a (11/150) | 22.0 ± 3.4 a (33/150) | 10.7 ± 2.5 a (16/150) |
| SCFP | 1.3 ± 0.9 b (2/150) | 12.0 ± 2.7 b (18/150) | 2.4 ± 1.4 b (3/125) | |
| * Salmonella Cecal Concentration (log10 MPN/g of culture-positive samples) | CON | 2.98 ± 0.91 | 4.72 ± 0.71 a | 3.88 ± 0.74 |
| SCFP | 1.67 ± 1.82 | 1.95 ± 0.77 b | 3.62 ± 1.18 | |
| Salmonella Est. Cecal Burden to Plant (log10 MPN) | CON | 4.59 ± 1.28 | 9.52 ± 1.28 | 7.81 ± 1.28 |
| SCFP | 0.92 ± 1.28 | 6.24 ±1.28 | 4.23 ± 1.40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaney, W.E.; Naqvi, S.A.; Gutierrez, M.; Gernat, A.; Johnson, T.J.; Petry, D. Dietary Inclusion of a Saccharomyces cerevisiae-Derived Postbiotic Is Associated with Lower Salmonella enterica Burden in Broiler Chickens on a Commercial Farm in Honduras. Microorganisms 2022, 10, 544. https://doi.org/10.3390/microorganisms10030544
Chaney WE, Naqvi SA, Gutierrez M, Gernat A, Johnson TJ, Petry D. Dietary Inclusion of a Saccharomyces cerevisiae-Derived Postbiotic Is Associated with Lower Salmonella enterica Burden in Broiler Chickens on a Commercial Farm in Honduras. Microorganisms. 2022; 10(3):544. https://doi.org/10.3390/microorganisms10030544
Chicago/Turabian StyleChaney, W. Evan, S. Ali Naqvi, Manuel Gutierrez, Abel Gernat, Timothy J. Johnson, and Derek Petry. 2022. "Dietary Inclusion of a Saccharomyces cerevisiae-Derived Postbiotic Is Associated with Lower Salmonella enterica Burden in Broiler Chickens on a Commercial Farm in Honduras" Microorganisms 10, no. 3: 544. https://doi.org/10.3390/microorganisms10030544
APA StyleChaney, W. E., Naqvi, S. A., Gutierrez, M., Gernat, A., Johnson, T. J., & Petry, D. (2022). Dietary Inclusion of a Saccharomyces cerevisiae-Derived Postbiotic Is Associated with Lower Salmonella enterica Burden in Broiler Chickens on a Commercial Farm in Honduras. Microorganisms, 10(3), 544. https://doi.org/10.3390/microorganisms10030544






