Epidemiology of Echovirus 30 Infections Detected in a University Hospital in Catalonia, Spain, in 1995–2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Samples
2.3. Methods
2.3.1. EV Isolation
2.3.2. EV Detection
2.3.3. EV Genotyping
2.3.4. Phylogenetic Analysis
2.3.5. Ethics Statement
3. Results
3.1. EV Detection and Genotyping
3.2. Demographic, Epidemiological and Clinical Characteristics of E30 Infections
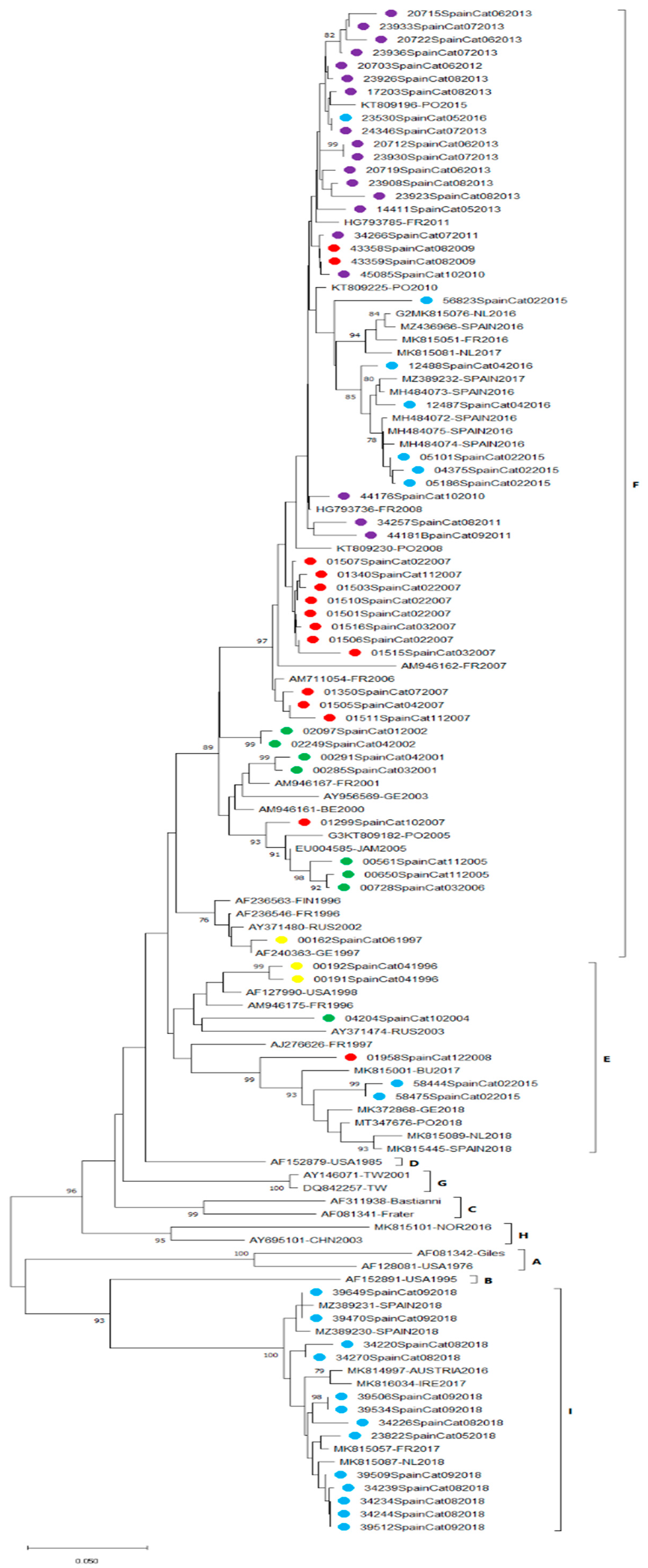
3.3. E30 Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Internationall Committee on Taxonomy of Viruses ICTV Picornaviridae. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sense-rna-viruses/w/picornaviridae (accessed on 15 November 2021).
- Palacios, G.; Casas, I.; Tenorio, A.; Freire, C. Molecular Identification of Enterovirus by Analyzing a Partial VP1 Genomic Region with Different Methods. J. Clin. Microbiol. 2002, 40, 182–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simmonds, P.; Gorbalenya, A.E.; Harvala, H.; Hovi, T.; Knowles, N.J.; Lindberg, A.M.; Oberste, M.S.; Palmenberg, A.C.; Reuter, G.; Skern, T.; et al. Recommendations for the nomenclature of enteroviruses and rhinoviruses. Arch. Virol. 2020, 165, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, S.; Xiong, Y.; Li, T.; Wen, Z.; Yan, M.; Qin, K.; Liu, Y.; Wu, J. Co-Circulation and Genomic Recombination of Coxsackievirus A16 and Enterovirus 71 during a Large Outbreak of Hand, Foot, and Mouth Disease in Central China. PLoS ONE 2014, 9, e96051. [Google Scholar] [CrossRef] [PubMed]
- Oberste, M.S.; Maher, K.; Pallansch, M.A. Evidence for Frequent Recombination within Species Human Enterovirus B Based on Complete Genomic Sequences of All Thirty-Seven Serotypes. J. Virol. 2004, 78, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Pons-Salort, M.; Parker, E.P.K.; Grassly, N.C. The epidemiology of non-polio enteroviruses: Recent Advances and Outstanding Questions. Curr. Opin. Infect. Dis. 2015, 28, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Palacios, G.; Oberste, M.S. Enteroviruses as agents of emerging infectious diseases. J. NeuroVirol. 2005, 11, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, W.Q.; Shulman, L.M. Polioviruses and Other Enteroviruses. In Global Water Pathogen Project; Rose, J.B., Jiménez-Cisneros, B., Eds.; Michigan State University: East Lansing, MI, USA; UNESCO: Paris, France, 2017. [Google Scholar]
- Racaniello, V.R. One hundred years of poliovirus pathogenesis. Virology 2006, 344, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Mirand, A.; Savy, N.; Henquell, C.; Maridet, S.; Perignon, R.; Labbé, A.; Peigue-Lafeuille, H. Acute flaccid paralysis following enterovirus D68 associated pneumonia, France, 2014. Eurosurveillance 2014, 19, 20952. [Google Scholar] [CrossRef]
- González-Sanz, R.; Casas-Alba, D.; Launes, C.; Muñoz-Almagro, C.; Ruiz-García, M.M.; Alonso, M.; González-Abad, M.J.; Megías, G.; Rabella, N.; Del Cuerpo, M.; et al. Molecular epidemiology of an enterovirus A71 outbreak associated with severe neurological disease, Spain, 2016. Eurosurveillance 2019, 24, 1800089. [Google Scholar] [CrossRef]
- Likosky, W.H.; Emmons, R.W.; Davis, L.E.; Thompson, R.S. U.S. cases in 1968: Epidemiology of echovirus 30 aseptic meningitis. Health Serv. Rep. 1972, 87, 638–642. [Google Scholar] [CrossRef]
- Wang, J.-R.; Tsai, H.-P.; Huang, S.-W.; Kuo, P.-H.; Kiang, D.; Liu, C.-C. Laboratory Diagnosis and Genetic Analysis of an Echovirus 30-Associated Outbreak of Aseptic Meningitis in Taiwan in 2001. J. Clin. Microbiol. 2002, 40, 4439–4444. [Google Scholar] [CrossRef] [PubMed]
- De Castro, C.M.O.; Oliveira, D.S.; Macedo, O.; Lima, M.J.L.; Santana, M.B.; Wanzeller, A.L.M.; Da Silveira, E.; Gomes, M.D.L.C. Echovirus 30 associated with cases of aseptic meningitis in state of Pará, Northern Brazil. Memórias do Instituto Oswaldo Cruz 2009, 104, 444–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiao, H.; Guan, D.; Chen, R.; Chen, P.; Monagin, C.; Li, W.; Su, J.; Ma, C.; Zhang, W.; Ke, C. Molecular characterization of echovirus 30-associated outbreak of aseptic meningitis in Guangdong in 2012. Virol. J. 2013, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Brunel, D.; Lévêque, N.; Jacques, J.; Renois, F.; Motte, J.; Andréoletti, L. Clinical and virological features of an aseptic meningitis outbreak in North-Eastern France, 2005. J. Clin. Virol. 2008, 42, 225–228. [Google Scholar] [CrossRef]
- Trallero, G.; Avellon, A.; Otero, A.; De Miguel, T.; Pérez, C.; Rabella, N.; Rubio, G.; Echevarría, J.E.; Cabrerizo, M. Enteroviruses in Spain over the decade 1998–2007: Virological and epidemiological studies. J. Clin. Virol. 2010, 47, 170–176. [Google Scholar] [CrossRef]
- Bailly, J.-L.; Mirand, A.; Henquell, C.; Archimbaud, C.; Chambon, M.; Charbonné, F.; Traore, O.; Peigue-Lafeuille, H. Phylogeography of circulating populations of human echovirus 30 over 50 years: Nucleotide polymorphism and signature of purifying selection in the VP1 capsid protein gene. Infect. Genet. Evol. 2009, 9, 699–708. [Google Scholar] [CrossRef]
- Gámbaro, F.; Pérez, A.B.; Agüera, E.; Prot, M.; Martínez-Martínez, L.; Cabrerizo, M.; Simon-Loriere, E.; Fernandez-Garcia, M.D. Genomic surveillance of enterovirus associated with aseptic meningitis cases in southern Spain, 2015–2018. Sci. Rep. 2021, 11, 21523. [Google Scholar] [CrossRef]
- Benschop, K.S.; Broberg, E.K.; Hodcroft, E.; Schmitz, D.; Albert, J.; Baicus, A.; Bailly, J.-L.; Baldvinsdottir, G.; Berginc, N.; Blomqvist, S.; et al. Molecular Epidemiology and Evolutionary Trajectory of Emerging Echovirus 30, Europe. Emerg. Infect. Dis. 2021, 27, 1616–1626. [Google Scholar] [CrossRef]
- Oberste, M.S.; Maher, K.; Kennett, M.L.; Campbell, J.J.; Carpenter, M.S.; Schnurr, D.; Pallansch, M.A. Molecular Epidemiology and Genetic Diversity of Echovirus Type 30 (E30): Genotypes Correlate with Temporal Dynamics of E30 Isolation. J. Clin. Microbiol. 1999, 37, 3928–3933. [Google Scholar] [CrossRef]
- Khetsuriani, N.; Lamonte-Fowlkes, A.; Oberst, S.; Pallansch, M.A.; Centers for Disease Control and Prevention. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill. Summ. 2006, 55, 1–20. [Google Scholar]
- Broberg, E.K.; Simone, B.; Jansa, J.; The Eu/Eea Member State Contributors. Upsurge in echovirus 30 detections in five EU/EEA countries, April to September, 2018. Eurosurveillance 2018, 23, 1800537. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://apps.who.int/iris/handle/10665/68762 (accessed on 9 December 2021).
- Prim, N.; Rodríguez, G.; Margall, N.; Del Cuerpo, M.; Trallero, G.; Rabella, N. Combining cell lines to optimize isolation of human enterovirus from clinical specimens: Report of 25 years of experience. J. Med. Virol. 2012, 85, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, M.; Echevarria, J.E.; González, I.; de Miguel, T.; Trallero, G. Molecular epidemiological study of HEV-B enteroviruses involved in the increase in meningitis cases occurred in Spain during 2006. J. Med. Virol. 2008, 80, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- González-Sanz, R.; Taravillo, I.; Reina, J.; Navascués, A.; Moreno-Docón, A.; Aranzamendi, M.; Romero, M.P.; Del Cuerpo, M.; Pérez-González, C.; Castro, S.P.; et al. Enterovirus D68-associated respiratory and neurological illness in Spain, 2014–2018. Emerg. Microbes Infect. 2019, 8, 1438–1444. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bubba, L.; Broberg, E.K.; Jasir, A.; Simmonds, P.; Harvala, H.; Enterovirus Study Collaborators. Circulation of Non-Polio Enteroviruses in 24 EU and EEA Countries between 2015 and 2017: A Retrospective Surveillance Study. Lancet Infect. Dis. 2020, 20, 350–361. [Google Scholar] [CrossRef]
- Hyeon, J.-Y.; Hwang, S.; Kim, H.; Song, J.; Ahn, J.; Kang, B.; Kim, K.; Choi, W.; Chung, J.K.; Kim, C.-H.; et al. Accuracy of Diagnostic Methods and Surveillance Sensitivity for Human Enterovirus, South Korea, 1999–2011. Emerg. Infect. Dis. 2013, 19, 1268–1275. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Yan, J.; Miao, Z.; Xu, C.; Zhang, Y.; Mao, H.; Gong, L. Molecular Epidemiology and Prevalence of Echovirus 30 in Zhejiang Province, China, from 2002 to 2015. J. Microbiol. Biotechnol. 2017, 27, 2221–2227. [Google Scholar] [CrossRef]
- Trallero, G.; Casas, I.; Tenorio, A.; Echevarria, J.E.; Castellanos, A.; Lozano, A.; Breña, P.P. Enteroviruses in Spain: Virological and epidemiological studies over 10 years (1988–97). Epidemiol. Infect. 2000, 124, 497–506. [Google Scholar] [CrossRef]
- Volle, R.; Bailly, J.-L.; Mirand, A.; Pereira, B.; Marque-Juillet, S.; Chambon, M.; Regagnon, C.; Brebion, A.; Henquell, C.; Peigue-Lafeuille, H.; et al. Variations in Cerebrospinal Fluid Viral Loads Among Enterovirus Genotypes in Patients Hospitalized with Laboratory-Confirmed Meningitis Due to Enterovirus. J. Infect. Dis. 2014, 210, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Rawlinson, W.D.; Andrews, P.I.; Stelzer-Braid, S. Global epidemiology of nonpolio enteroviruses causing severe neurological complications: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, e2082. [Google Scholar] [CrossRef] [PubMed]
- Keeren, K.; Böttcher, S.; Diedrich, S. Enterovirus Surveillance (EVSurv) in Germany. Microorganisms 2021, 9, 2005. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, Z.; Wu, H.; Xu, W.; Yu, D.; Zhang, Y. A Large-Scale Outbreak of Echovirus 30 in Gansu Province of China in 2015 and Its Phylodynamic Characterization. Front. Microbiol. 2020, 11, 1137. [Google Scholar] [CrossRef] [PubMed]
- Strikas, R.A.; Anderson, L.J.; Parker, R.A. Temporal and Geographic Patterns of Isolates of Nonpolio Enterovirus in the United States, 1970–1983. J. Infect. Dis. 1986, 153, 346–351. [Google Scholar] [CrossRef]
- Rasti, M.; Makvandi, M.; Neisi, N.; Azaran, A.; Rastegarvand, N.; Khalafkhany, D.; Jahangirnezhad, E.; Teimoori, A.; Hadian, M.; Shabani, A.; et al. Three cases of mumps virus and enterovirus coinfection in children with enteroviral meningitis. Medicine 2016, 95, e5610. [Google Scholar] [CrossRef]
- Choi, S.-H.; Shin, S.H.; Kim, H.S. Respiratory Enterovirus Infections and Other Respiratory Virus Coinfections in Children. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2016; Volume 3. [Google Scholar] [CrossRef]
- Ooi, M.H.; Wong, S.C.; Podin, Y.; Akin, W.; Del Sel, S.; Mohan, A.; Chieng, C.H.; Perera, D.; Clear, D.; Wong, D.; et al. Human Enterovirus 71 Disease in Sarawak, Malaysia: A Prospective Clinical, Virological, and Molecular Epidemiological Study. Clin. Infect. Dis. 2007, 44, 646–656. [Google Scholar] [CrossRef]
- Makimaa, H.; Ingle, H.; Baldridge, M.T. Enteric Viral Co-Infections: Pathogenesis and Perspective. Viruses 2020, 12, 904. [Google Scholar] [CrossRef]
- Yang, F.; Du, J.; Hu, Y.; Wang, X.; Xue, Y.; Dong, J.; Sun, L.; Li, Z.; Li, Y.; Sun, S.; et al. Enterovirus Coinfection During an Outbreak of Hand, Foot, and Mouth Disease in Shandong, China. Clin. Infect. Dis. 2011, 53, 400–401. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.F.; Yang, F.; Du, J.; Dong, J.; Zhang, T.; Wu, Z.Q.; Xue, Y.; Jin, Q. Complete Genome Analysis of Coxsackievirus A2, A4, A5, and A10 Strains Isolated from Hand, Foot, and Mouth Disease Patients in China Revealing Frequent Recombination of Human Enterovirus A. J. Clin. Microbiol. 2011, 49, 2426–2434. [Google Scholar] [CrossRef]
- Messacar, K.; Burakoff, A.; Nix, W.A.; Rogers, S.; Oberste, M.S.; Gerber, S.I.; Spence-Davizon, E.; Herlihy, R.; Dominguez, S.R. Notes from the Field: Enterovirus A71 Neurologic Disease in Children—Colorado, 2018. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Taravilla, C.N.; Pérez-Sebastián, I.; Salido, A.G.; Serrano, C.V.; Extremera, V.C.; Rodríguez, A.D.; Marín, L.L.; Sanz, M.A.; Traba, O.M.S.; González, A.S. Enterovirus A71 Infection and Neurologic Disease, Madrid, Spain, 2016. Emerg. Infect. Dis. 2019, 25, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-I.; Shih, S.-R. Neurotropic Enterovirus Infections in the Central Nervous System. Viruses 2015, 7, 6051–6066. [Google Scholar] [CrossRef] [PubMed]


| Age Group | E30 Infections | Percentage (%) |
|---|---|---|
| 0–3 months | 28 | 16.2 |
| 4–23 months | 25 | 14.5 |
| 2–3 years | 34 | 19.7 |
| 4–5 years | 24 | 13.9 |
| 6–10 years | 28 | 16.2 |
| 11–17 years | 10 | 5.8 |
| 18–40 years | 16 | 9.2 |
| >40 years | 6 | 3.4 |
| not specified | 2 | 1.1 |
| Sex | ||
| Female | 67 | 38.7 |
| Male | 106 | 61.3 |
| Patients with clinical information | 158 | 91.3 |
| Non-specific febrile syndrome | 34 | 21.5 |
| Exanthema | 5 | 3.1 |
| Respiratory symptoms | 12 | 7.6 |
| Neurological symptoms | 107 | 67.8 |
| Meningitis | 103 | 96.2 |
| Meningoencephalitis | 2 | 1.9 |
| Encephalitis | 2 | 1.9 |
| Sample | n | E30-Positive | Percentage (%) | ||
|---|---|---|---|---|---|
| CSF * | 97 | 87 | 89.7 | ||
| Respiratory sample | 108 | 99 | 91.7 | ||
| Feces | 42 | 42 | 100 | ||
| Urine | 5 | 2 | 40 | ||
| Skin exudates | 2 | 2 | 100 | ||
| Total | 254 | 232 | 91.3 | ||
| Cases with multiple specimens analyzed (n = 48) | |||||
| Cases | Percentage (%) | ||||
| CSF + RS ** + feces + urine | 1 | 0.6 | |||
| CSF + RS + urine | 2 | 1.1 | |||
| CSF + RS + feces | 22 | 12.7 | |||
| CSF + RS | 10 | 5.8 | |||
| CSF + feces | 3 | 1.7 | |||
| RS + feces | 8 | 4.6 | |||
| RS + feces + urine | 1 | 0.6 | |||
| RS + urine | 1 | 0.6 | |||
| Year | EV Detection | Genotyped EV (%) | E30 Detection (%) |
|---|---|---|---|
| 1995 | 23 | 19 (82.6) | 0 |
| 1996 | 42 | 19 (45.2) | 7 (36.8) |
| 1997 | 76 | 35 (46) | 3 (8.6) |
| 1998 | 76 | 28 (36.8) | 0 |
| 1999 | 115 | 52 (45.2) | 0 |
| 2000 | 111 | 62 (55.9) | 13 (21) |
| 2001 | 93 | 70 (75.3) | 9 (12.9) |
| 2002 | 98 | 68 (69.4) | 4 (5.9) |
| 2003 | 96 | 85 (88.5) | 0 |
| 2004 | 74 | 54 (73) | 1 (1.8) |
| 2005 | 151 | 119 (78.8) | 17 (14.3) |
| 2006 | 132 | 94 (71.2) | 2 (2.1) |
| 2007 | 137 | 91 (66.4) | 48 (52.7) |
| 2008 | 129 | 78 (60.5) | 1 (1.3) |
| 2009 | 119 | 92 (77.3) | 3 (3.3) |
| 2010 | 162 | 116 (71.6) | 7 (6) |
| 2011 | 122 | 74 (60.6) | 1 (1.4) |
| 2012 | 130 | 67 (51.5) | 7 (10.4) |
| 2013 | 63 | 43 (68.2) | 15 (34.9) |
| 2014 | 64 | 42 (65.6) | 0 |
| 2015 | 70 | 52 (74.3) | 8 (15.4) |
| 2016 | 91 | 67 (73.6) | 11 (16.4) |
| 2017 | 92 | 71 (77.2) | 2 (2.8) |
| 2018 | 72 | 61 (84.7) | 13 (21.3) |
| 2019 | 48 | 47 (98) | 1 (2.1) |
| 2020 | 16 | 15 (93.7) | 0 |
| Total | 2402 | 1619 (67.4) | 173 (10.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Cuerpo, M.; Gonzalez de Audicana, J.; Fernandez-Garcia, M.D.; Marín, P.; Esteban, M.; Español, M.; Cabrerizo, M.; Rabella, N. Epidemiology of Echovirus 30 Infections Detected in a University Hospital in Catalonia, Spain, in 1995–2020. Microorganisms 2022, 10, 592. https://doi.org/10.3390/microorganisms10030592
del Cuerpo M, Gonzalez de Audicana J, Fernandez-Garcia MD, Marín P, Esteban M, Español M, Cabrerizo M, Rabella N. Epidemiology of Echovirus 30 Infections Detected in a University Hospital in Catalonia, Spain, in 1995–2020. Microorganisms. 2022; 10(3):592. https://doi.org/10.3390/microorganisms10030592
Chicago/Turabian Styledel Cuerpo, Margarita, Jon Gonzalez de Audicana, Maria Dolores Fernandez-Garcia, Pilar Marín, Montserrat Esteban, Montserrat Español, María Cabrerizo, and Núria Rabella. 2022. "Epidemiology of Echovirus 30 Infections Detected in a University Hospital in Catalonia, Spain, in 1995–2020" Microorganisms 10, no. 3: 592. https://doi.org/10.3390/microorganisms10030592
APA Styledel Cuerpo, M., Gonzalez de Audicana, J., Fernandez-Garcia, M. D., Marín, P., Esteban, M., Español, M., Cabrerizo, M., & Rabella, N. (2022). Epidemiology of Echovirus 30 Infections Detected in a University Hospital in Catalonia, Spain, in 1995–2020. Microorganisms, 10(3), 592. https://doi.org/10.3390/microorganisms10030592






