Cyanobacteria: Model Microorganisms and Beyond
Abstract
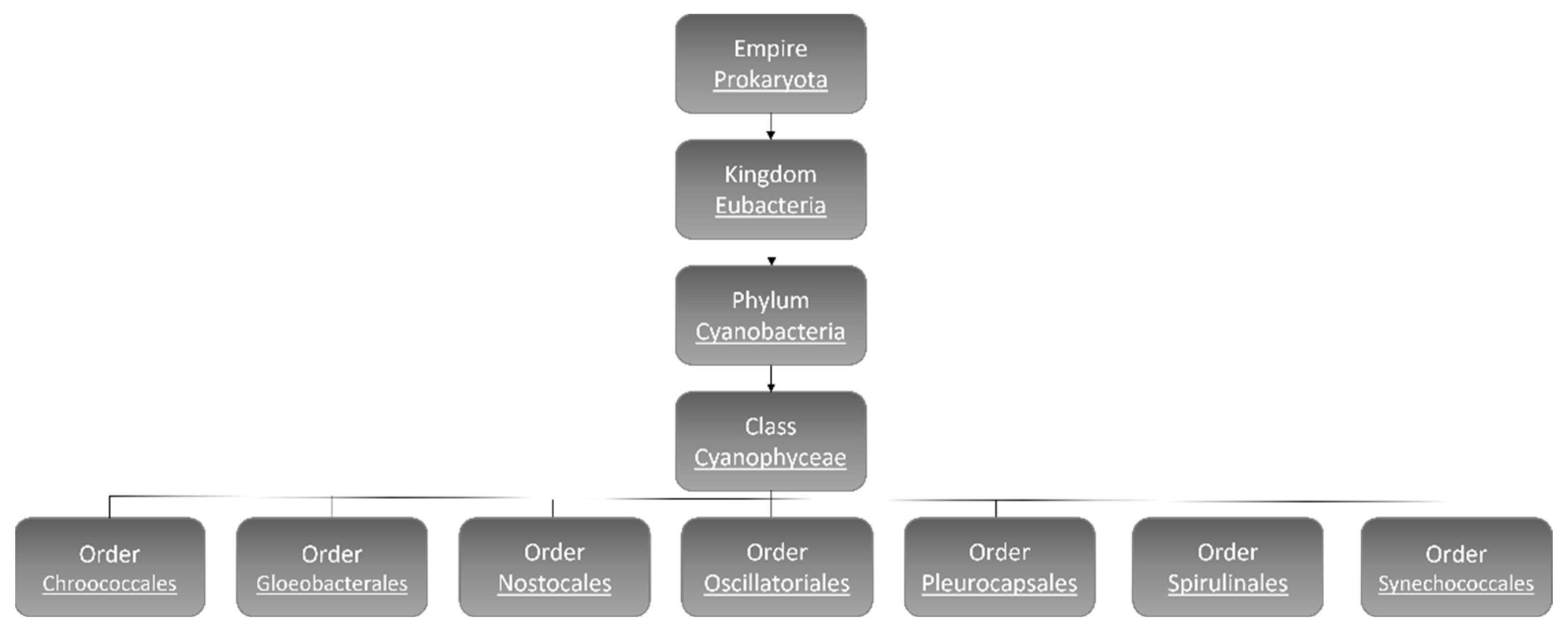
:1. Cyanobacteria
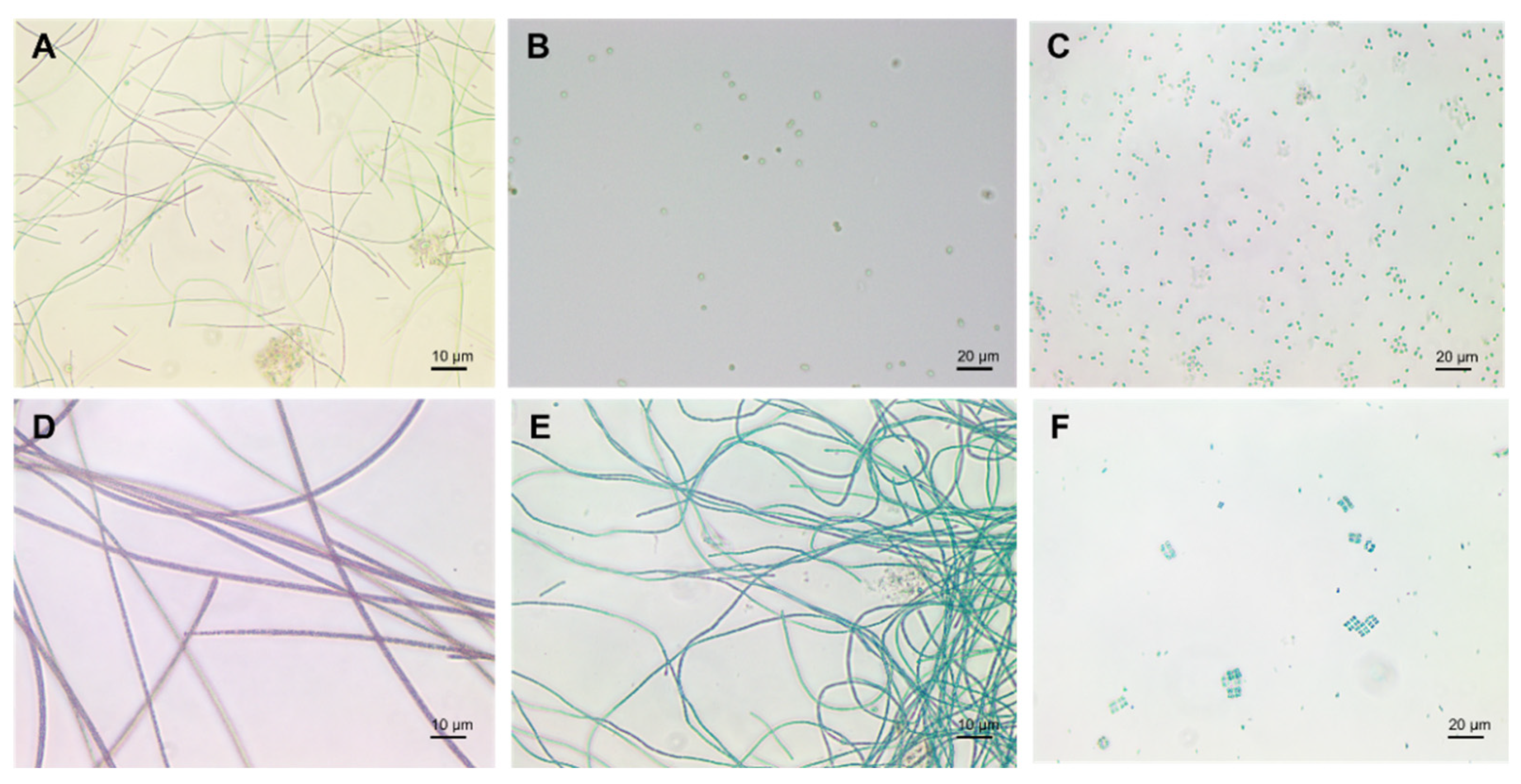
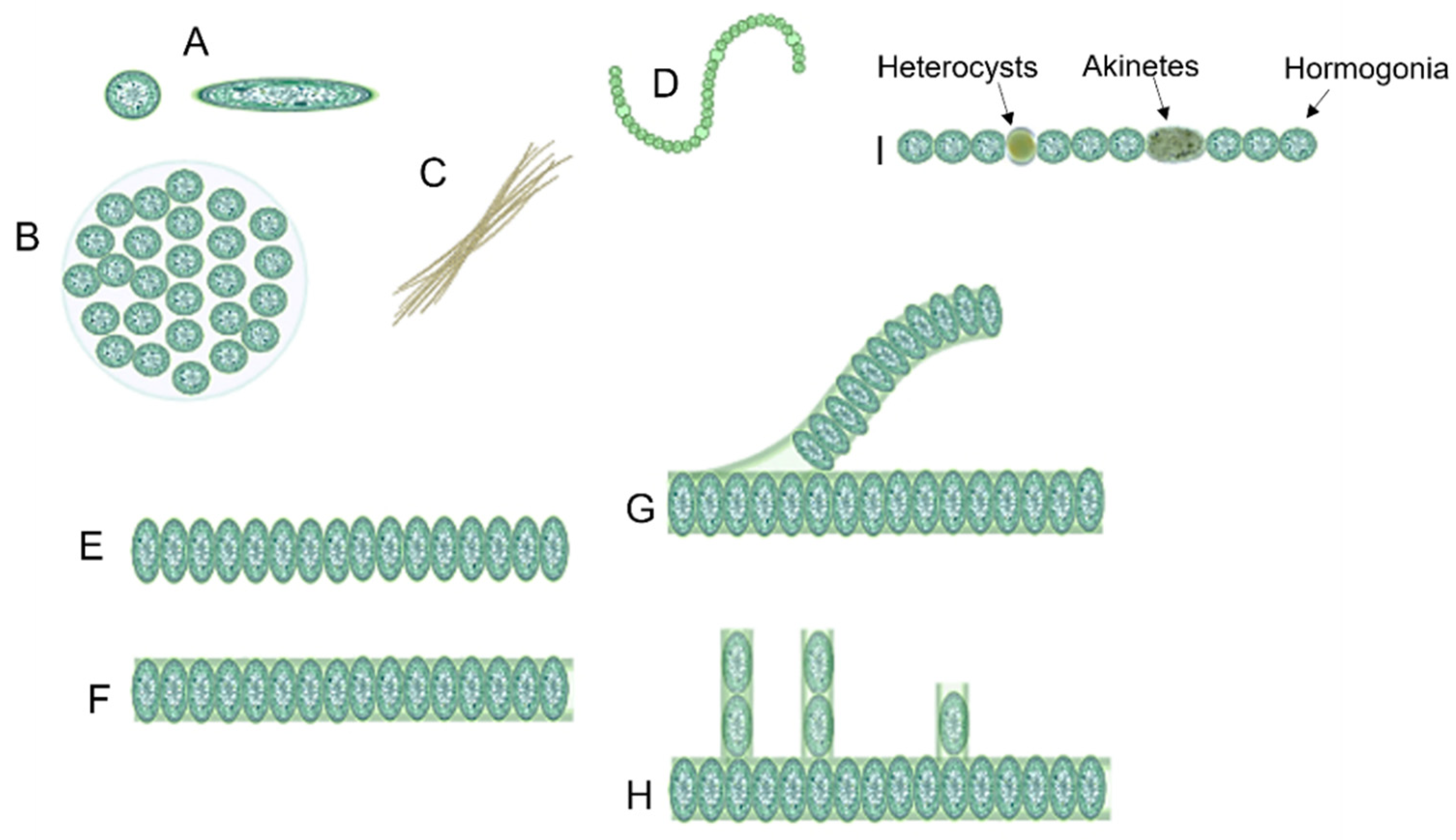
1.1. Cyanobacteria’ Characteristics
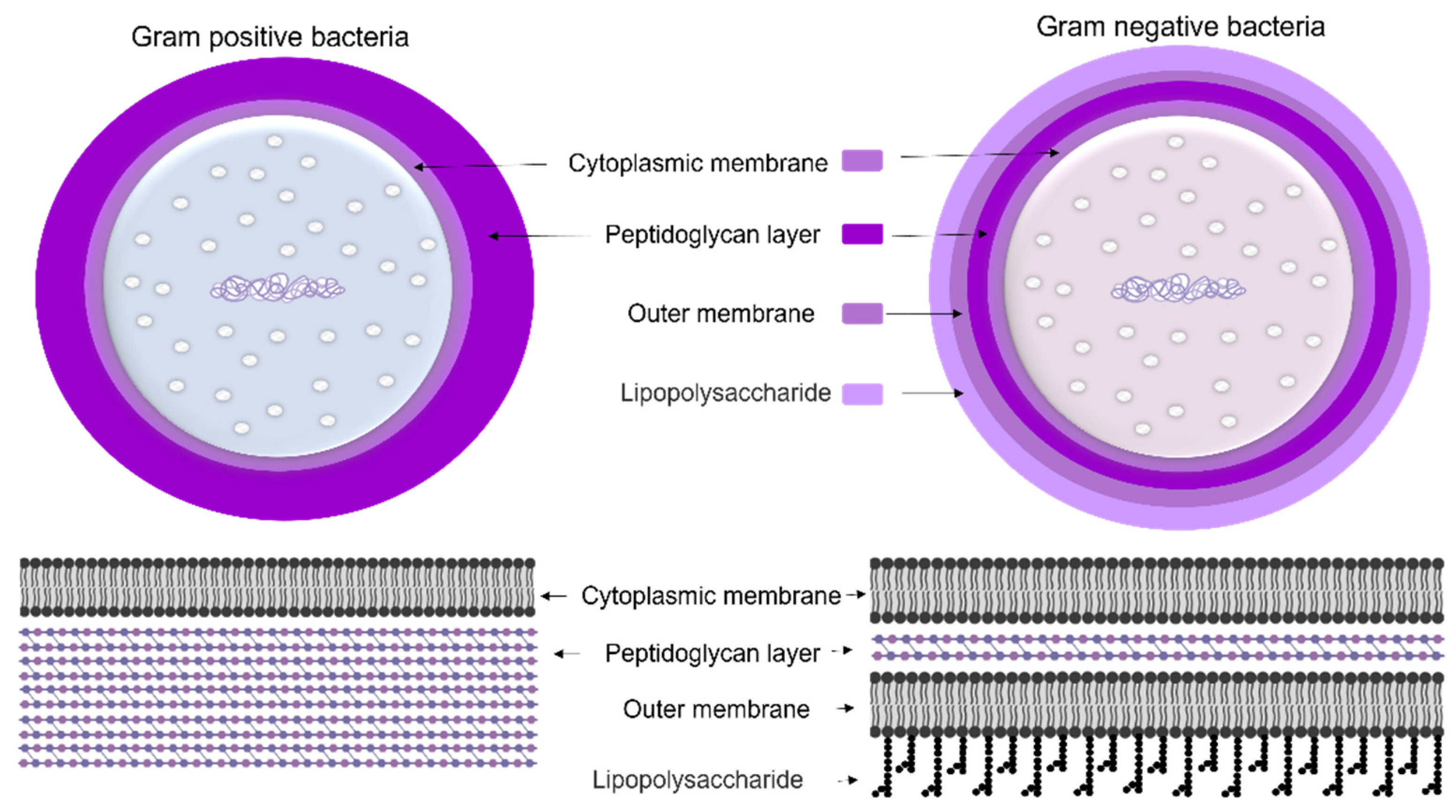
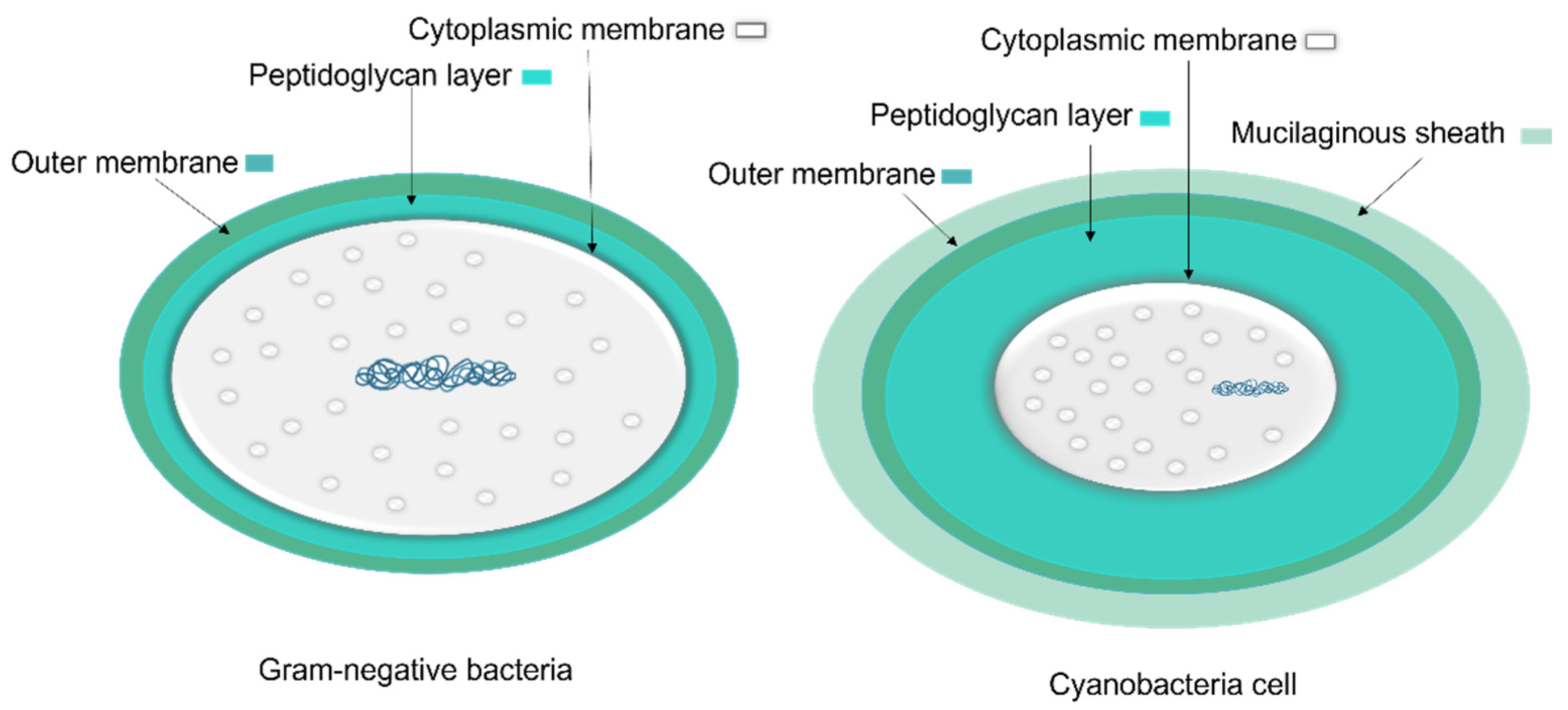
1.1.1. Cyanobacteria’ Cell Membrane
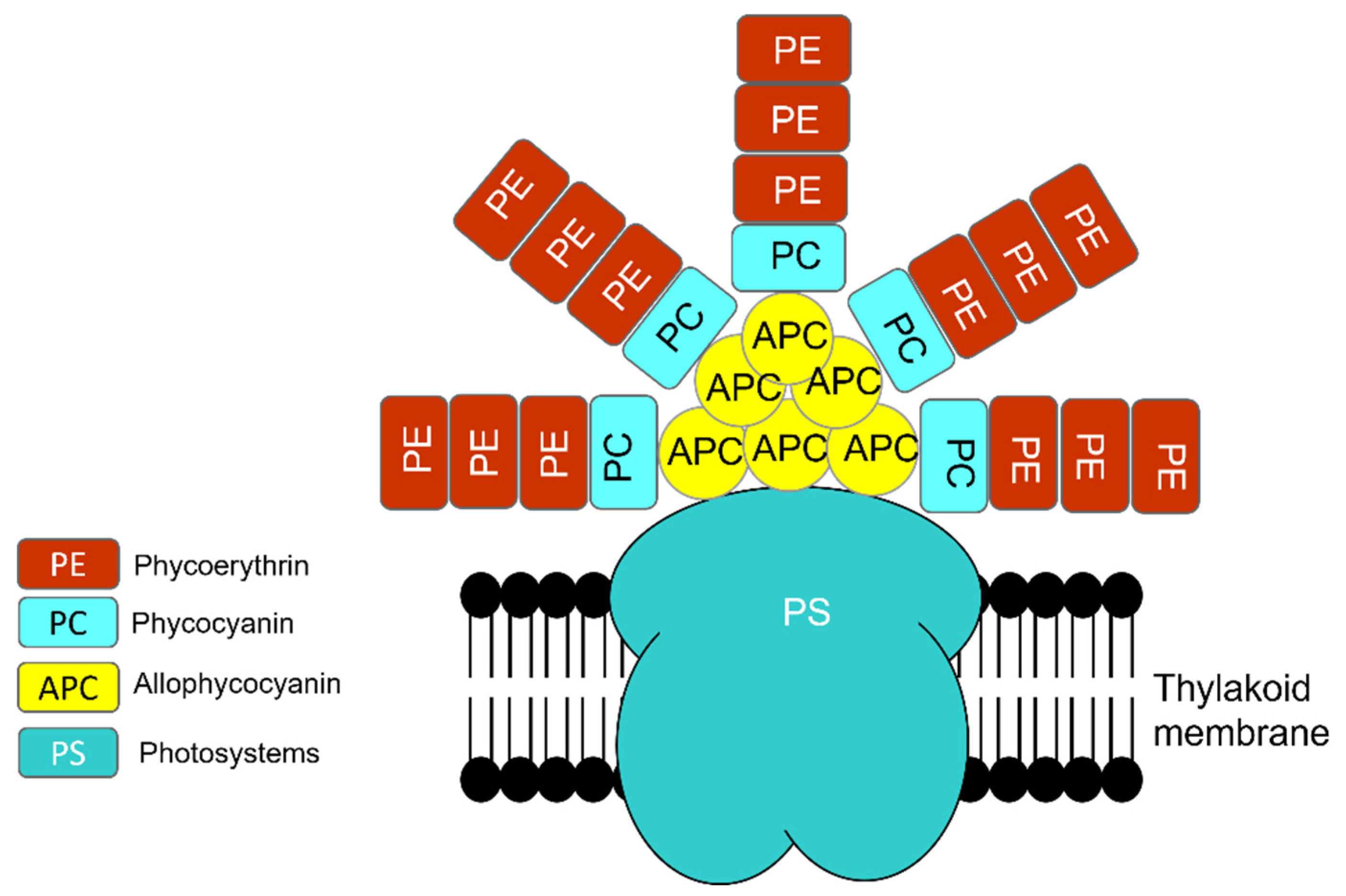
1.1.2. Photosynthesis
1.1.3. Production of Secondary Metabolites
1.2. Cyanobacteria in Nature and Industry
1.2.1. Algal Bloom
1.2.2. Usage of Cyanobacteria
2. Synechocystis sp.: A Model Microorganism
2.1. Motility
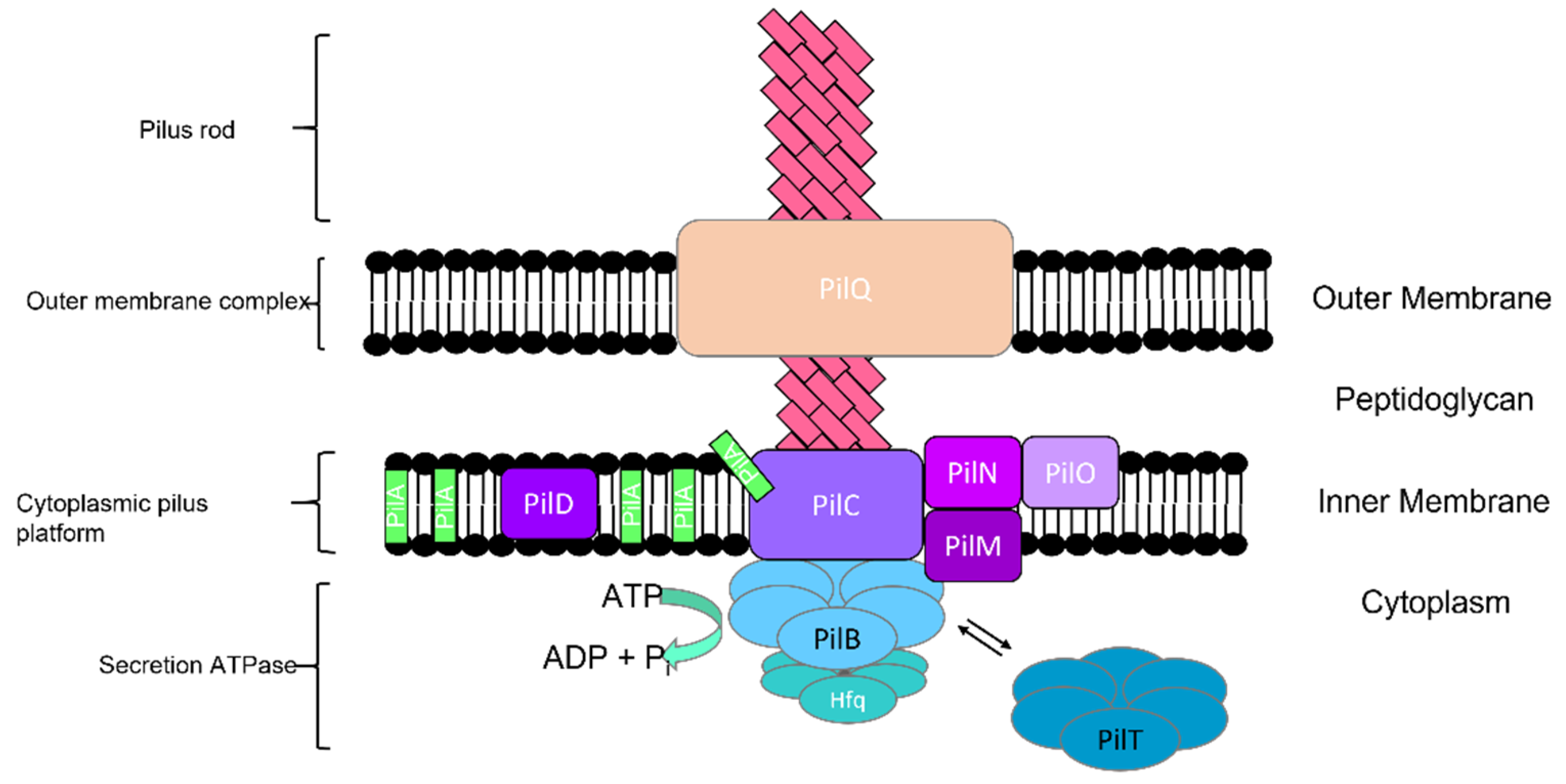
2.1.1. Type IV Pili
2.1.2. Cell Movement
2.2. Biofilm Formation
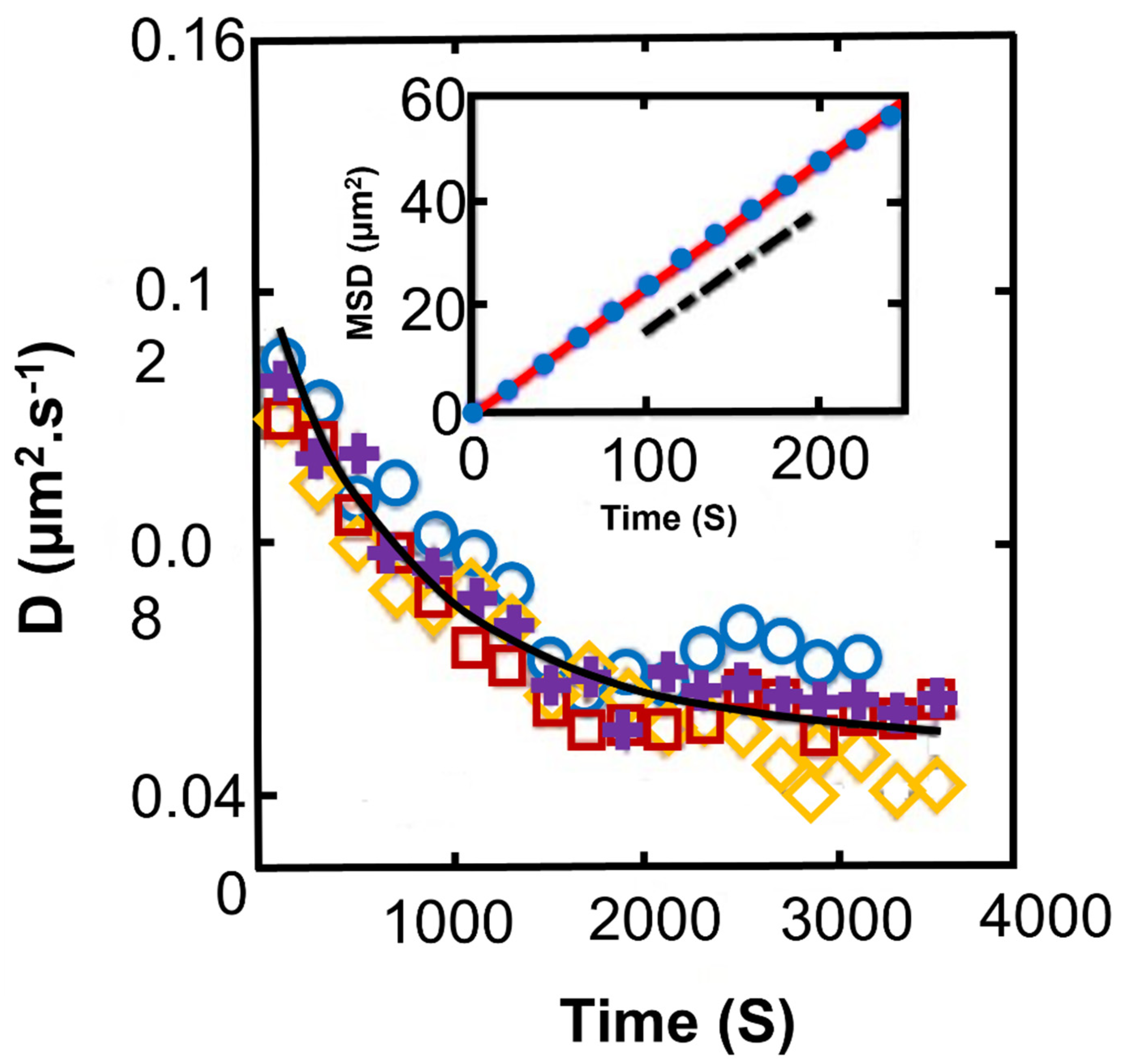
2.3. Synechocystis in Suspensions
3. Summary
Funding
Conflicts of Interest
References
- Schopf, J.W. Microfossils of the early Archean Apex Chert: New evidence of the antiquity of life. Science 1993, 260, 640–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shestakov, S.V.; Karbysheva, E.A. The origin and evolution of cyanobacteria. Biol. Bull. Rev. 2017, 7, 259–272. [Google Scholar] [CrossRef]
- Whitton, B.A. Diversity, ecology, and taxonomy of the cyanobacteria. In Photosynthetic Prokaryotes; Mann, N.H., Carr, N.G., Eds.; Plenum Press: New York, NY, USA, 1992; pp. 1–51. [Google Scholar]
- Lang-Yona, N.; Kunert, A.T.; Vogel, L.; Kampf, C.J.; Bellinghausen, I.; Saloga, J.; Schink, A.; Ziegler, K.; Lucas, K.; Schuppan, D.; et al. Fresh water, marine and terrestrial cyanobacteria display distinct allergen characteristics. Sci. Total Environ. 2018, 612, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Castenholz, R.W.; Miller, S.R. Cyanobacteria in geothermal habitats. In Ecology of Cyanobacteria II, Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 39–64. [Google Scholar]
- Quesada, A.; Vincent, W.F. Cyanobacteria in the cryosphere: Snow, ice and extreme cold. In Ecology of Cyanobacteria II, Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 387–400. [Google Scholar]
- Oren, A. Salts and brines. In Ecology of Cyanobacteria II, Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 401–426. [Google Scholar]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: https://www.algaebase.org/browse/taxonomy/#4351 (accessed on 21 March 2022).
- Komárek, J. Coccoid and colonial cyanobacteria. In Freshwater Algae of North America, Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 59–116. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2015. Available online: https://www.algaebase.org/search/species/detail/?species_id=153246 (accessed on 21 March 2022).
- Dolman, A.M.; Rücker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar] [CrossRef]
- Vidal, L.; Ballot, A.; Azevedo, S.M.F.O.; Padisák, J.; Welker, M. Introduction to cyanobacteria. In Toxic Cyanobacteria in Water, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 163–211. [Google Scholar]
- Vincent, W.F. Cyanobacteria. In Plankton of Inland Waters; Liknes, G.E., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 142–148. [Google Scholar]
- Dvořák, P.; Casamatta, D.A.; Hašler, P.; Jahodářová, E.; Norwich, A.R.; Poulíčková, A. Diversity of the cyanobacteria. In Modern Topics in the Phototrophic Prokaryotes; Hallenbeck, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 3–46. [Google Scholar]
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Holland, H.D. Volcanic gases, black smokers, and the great oxidation event. Geochim. Cosmochim. Acta 2002, 66, 3811–3826. [Google Scholar] [CrossRef]
- Tyrrell, T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 1999, 400, 525–531. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Tabata, S. Synechocystis sp. PCC 6803—A useful tool in the study of the genetics of cyanobacteria. Photosynth. Res. 2001, 70, 73–83. [Google Scholar] [CrossRef]
- Lindberg, P.; Park, S.; Melis, A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 2010, 12, 70–79. [Google Scholar] [CrossRef]
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, J.F.; Fastner, J. Ecology of cyanobacteria. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 11–18. [Google Scholar]
- Schulz-Vogt, H.N.; Angert, E.R.; Garcia-Pichel, F. Giant Bacteria. eLS 2007, 1–7. [Google Scholar] [CrossRef]
- Jasser, I.; Callieri, C. Picocyanobacteria: The smallest cell-size cyanobacteria. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 19–27. [Google Scholar]
- Mur, L.R.; Skulberg, O.M.; Utkilen, H. Cyanobacteria in the environment. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 15–40. [Google Scholar]
- Uyeda, J.C.; Harmon, L.J.; Blank, C.E. A comprehensive study of cyanobacterial morphological and ecological evolutionary dynamics through deep geologic time. PLoS ONE 2016, 9, e0162539. [Google Scholar] [CrossRef]
- Singh, S.P.; Montgomery, B.L. Determining cell shape: Adaptive regulation of cyanobacterial cellular differentiation and morphology. Trends. Microbiol. 2011, 19, 278–285. [Google Scholar]
- Dvořák, P.; Poulíčková, A.; Hasler, P.; Belli, M.; Casamatta, D.A.; Papini, A. Species concepts and speciation factors in cyanobacteria, with connection to the problems of diversity and classification. Biodivers. Conserv. 2015, 24, 739–757. [Google Scholar] [CrossRef] [Green Version]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Meeks, J.C.; Elhai, J. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol. Mol. Biol. R. 2002, 66, 94–121. [Google Scholar] [CrossRef] [Green Version]
- Wong, F.C.Y.; Meeks, J.C. Establishment of a functional symbiosis between the cyanobacterium Nostoc punctiforme and the bryophyte Anthoceros punctatus requires genes involved in nitrogen control and initiation of heterocyst differentiation. Microbiology 2002, 148, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.G.; Duggan, P.S. Cyanobacteria–bryophyte symbioses. J. Exp. Bot. 2008, 59, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Mella-Herrera, R.A.; Golden, J.W. Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2010, 2, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Wolk, C.P.; Ernst, A.; Elhai, J. Heterocyst metabolism and development. In the Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 769–823. [Google Scholar]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Woese, C.R. Bacterial Evolution. Microbiol. Rev. 1987, 51, 221–271. [Google Scholar] [PubMed]
- Hoiczyk, E.; Hansel, A. Cyanobacterial cell walls: News from an unusual prokaryotic envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durai, P.; Batool, M.; Choi, S. Structure and effects of cyanobacterial lipopolysaccharides. Mar. Drugs 2015, 13, 4217–4230. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A.D.T.; Petersen, J.D.; Reese, T.S. Envelope structure of Synechococcus sp. WH8113, a nonflagellated swimming cyanobacterium. BMC Microbiol. 2001, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Hoiczyk, E.; Baumeister, W. Envelope structure of four gliding filamentous cyanobacteria. J. Bacteriol. 1995, 177, 2387–2395. [Google Scholar] [CrossRef] [Green Version]
- Schuergers, N.; Wilde, A. Appendages of the cyanobacterial cell. Life 2015, 5, 700–715. [Google Scholar] [CrossRef]
- Bhaya, D.; Bianco, N.R.; Bryant, D.; Grossman, A.R. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 2000, 37, 941–951. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, S.; Geng, X.X.; Okamoto, S.; Yura, K.; Murata, T.; Go, M.; Ohmori, M.; Ikeuchi, M. Mutational analysis of genes involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2001, 42, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Angermayr, S.A.; Hellingwerf, K.J.; Lindblad, P.; de Mattos, M.J.T. Energy biotechnology with cyanobacteria. Curr. Opin. Biotech. 2008, 20, 257–263. [Google Scholar] [CrossRef]
- Durall, C.; Lindblad, P. Mechanisms of carbon fixation and engineering for increased carbon fixation in cyanobacteria. Algal Res. 2015, 11, 263–270. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. General overview. In Algae, Anatomy, Biotechemisty and Bioteconlogy, 2nd ed.; Barsanti, L., Gualtieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–48. [Google Scholar]
- Zakar, T.; Laczko-Dobos, H.; Toth, T.N.; Gombos, Z. Carotenoids assist in cyanobacterial photosystem II assembly and function. Front. Plant Sci. 2016, 7, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, A. Chlorophylls and carotenoids. In Handbook of Phycological Methods. Physiological and Biochemical Methods; Hellebust, J.A., Craigie, J.S., Eds.; Cambridge University Press: New York, NY, USA, 1978; pp. 59–70. [Google Scholar]
- Jaiswal, A.; Koli, D.K.; Kumar, A.; Kumar, S.; Sagar, S. Pigments analysis of cyanobacterial strains. Int. J. Chem. Stud. 2018, 6, 1248–1251. [Google Scholar]
- Grossman, A.R.; Bhaya, D.; Apt, K.E.; Kehoe, D.M. Light-harvesting complexes in oxygenic photosynthesis: Diversity, control, and evolution. Annu. Rev. Genet. 1995, 29, 231–287. [Google Scholar] [CrossRef]
- Stomp, M.; Huisman, J.; de Jongh, F.; Veraart, A.J.; Gerla, D.; Rijkeboer, M.; Ibelings, B.W.; Wollenzien, U.I.A.; Stal, L.J. Adaptive divergence in pigment composition promotes phytoplankton biodiversity. Nature 2004, 432, 5–8. [Google Scholar] [CrossRef]
- Oliver, R.L.; Hamilton, D.P.; Brookes, J.D.; Ganf, G.G. Physiology, blooms and prediction of planktonic cyanobacteria. In Ecology of Cyanobacteria II, Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 155–194. [Google Scholar]
- Singh, A.K.; Elvitigala, T.; Bhattacharyya-Pakrasi, M.; Aurora, R.; Ghosh, B.; Pakrasi, H.B. Integration of carbon and nitrogen metabolism with energy production is crucial to light acclimation in the cyanobacterium Synechocystis. Plant Physiol. 2008, 148, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.A.; Altus, S.; Tay, J.W.; Meehl, J.B.; Johnson, E.B.; Bortz, D.M.; Cameron, J.C. Mechanical regulation of photosynthesis in cyanobacteria. Nat. Microbiol. 2020, 5, 757–767. [Google Scholar] [CrossRef]
- Moore, R.E. Cyclic peptides and depsipeptides from cyanobacteria: A review. J. Ind. Microbiol. 1996, 16, 134–143. [Google Scholar] [CrossRef]
- Rawat, D.; Bhargava, S. Bioactive compounds from Nostoc species. Curr. Res. Pharm. Sci. 2011, 2, 48–54. [Google Scholar]
- Sharma, N.K.; Tiwari, S.P.; Tripathi, K.; Rai, A.K. Sustainability and cyanobacteria (blue-green algae): Facts and challenges. J. Appl. Phycol. 2011, 23, 1059–1081. [Google Scholar] [CrossRef]
- Yadav, S.; Sinha, R.P.; Tyagi, M.B. Antimicrobial activity of some cyanobacteria. Int. J. Pharm. Pharm. Sci. 2012, 4, 631–635. [Google Scholar]
- Méjean, A.; Payraud, T.H.; Kerbrat, A.S.; Goulbic, S.; Pauillac, S.; Chinain, M.; Laurent, D. First identification of the neurotoxin homoanatoxin-a from mast of Hydrocoleum lyngbyaceum (marine cyanobacterium) possibly liked to giant calm poisoning in New Caledonia. Toxicon 2010, 56, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Cadel-Six, S.; Peyraud-Thomas, C.; Brient, L.; de Marsac, N.T.; Ripka, R.; Méjean, A. Different genotypes of anatoxin-producing cyanobacteria coexist in the Tarn River, France. Appl. Environ. Microbiol. 2007, 73, 7605–7614. [Google Scholar] [CrossRef]
- Méjean, A.; Mazmouz, R.; Essadik, I.; Ploux, O. Anatoxin-a and analogs: Occurrence, biosynthesis and detection. Toxicon 2018, 149, 107. [Google Scholar] [CrossRef]
- Méjean, A.; Mazmouz, R.; Mann, S.; Calteau, A.; Medigues, C.; Ploux, O. The genome sequence of the cyanobacterium oscillatoria sp. 6506 reveals several gene clusters responsible for the biosynthesis of toxins and secondary metabolites. J. Bacteriol. 2010, 192, 5264–5265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maschek, J.A.; Baker, B.J. The chemistry of algal secondary metabolism. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer: Berlin, Germany, 2008; pp. 1–24. [Google Scholar]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van de Waal, D.B.; Smith, V.H.; Declerck, S.A.J.; Stam, E.C.M.; Elser, J.J. Stoichiometric regulation of phytoplankton toxins. Ecol. Lett. 2014, 17, 736–742. [Google Scholar] [CrossRef]
- Picardo, M.; Filatova, D.; Nunez, O.; Farré, M. Recent advances in the detection of natural toxins in freshwater environments. Trends Analyt. Chem. 2019, 112, 75–86. [Google Scholar] [CrossRef]
- Chauvat, F.; Cassier-Chauvat, C. Genomics of cyanobacteria: New insights and lessons for shaping our future—A follow-up of volume 65: Genomics of cyanobacteria. In Advances in Botanical Research; Jacquot, J.P., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 100, pp. 213–235. [Google Scholar] [CrossRef]
- Filatova, D.; Jones, M.R.; Haley, J.A.; Núñez, O.; Farré, M.; Janssen, E.M.L. Cyanobacteria and their secondary metabolites in three freshwater reservoirs in the United Kingdom. Environ. Sci. Eur. 2021, 33, 29. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7, S4. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayers, M.J.; Grimm, A.G.; Shuchman, R.A.; Bosse, K.R.; Fahnenstiel, G.L.; Ruberg, S.A.; Leshkevich, G.A. Satellite monitoring of harmful algal blooms in the Western Basin of Lake Erie: A 20-year time-series. J. Great Lakes Res. 2019, 45, 508–521. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Elsevier: Oxford, UK, 2009; pp. 290–307. [Google Scholar]
- Schembri, M.A.; Neilan, B.A.; Saint, C.P. Identification of genes implicated in toxin production in the cyanobacterium Cylindrospermopsis raciborskii. Environ. Toxicol. 2001, 16, 413–421. [Google Scholar] [CrossRef]
- Preußel, K.; Stüken, A.; Wiedner, C.; Chorus, I.; Fastner, J. First report on cylindrospermopsin producing Aphanizomenon flos-aquae (cyanobacteria ) isolated from two German lakes. Toxicon 2006, 47, 156–162. [Google Scholar] [CrossRef]
- Codd, G.A.; Meriluoto, J.; Metcalf, J.S. Introduction: Cyanobacteria, cyanotoxins, their human impact, and risk management. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 3–8. [Google Scholar]
- Wang, C.C.; Petty, E.E.; Smith, C.M. Rapid and efficient analysis of microcystins, nodularin, cylindrospermopsin, and anatoxin-a in drinking water by LC-MS-MS. J. AOAC Int. 2016, 99, 1565–1571. [Google Scholar] [CrossRef]
- Sarsekeyeva, F.; Zayadan, B.K.; Usserbaeva, A.; Bedbenov, V.S.; Sinetova, M.A.; Los, D.A. Cyanofuels: Biofuels from cyanobacteria. Reality and perspectives. Photosynth. Res. 2015, 125, 329–340. [Google Scholar] [CrossRef]
- Quintana, N.; Van der Kooy, F.; Van de Rhee, M.D.; Voshol, G.P.; Verpoorte, R. Renewable energy from cyanobacteria: Energy production optimization by metabolic pathway engineering. Appl. Microbiol. Biot. 2011, 91, 471–490. [Google Scholar] [CrossRef] [Green Version]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Barka, A.; Blecker, C. Microalgae as a potential source of single-cell proteins. Biotechnol. Agron. Soc. Environ. 2016, 20, 427–436. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yuan, W.; Pei, Z.J.; Wu, Q.; Mao, E. Microalgae mass production methods. Trans. ASABE 2009, 52, 1275–1287. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Microalgal reactors: A review of enclosed system designs and performances. Biotechnol. Progr. 2006, 22, 1490–1506. [Google Scholar] [CrossRef]
- Yadala, S.; Cremaschi, S. Design and optimization of artificial cultivation units for algae production. Energy 2014, 78, 23–39. [Google Scholar] [CrossRef]
- Pruvost, J.; Cornet, J.F.; Goetz, V.; Legrand, J. Theoretical investigation of biomass productivities achievable in solar rectangular photobioreactors for the cyanobacterium Arthrospira platensis. Biotechnol. Prog. 2012, 28, 699–714. [Google Scholar] [CrossRef]
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny microbes with a big impact: The role of cyanobacteria and their metabolites in shaping our future. Mar. Drugs 2016, 14, 97. [Google Scholar] [CrossRef] [Green Version]
- IBA Hamburg—BIQ. Bio Intelligent Quotient (BIQ) House in Hamburg. Smart Material Houses. 2020. Available online: https://www.internationale-bauausstellung-hamburg.de/en/projects/the-building-exhibition-within-the-building-exhibition/smart-material-houses/biq/projekt/biq.html (accessed on 21 March 2022).
- Gupta, R.K.; Chaudhary, K.K.; Kumar, M.; Negi, A.; Rai, H. Bioremediation and cyanobacteria: An overview. BioNano Front. 2012, 9, 190–196. [Google Scholar] [CrossRef]
- Hall, D.O.; Markov, S.A.; Watanabe, Y.; Rao, K.K. The potential applications of cyanobacterial photosynthesis for clean technologies. Photosynth. Res. 1995, 46, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Herrero, A. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 2005, 33, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Cuello, J.L. Carbon dioxide mitigation using thermophilic cyanobacteria. Biosyst. Eng. 2007, 96, 129–134. [Google Scholar] [CrossRef]
- Roeselers, G.; van Loosdrecht, M.C.M.; Muyzer, G. Phototrophic biofilms and their potential applications. J. Appl. Phycol. 2008, 20, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.K.; Dubey, J.; Mehra, S.; Tiwari, P.; Bishwas, A.J. Potential use of cyanobacterial species in bioremediation of industrial effluents. Afr. J. Biotechnol. 2011, 10, 1125–1132. [Google Scholar] [CrossRef]
- Vijayakumar, S. Potential applications of cyanobacteria in industrial effluents—A review. J. Bioremed. Biodegrad. 2012, 3, 2–4. [Google Scholar] [CrossRef]
- Kumar, B.N.P.; Mahaboobi, S.; Satyam, S. Cyanobacteria: A potential natural source for drug discovery and bioremediation. J. Ind. Pollut. Control 2016, 32, 508–517. [Google Scholar]
- Dittmann, M.E. Molecular biology of peptide and polyketide biosynthesis in cyanobacteria. Appl. Microbiol. Biot. 2001, 57, 467–473. [Google Scholar] [CrossRef]
- Simmons, T.L.; Andrianasolo, E.; Mcphail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–343. [Google Scholar]
- Volk, R. Screening of microalgae for species excreting norharmane, a manifold biologically active indole alkaloid. Microbiol. Res. 2008, 163, 307–313. [Google Scholar] [CrossRef]
- Wase, N.V.; Wright, P.C. Systems biology of cyanobacterial secondary metabolite production and its role in drug discovery. Expert Opin. Drug Dis. 2008, 3, 903–930. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Hamann, M.T. Marine pharmacology in 2005–2006: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakumar, S.; Menakha, M. Pharmaceutical applications of cyanobacteria—A review. J. Acute Med. 2015, 5, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Mukund, S.; Sivasubramanian, V. Anticancer activity of Oscillatoria terebriformis cyanobacteria in human lung cancer cell line A549. Int. J. Appl. Biol. Pharm. 2014, 5, 34–45. [Google Scholar]
- Chauhan, A.; Chauhan, G.; Gupta, P.C.; Goyal, P.; Kaushik, P. In vitro antibacterial evaluation of Anabaena sp. against several clinically significant microflora and HPTLC analysis of its active crude extracts. Indian J. Pharmacol. 2010, 42, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Jaki, B.; Zerbe, O.; Heilmann, J.; Sticher, O. Two novel cyclic peptides with antifungal activity from the cyanobacterium Tolypothrix byssoidea (EAWAG 195). J. Nat. Prod. 2001, 64, 154–158. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Kang, S.M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Nasra Reddy, M.; Reddanna, P.; Madyastha, K.M. Selective inhibition of cyclooxygenase-2 by c-phycocyanin, a biliprotein from Spirulina platensis. Biochem. Bioph. Res. Commun. 2000, 603, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Cohen, Z. The chemical of Spirulina. In Spirulina platensis (Arthrospira) Physiology, Cell-Biology and Biotechnology; Vonshak, A., Ed.; Taylor and Francis Ltd.: London, UK, 1997; pp. 175–204. [Google Scholar]
- Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Microalgal rainbow colours for nutraceutical and pharmaceutical applications. In Plant Biology and Biotechnology; Volume I: Plant Diversity, Organization, Function and Improvement; Bahadur, B., Rajam, M.V., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer: New Delhi, India, 2015; pp. 777–791. [Google Scholar]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Cyanobacterial sunscreen scytonemin: Role in photoprotection and biomedical research. Appl. Biochem. Biotechnol. 2015, 176, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Madamwar, D. Cyanobacteria synthesize their own UV-sunscreens for photoprotection. Bioenergetics 2016, 5, 1000138. [Google Scholar]
- Derikvand, P.; Llewellyn, C.A.; Purton, S. Cyanobacterial metabolites as a source of sunscreens and moisturizers: A comparison with current synthetic compounds. Eur. J. Phycol. 2017, 52, 43–56. [Google Scholar] [CrossRef]
- Valiente, E.F.; Ucha, A.; Quesada, A.; Leganés, F.; Carreres, R. Contribution of N2 fixing cyanobacteria to rice production: Availability of nitrogen from 15N-labelled cyanobacteria and ammonium sulphate to rice. Plant Soil 2000, 1, 107–112. [Google Scholar] [CrossRef]
- Prasanna, R.; Sharma, E.; Sharma, P.; Kumar, A.; Kumar, R.; Gupta, V.; Pal, R.K.; Singh Shivay, Y.; Nain, L. Soil fertility and establishment potential of inoculated cyanobacteria in rice crop grown under non-flooded conditions. Paddy Water Environ. 2013, 11, 175–183. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, A.L. The use of microalgae and cyanobacteria in the improvement of agricultural practices: A review on their biofertilising, biostimulating and biopesticide roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Henrikson, R. A nutrient rich supper food for super health. In Spirulina World Food; Henrikson, R., Ed.; Ronore Enterprises, Inc.: Hawaii, HI, USA, 2010; pp. 25–42. [Google Scholar]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Liberton, M.; Berg, R.H.; Heuser, J.; Roth, R.; Pakrasi, H.B. Ultrastructure of the membrane systems in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. Protoplasma 2006, 227, 129–138. [Google Scholar] [CrossRef]
- Liberton, M.; Austin, J.R.; Berg, R.H.; Pakrasi, H.B. Unique thylakoid membrane architecture of a unicellular N2 -fixing cyanobacterium revealed by electron tomography. Plant Physiol. 2011, 155, 1656–1666. [Google Scholar] [CrossRef] [Green Version]
- Demé, B.; Cataye, C.; Block, M.A.; Maréchal, E.; Jouhet, J. Contribution of galactoglycerolipids to the 3-dimensional architecture of thylakoids. FASEB 2014, 28, 3373–3383. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, D.D. Thylakoid centers: Structures associated with the cyanobacterial photosynthetic membrane system. Arch. Microbiol. 1982, 133, 97–99. [Google Scholar] [CrossRef]
- van de Meene, A.M.L.; Hohmann-Marriott, M.H.; Vermaas, W.F.J.; Roberson, R.W. The three-dimensional structure of the cyanobacterium Synechocystis sp. PCC 6803. Arch. Microbiol. 2006, 184, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.G.; Zwart, P.; Bagby, S.C.; Cai, F.; Chisholm, S.W.; Heinhorst, S.; Cannon, G.C.; Kerfeld, C.A. Identification and structural analysis of a novel carboxysome shell protein with implications for metabolite transport. J. Mol. Biol. 2009, 392, 319–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilo-Ferretjans, M.D.M.; Bosch, R.; Puxty, R.J.; Latva, M.; Zadjelovic, V.; Chhun, A.; Sousoni, D.; Polin, M.; Scanlan, D.J.; Christie-Oleza, J.A. Pili allow dominant marine cyanobacteria to avoid sinking and evade predation. Nat. Commun. 2021, 12, 1857. [Google Scholar] [CrossRef]
- Vourc’h, T.; Peerhossaini, H.; Léopoldès, J.; Mejean, A.; Chauvat, F.; Cassier-Chauvat, C. Slowdown of the surface diffusion during early stages of bacterial colonization. Phys. Rev. E 2018, 97, 032407. [Google Scholar] [CrossRef] [Green Version]
- Plohnke, N.; Seidel, T.; Kahmann, U.; Rögner, M.; Schneider, D.; Rexroth, S. The proteome and lipidome of Synechocystis sp. PCC 6803 cells grown under light-activated heterotrophic conditions. Mol. Cell Proteom. 2015, 14, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Wang, B.; Yang, L.; Hu, L.Z.; Yi, L.; Wang, Y.; Chen, S.; Emili, A.; Wan, C. Global landscape of native protein complexes in Synechocystis sp. PCC 6803. Mol. Cell Proteom. 2021; in press. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Sun, T.; Jiang, J.; Tian, L.; Cui, J.; Zhang, W. A transporter Slr1512 involved in bicarbonate and pH-dependent acclimation mechanism to high light stress in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148336. [Google Scholar] [CrossRef]
- Eungrasamee, K.; Miao, R.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Improved lipid production via fatty acid biosynthesis and free fatty acid recycling in engineered Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Pembroke, J.T.; Armshaw, P.; Ryan, M.P. Metabolic engineering of the model photoautotrophic cyanobacterium synechocystis for ethanol production: Optimization strategies and challenges. In Fuel Ethanol Production from Sugarcane; Basso, T.P., Basso, L.C., Eds.; IntechOpen: London, UK, 2019; pp. 199–219. [Google Scholar] [CrossRef] [Green Version]
- Utharn, S.; Yodsang, P.; Incharoensakdi, A.; Jantaro, S. Cyanobacterium Synechocystis sp. PCC 6803 lacking adc1 gene produces higher polyhydroxybutyrate accumulation under modified nutrients of acetate supplementation and nitrogen-phosphorus starvation. Biotechnol. Rep. 2021, 31, e00661. [Google Scholar] [CrossRef]
- Mittermair, S.; Lakatos, G.; Nicoletti, C.; Ranglová, K.; Manoel, J.C.; Grivalský, T.; Kozhan, D.M.; Masojídek, J.; Richter, J. Impact of glgA1, glgA2 or glgC overexpression on growth and glycogen production in Synechocystis sp. PCC 6803. J. Biotechnol 2021, 340, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Volkmann, N.; Arvai, A.S.; Pique, M.E.; Yeager, M.; Egelman, E.H.; Tainer, J.A. Type IV pilus structure by cryo-electron microscopy and crystallography: Implications for pilus assembly and functions. Mol Cell 2006, 23, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Li, J. Type IV pili: Paradoxes in form and function. Curr. Opin. Struct. Biol. 2009, 18, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Bhaya, D.; Watanabe, N.; Ogawa, T.; Grossman, A.R. The role of an alternative sigma factor in motility and pilus formation in the cyanobacterium Synechocystis sp. strain PCC6803. Proc. Natl. Acad. Sci. USA 1999, 96, 3188–3193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuergers, N.; Nürnberg, D.J.; Wallner, T.; Mullineaux, C.W.; Wilde, A. PilB localization correlates with the direction of twitching motility in the cyanobacterium. Microbiology 2015, 161, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Schuergers, N.; Mullineaux, C.W.; Wilde, A. Cyanobacteria in motion. Curr. Opin. Plant Biol. 2017, 37, 109–115. [Google Scholar] [PubMed]
- Maier, B.; Wong, G.C.L. How bacteria use type IV pili machinery on surfaces. Trends Microbiol. 2015, 23, 775–788. [Google Scholar] [CrossRef]
- Nakane, D.; Nishizaka, T. Asymmetric distribution of type IV pili triggered by directional light in unicellular cyanobacteria. Proc. Natl. Acad. Sci. USA 2017, 114, 6593–6598. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Zang, S.; Li, Z.; Dai, G.; Liu, K.; Chen, M.; Qiu, B. Sycrp2 is essential for twitching motility in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2018, 200, e00436-18. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, S.; Ikeuchi, M. Phototactic motility in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. Sci. 2004, 3, 512–518. [Google Scholar] [CrossRef]
- Bhaya, D.; Takahashi, A.; Grossman, A.R. Light regulation of type IV pilus-dependent motility by chemosensor-like elements in Synechocystis PCC6803. Proc. Natl. Acad. Sci. USA 2001, 98, 7540–7545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaya, D.; Takahashi, A.; Shahi, P.; Grossman, A.R. Novel motility mutants of Synechocystis strain PCC 6803 generated by in vitro transposon mutagenesis. J. Bacteriol. 2001, 183, 6140–6143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vourc’h, T.; Léopoldès, J.; Peerhossaini, H. Light control of the diffusion coefficient of active fluids. J. Fluid Eng. 2020, 142, 031109-1–031109-7. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Joubert, L.; Bhaya, D. Modulation of type IV pili phenotypic plasticity through a novel Chaperone-Usher system in Synechocystis sp. BioRxiv 2017, 130278. [Google Scholar] [CrossRef] [Green Version]
- Wadhams, G.H.; Armitage, J.P. Making sense of it all: Bacterial chemotaxis. Nature 2004, 5, 1024–1037. [Google Scholar] [CrossRef]
- Mattick, J.S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 2002, 56, 289–314. [Google Scholar] [CrossRef]
- Wall, D.; Kaiser, D. Type IV pili and cell motility. Mol. Microbiol. 1999, 32, 1–10. [Google Scholar] [CrossRef]
- Ursell, T.; Chau, R.M.W.; Wisen, S.; Bhaya, D.; Huang, K.C. Motility enhancement through surface modification is sufficient for cyanobacterial community organization during phototaxis. PLoS Comput. Biol. 2013, 9, e1003205. [Google Scholar] [CrossRef]
- Samadi, Z.; Johlin, E.; DeGroot, C.; Peerhossaini, H. Modelling optical properties of algae using the finite-difference time domain method. In Proceedings of the Fluids Engineering Division Summer Meeting, Online, 10–12 August 2021; p. 85307, V003T05A016. [Google Scholar] [CrossRef]
- Schuergers, N.; Lenn, T.; Kampmann, R.; Meissner, M.V.; Esteves, T.; Temerinac-ott, M.; Korvink, J.G.; Lowe, A.R.; Mullineaux, C.W.; Wilde, A. Cyanobacteria use micro-optics to sense light direction. eLife 2016, 5, e12620. [Google Scholar] [CrossRef]
- Mullineaux, C.W. How do cyanobacteria sense and respond to light? Mol. Microbiol. 2001, 41, 965–971. [Google Scholar] [CrossRef]
- Chau, R.M.W.; Bhaya, D.; Huang, K.C. Emergent phototactic responses of cyanobacteria under complex light regimes. ASM 2017, 8, 1–15. [Google Scholar]
- Nilsson, D.E.; Colley, N.J. Comparative vision: Can bacteria really see? Curr. Biol. 2016, 26, R369–R371. [Google Scholar] [CrossRef] [PubMed]
- Burriesci, M.; Bhaya, D. Tracking phototactic responses and modeling motility of Synechocystis sp. strain PCC6803. J. Photochem. Photobiol. B 2008, 91, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.; Grossman, A.R.; Bhaya, D. Multiple light inputs control phototaxis in Synechocystis sp. strain PCC6803. J. Bacteriol. 2003, 185, 1599–1607. [Google Scholar] [CrossRef] [Green Version]
- Moon, Y.; Kim, S.; Chung, Y. Sensing and responding to UV-A in cyanobacteria. Int. J. Mol. Sci. 2012, 13, 16303–16332. [Google Scholar] [CrossRef]
- Choi, J.; Chungl, Y.; Moon, Y.; Kim, C.; Watanabe, M.; Song, P.; Joe, C.; Bogorad, L.; Park, Y.M. Photomovement of the Gliding Cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. 1999, 70, 95–102. [Google Scholar] [CrossRef]
- Webre, D.J.; Wolanin, P.M.; Stock, J.B. Bacterial chemotaxis. Curr. Biol. 2003, 13, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Parkinson, J.S. Signal transduction schemes of bacteria. Cell 1993, 73, 857–871. [Google Scholar] [CrossRef]
- Copeland, M.F.; Weibel, D.B. Bacterial swarming: A model system for studying dynamic self-assembly. Soft Matter 2013, 5, 1174–1187. [Google Scholar] [CrossRef]
- Sourjik, V.; Wingreen, N.S. Responding to chemical gradients: Bacterial chemotaxis. Curr. Opin. Cell Biol. 2012, 24, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 28, 1318–1323. [Google Scholar] [CrossRef] [Green Version]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nature 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Bender, J.; Phillips, P. Microbial mats for multiple applications in aquaculture and bioremediation. Bioresour. Technol. 2004, 94, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ivnitsky, H.; Katz, I.; Minz, D.; Volvovic, G.; Shimoni, E.; Kesselman, E.; Semiat, R.; Dosoretz, C.G. Bacterial community composition and structure of biofilms developing on nanofiltration membranes applied to wastewater treatment. Water Res. 2007, 41, 3924–3935. [Google Scholar] [CrossRef]
- Mazza, M.G. The physics of biofilms-an introduction. J. Phys. D Appl. Phys. 2016, 49, 203001. [Google Scholar] [CrossRef]
- Taktikos, J.; Lin, Y.T.; Stark, H.; Biais, N.; Zaburdaev, V. Pili-induced clustering of N. gonorrhoeae Bacteria. PLoS ONE 2015, 10, e0137661. [Google Scholar] [CrossRef]
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013, 11, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Vourc’h, T.; Léopoldès, J.; Peerhossaini, H. Clustering of bacteria with heterogeneous motility. Phys. Rev. E 2020, 101, 022612. [Google Scholar] [CrossRef] [Green Version]
- Vermaas, W. Molecular genetics of the cyanobacterium Synechocystis sp. PCC 6803: Principles and possible biotechnology applications. J. Appl. Phycol. 1996, 8, 263–273. [Google Scholar] [CrossRef]
- Allen, R.; Rittmann, B.E.; Curtiss, R. Axenic biofilm formation and aggregation by Synechocystis concentration and require cell surface structures. Appl. Environ. Microb. 2019, 85, e02192-18. [Google Scholar] [CrossRef] [Green Version]
- Des Marais, D.J. Microbial mats and the early evolution of life. Trends Ecol. Evol 1990, 5, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lichter, J.A.; Thompson, M.T.; Delgadillo, M.; Nishikawa, T.; Rubner, M.F.; Van Vliet, K.J. Substrata mechanical stiffness can regulate adhesion of viable bacteria. Biomacromolecules 2008, 9, 1571–1578. [Google Scholar] [CrossRef] [Green Version]
- Guégan, C.; Garderes, J.; Le Pennec, G.; Gaillard, F.; Fay, F.; Linossier, I.; Herry, J.M.; Bellon Fontaine, V.R.K. Alteration of bacterial adhesion induced by the substrate stiffness. Colloid Surface B 2014, 114, 193–200. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.I. Models for the specific adhesion of cells to cells. Science 1978, 200, 618–627. [Google Scholar] [CrossRef]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins involved in attachment to abiotic surfaces by gram-negative bacteria. Microbiol. Spectr. 2015, 3, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Okuda, Y.; Enomoto, G.; Watanabe, S.; Ikeuchi, M. Biosynthesis of a sulfated exopolysaccharide, synechan, and bloom formation in the model cyanobacterium Synechocystis sp. strain PCC 6803. eLife 2021, 10, e66538. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectrum. 2015, 3, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Planchon, M.; Jittawuttipoka, T.; Cassier-Chauvat, C.; Guyot, F.; Gelabert, A.; Benedetti, M.F.; Chauvat, F.; Spalla, O. Exopolysaccharides protect Synechocystis against the deleterious effects of titanium dioxide nanoparticles in natural and artificial waters. J. Colloid Interface Sci. 2013, 405, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dervaux, J.; Mejean, A.; Brunet, P. Irreversible collective migration of cyanobacteria in eutrophic conditions. PLoS ONE 2015, 10, e0120906. [Google Scholar] [CrossRef]
- Rao, D.L.N.; Burns, R.G. The effect of surface growth of blue-green algae and bryophytes on some microbiological, biochemical, and physical soil properties. Biol. Fertil. Soils 1990, 9, 239–244. [Google Scholar] [CrossRef]
- Šmarda, J.; Šmajs, D.; Komrska, J.; Krzyžánek, V. S-layers on cell walls of cyanobacteria. Micron 2002, 33, 257–277. [Google Scholar] [CrossRef]
- Panoff, J.; Priem, B.; Morvan, H.; Joset, F. Sulphated exopolysaccharides produced by two unicellular strains of cyanobacteria, Synechocystis PCC 6803 and 6714. Arch. Microbiol. 1988, 150, 558–563. [Google Scholar] [CrossRef]
- Prieto-Barajas, C.M.; Valencia-Cantero, E.; Santoyo, G. Microbial mat ecosystems: Structure types, functional diversity, and biotechnological application. Electron. J. Biotechnol. 2018, 31, 48–56. [Google Scholar] [CrossRef]
- van Gemerden, H. Microbial mats: A joint venture. Mar. Geol. 1993, 113, 3–25. [Google Scholar] [CrossRef]
- Klatt, J.M.; Meyer, S.; Häusler, S.; Macalady, J.L.; de Beer, D.; Polerecky, L. Structure and function of natural sulphide-oxidizing microbial mats under dynamic input of light and chemical energy. ISME J. 2016, 10, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.S.; Dudley, L.Y. Biofouling in membrane systems—A review. Desalination 1998, 118, 81–90. [Google Scholar] [CrossRef]
- Parnasa, R.; Nagar, E.; Sendersky, E.; Reich, Z.; Simkovsky, R.; Golden, S.; Schwarz, R. Small secreted proteins enable biofilm development in the cyanobacterium Synechococcus elongatus. Sci. Rep. 2016, 6, 32209. [Google Scholar] [CrossRef]
- Dahms, H.; Ying, X.; Pfeiffer, C. Antifouling potential of cyanobacteria: A mini-review. Biofouling 2006, 22, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh Allaf, M.; Habib, Z.; de Bruyn, J.R.; DeGroot, C.T.; Peerhossaini, H. Rheological and Biophysical Properties of Living Fluids Under Shear: Active Suspensions of Synechocystis sp. CPCC 534. J. Fluid Eng. 2022, 144, 021208. [Google Scholar] [CrossRef]
- Samadi, Z.; Mehdizadeh Allaf, M.; Saifi, R.; De Groot, C.T.; Peerhossaini, H. Effects of turbulent mixing and orbitally shaking on cell growth and biomass production in active fluids. AJBSR 2022, 15, 396–404. [Google Scholar] [CrossRef]
- Mehdizadeh Allaf, M.; Fadlallah, H.; Jarrahi, M.; Peerhossaini, H. Growth and pigment production of Synechocystis sp. PCC 6803 under shear stress. Can. J. Chem. Eng. 2022; in press. [Google Scholar]
- Gao, T.; Hu, H.H.; Castaneda, P.P. Shape dynamics and rheology of soft elastic particles in a shear flow. Phys. Rev. Lett. 2012, 108, 058302. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdizadeh Allaf, M.; Peerhossaini, H. Cyanobacteria: Model Microorganisms and Beyond. Microorganisms 2022, 10, 696. https://doi.org/10.3390/microorganisms10040696
Mehdizadeh Allaf M, Peerhossaini H. Cyanobacteria: Model Microorganisms and Beyond. Microorganisms. 2022; 10(4):696. https://doi.org/10.3390/microorganisms10040696
Chicago/Turabian StyleMehdizadeh Allaf, Malihe, and Hassan Peerhossaini. 2022. "Cyanobacteria: Model Microorganisms and Beyond" Microorganisms 10, no. 4: 696. https://doi.org/10.3390/microorganisms10040696
APA StyleMehdizadeh Allaf, M., & Peerhossaini, H. (2022). Cyanobacteria: Model Microorganisms and Beyond. Microorganisms, 10(4), 696. https://doi.org/10.3390/microorganisms10040696






