Evaluation of UV-C Radiation Efficiency in the Decontamination of Inanimate Surfaces and Personal Protective Equipment Contaminated with Phage ϕ6
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Preparation of Virus Suspension
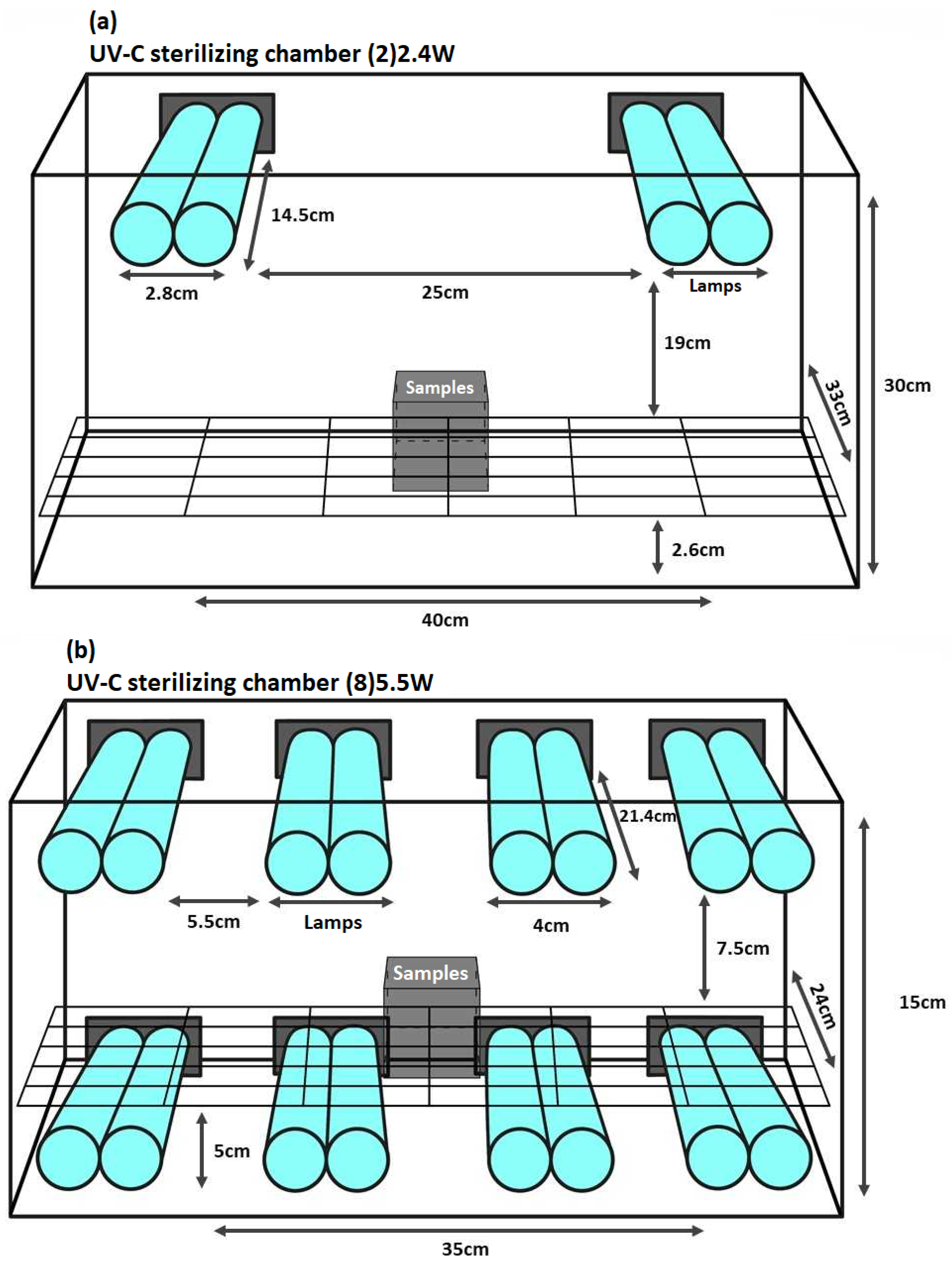
2.3. UV-C Light System, Lamps Characteristics, and Irradiation Systems
2.4. Inanimate Surfaces and Personal Protective Equipment Preparation
2.5. UV-C Irradiation Assays
2.6. Statistical Analysis
3. Results
3.1. UV-C Irradiation Assays
3.1.1. Inanimate Surfaces
3.1.2. Personal Protective Equipment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raeiszadeh, M.; Adeli, B. A Critical review on ultraviolet disinfection systems against COVID-19 outbreak: Applicability, validation, and safety considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef]
- Subbarao, K.; Mahanty, S. Respiratory virus infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus Disease (COVID-19): How Is It Transmitted? Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-how-is-it-transmitted (accessed on 12 February 2022).
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, A.; Chu, J.; Perera, M.; Hui, K.; Yen, H.-L.; Chan, M.; Peiris, M.; Poon, L. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Doremalen, N.; Bushmaker, T.; Morris, D.; Holbrook, M.; Gamble, A.; Williamson, B.; Tamin, A.; Harcourt, J.; Thornburg, N.; Gerber, S.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2010, 382, 1564–1567. [Google Scholar] [CrossRef]
- Gidari, A.; Sabbatini, S.; Bastianelli, S.; Pierucci, S.; Busti, C.; Bartolini, D.; Stabile, A.M.; Monari, C.; Galli, F.; Rende, M.; et al. SARS-CoV-2 survival on surfaces and the effect of UV-C light. Viruses 2021, 13, 408. [Google Scholar] [CrossRef]
- Gonçalves, J.; Da Silva, P.; Reis, L.; Nascimento, M.; Koritnik, T.; Paragi, M.; Mesquita, J. Surface contamination with SARS-CoV-2: A systematic review. Sci. Total Environ. 2021, 798, 149231. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 19–21. [Google Scholar] [CrossRef] [Green Version]
- Kaba, H.E.J.; Steinmann, J.; Pfaender, S.; Kampf, G.; Bru, Y.; Scheithauer, S.; Steinmann, E. Potential sources, modes of transmission and effectiveness of prevention measures against SARS-CoV-2. J. Hosp. Infect. 2020, 106, 678–697. [Google Scholar] [CrossRef]
- Lv, J.; Yang, J.; Xue, J.; Zhu, P.; Liu, L.; Li, S. Detection of SARS-CoV-2 RNA residue on object surfaces in nucleic acid testing laboratory using droplet digital PCR. Sci. Total Environ. 2020, 742, 140370. [Google Scholar] [CrossRef]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Magurano, F.; Baggieri, M.; Marchi, A.; Rezza, G.; Nicoletti, L.; Eleonora, B.; Concetta, F.; Stefano, F.; Maedeh, K.; Paola, B.; et al. SARS-CoV-2 infection: The environmental endurance of the virus can be influenced by the increase of temperature. Clin. Microbiol. Infect. 2021, 27, 289.e5–289.e7. [Google Scholar] [CrossRef] [PubMed]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. mSphere 2020, 5, e00441-20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, R.; Kumari, N.; Karmakar, S.; Behera, M.; Siddiqui, A.J. Current understanding of the influence of environmental factors on SARS-CoV-2 transmission, persistence, and infectivity. Environ. Sci. Pollut. Res. 2021, 28, 6267–6288. [Google Scholar] [CrossRef]
- Heßling, M.; Hönes, K.; Vatter, P.; Lingenfelder, C. Ultraviolet irradiation doses for coronavirus inactivation—Review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control 2020, 15, Doc08. [Google Scholar] [CrossRef] [PubMed]
- Beggs, C.B.; Avital, E.J. Upper-room ultraviolet air disinfection might help to reduce COVID-19 transmission in buildings: A feasibility study. PeerJ 2020, 8, e10196. [Google Scholar] [CrossRef]
- Hadi, J.; Dunowska, M.; Wu, S.; Brightwell, G. Control measures for SARS-V-2: A review on light-based inactivation of single-stranded rna viruses. Pathogens 2020, 9, 737. [Google Scholar] [CrossRef]
- Cutler, T.; Zimmerman, J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Wurtmann, E.J.; Wolin, S.L. RNA under attack: Cellular handling of RNA damage. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Wigginton, K.R.; Menin, L.; Sigstam, T.; Gannon, G.; Cascella, M.; Hamidane, H.B.; Tsybin, Y.O.; Waridel, P.; Kohn, T. UV radiation induces genome-mediated, site-specific cleavage in viral proteins. ChemBioChem 2012, 13, 837–845. [Google Scholar] [CrossRef]
- Reed, N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W.J.; Bahnfleth, W.P.; Hernandez, M.T. A genomic model for predicting the ultraviolet susceptibility of viruses and bacteria. In Proceedings of the IUVA Conference, Amsterdam, The Netherlands, 21–25 September 2009; Volume 11, pp. 15–28. [Google Scholar]
- Mojica, K.D.A.; Brussaard, C.P.D. Factors affecting virus dynamics and microbial host-virus interactions in marine environments. FEMS Microbiol. Ecol. 2014, 89, 495–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lytle, C.D.; Sagripanti, J.-L. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J. Virol. 2005, 79, 14244–14252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlmutter, J.; Hagan, M. Mechanisms of virus assembly. Annu. Rev. Phys. Chem. 2015, 66, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meo, S.A.; Alhowikan, A.M.; Khlaiwi, T.A.L.; Meo, I.M.; Halepoto, D.M.; Iqbal, M.; Usmani, A.M.; Hajjar, W.; Ahmed, N. Novel coronavirus 2019-nCoV: Prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Pinon, A.; Vialette, M. Survival of viruses in water. Intervirology 2019, 61, 214–222. [Google Scholar] [CrossRef]
- Santos, A.L.; Moreirinha, C.; Lopes, D.; Esteves, A.C.; Henriques, I.; Almeida, A.; Domingues, M.R.M.; Delgadillo, I.; Correia, A.; Cunha, A. Effects of UV radiation on the lipids and proteins of bacteria studied by mid-infrared spectroscopy. Environ. Sci. Technol. 2013, 47, 6306–6315. [Google Scholar] [CrossRef]
- Sabino, C.P.; Sellera, F.P.; Sales-Medina, D.F.; Machado, R.R.G.; Durigon, E.L.; Freitas-Junior, L.H.; Ribeiro, M.S. UV-C (254 nm) lethal doses for SARS-CoV-2. Photodiagn. Photodyn. Ther. 2020, 32, 101995. [Google Scholar] [CrossRef]
- Inagaki, H.; Saito, A.; Sugiyama, H.; Okabayashi, T.; Fujimoto, S. Rapid inactivation of SARS-CoV-2 with Deep-UV LED irradiation. Emerg. Microbes Infect. 2020, 9, 1744–1747. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Ozog, D.M.; Sexton, J.Z.; Narla, S.; Pretto-Kernahan, C.D.; Mirabelli, C.; Lim, H.W.; Hamzavi, I.H.; Tibbetts, R.J.; Mi, Q.S. The effect of ultraviolet C radiation against different N95 respirators inoculated with SARS-CoV-2. Int. J. Infect. Dis. 2020, 100, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.R.; Yoichi, M.; Unno, H.; Tanji, Y. The coexistence of Escherichia coli serotype O157:H7 and its specific bacteriophage in continuous culture. FEMS Microbiol. Lett. 2004, 241, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, J.; Donskey, C. Understanding ultraviolet light surface decontamination in hospital rooms: A primer. Infect. Control Hosp. Epidemiol. 2019, 40, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Donskey, C.J. Decontamination devices in health care facilities: Practical issues and emerging applications. Am. J. Infect. Control 2019, 47, A23–A28. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.; Weaver, S. Evaluation of eluents for the recovery of an enveloped virus from hands by whole-hand sampling. J. Appl. Microbiol. 2015, 118, 1210–1216. [Google Scholar] [CrossRef]
- Whitworth, C.; Mu, Y.; Houston, H.; Martinez-Smith, M.; Noble-Wang, J.; Coulliette-Salmond, A.; Rose, L. Persistence of bacteriophage phi 6 on porous and nonporous surfaces and the potential for its use as an ebola virus or coronavirus surrogate. Appl. Environ. Microbiol. 2020, 86, e01482-20. [Google Scholar] [CrossRef]
- Silverman, A.I.; Boehm, A.B. Systematic review and meta-analysis of the persistence and disinfection of human coronaviruses and their viral surrogates in water and wastewater. Environ. Sci. Technol. Lett. 2020, 7, 544–553. [Google Scholar] [CrossRef]
- Vejerano, E.P.; Marr, L.C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J. R. Soc. Interface 2018, 15, 20170939. [Google Scholar] [CrossRef] [Green Version]
- Prussin, A.J.; Schwake, D.O.; Lin, K.; Gallagher, D.L.; Buttling, L.; Marr, L.C. Survival of the enveloped virus phi6 in droplets as a function of relative humidity, absolute humidity, and temperature. Appl. Environ. Microbiol. 2018, 84, e00551-18. [Google Scholar] [CrossRef] [Green Version]
- Turgeon, N.; Toulouse, M.-J.; Martel, B.; Moineau, S.; Duchaine, C. Comparison of five bacteriophages as models for viral aerosol studies. Appl. Environ. Microbiol. 2014, 80, 4242–4250. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, N.A.; Stachler, E.N.; Cimabue, N.; Bibby, K. Evaluation of phi6 persistence and suitability as an enveloped virus surrogate. Environ. Sci. Technol. 2017, 51, 8692–8700. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.; Grinberg, M.; Orevi, T.; Kashtan, N. Survival of the enveloped bacteriophage phi6 (a surrogate for SARS-CoV-2) in evaporated saliva microdroplets deposited on glass surfaces. Sci. Rep. 2020, 10, 22419. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Pandey, N.; Shukla, A.; Singh, S.K. SARS coronavirus 2: From genome to infectome. Respir. Res. 2020, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Mindich, L. Precise packaging of the three genomic segments of the double-stranded-RNA bacteriophage φ6. Microbiol. Mol. Biol. Rev. 1999, 63, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, L.; Pereira, C.; Frazão, C.; Balcão, V.; Almeida, A. Efficiency of phage φ6 for biocontrol of Pseudomonas syringae pv. syringae: An in vitro preliminary study. Microorganisms 2019, 7, 1319–1330. [Google Scholar] [CrossRef]
- Callanan, J.; Stockdale, S.R.; Shkoporov, A.; Draper, L.A.; Ross, R.P.; Hill, C. RNA phage biology in a metagenomic era. Viruses 2018, 10, 386. [Google Scholar] [CrossRef] [Green Version]
- Mäntynen, S.; Sundberg, L.R.; Poranen, M.M. Recognition of six additional cystoviruses: Pseudomonas virus phi6 is no longer the sole species of the family Cystoviridae. Arch. Virol. 2018, 163, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.H. Bacteriophages; Interscience Publishers, John Wiley and Sons Inc.: New York, NY, USA, 1959. [Google Scholar]
- Tseng, C.C.; Li, C.S. Inactivation of viruses on surfaces by ultraviolet germicidal irradiation. J. Occup. Environ. Hyg. 2007, 4, 400–405. [Google Scholar] [CrossRef]
- Ma, B.; Linden, Y.S.; Gundy, P.M.; Gerba, C.P.; Sobsey, M.D.; Linden, K.G. Inactivation of coronaviruses and phage phi6 from irradiation across UVC wavelengths. Environ. Sci. Technol. Lett. 2021, 8, 425–430. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Bounty, S.; Beck, S.; Chan, C.; McGuire, C.; Linden, K.G. Photoreactivation of bacteriophages after UV disinfection: Role of genome structure and impacts of UV source. Water Res. 2014, 55, 143–149. [Google Scholar] [CrossRef]
- Córdoba-Lanús, E.; García-Pérez, O.; Cazorla-Rivero, S.; Rodríguez-Esparragón, F.; Piñero, J.E.; Clavo, B.; Lorenzo-Morales, J. Persistence of SARS-CoV-2 infection on personal protective equipment (PPE). BMC Infect. Dis. 2021, 21, 1169. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.M.; Shaffer, R.E. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J. Appl. Microbiol. 2011, 110, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Biasin, M.; Bianco, A.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, C.; Galli, P.; Lessio, L.; Lualdi, M.; Tombetti, E.; et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021, 11, 6260. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomeu, M.; Braz, M.; Costa, P.; Duarte, J.; Pereira, C.; Almeida, A. Evaluation of UV-C Radiation Efficiency in the Decontamination of Inanimate Surfaces and Personal Protective Equipment Contaminated with Phage ϕ6. Microorganisms 2022, 10, 593. https://doi.org/10.3390/microorganisms10030593
Bartolomeu M, Braz M, Costa P, Duarte J, Pereira C, Almeida A. Evaluation of UV-C Radiation Efficiency in the Decontamination of Inanimate Surfaces and Personal Protective Equipment Contaminated with Phage ϕ6. Microorganisms. 2022; 10(3):593. https://doi.org/10.3390/microorganisms10030593
Chicago/Turabian StyleBartolomeu, Maria, Márcia Braz, Pedro Costa, João Duarte, Carla Pereira, and Adelaide Almeida. 2022. "Evaluation of UV-C Radiation Efficiency in the Decontamination of Inanimate Surfaces and Personal Protective Equipment Contaminated with Phage ϕ6" Microorganisms 10, no. 3: 593. https://doi.org/10.3390/microorganisms10030593
APA StyleBartolomeu, M., Braz, M., Costa, P., Duarte, J., Pereira, C., & Almeida, A. (2022). Evaluation of UV-C Radiation Efficiency in the Decontamination of Inanimate Surfaces and Personal Protective Equipment Contaminated with Phage ϕ6. Microorganisms, 10(3), 593. https://doi.org/10.3390/microorganisms10030593









