The Tip of Brucella O-Polysaccharide Is a Potent Epitope in Response to Brucellosis Infection and Enables Short Synthetic Antigens to Be Superior Diagnostic Reagents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of Bacterial Antigens
2.2. Extraction and Preparation of Native Antigens
2.3. Synthetic Antigens
2.4. Serum Samples
2.5. Serological Methods
2.6. Data Analysis
3. Results
3.1. Magnitude of Serological Response
3.2. Quantification of Impact of OPS Tip Epitope on Antibody Binding
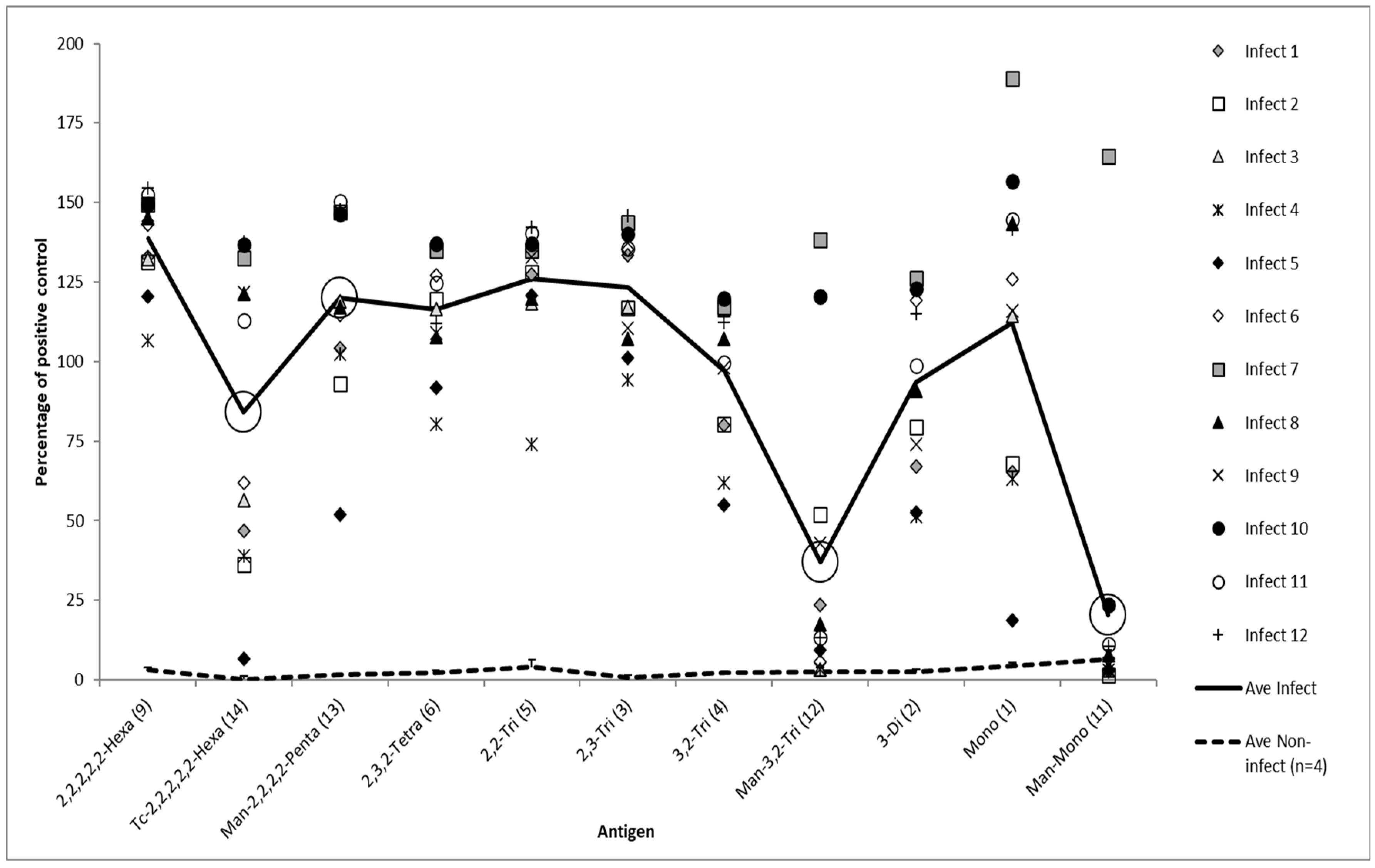
3.3. Diagnostic Impact of OPS Tip Epitope: Experimental Infection
3.4. Diagnostic Impact of OPS Tip Epitope: Field Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Godfroid, J.; Nielsen, K.; Saegerman, C. Diagnosis of brucellosis in livestock and wildlife. Croat. Med. J. 2010, 51, 296–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, P.; Macedo, G.; Azevedo, V.; Oliveira, S. Brucella spp. noncanonical LPS: Structure, biosynthesis, and interaction with host immune system. Microb. Cell Factories 2006, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haag, A.F.; Myka, K.K.; Arnold, M.F.F.; Caro-Hernandez, P.; Ferguson, G.P. Importance of Lipopolysaccharide and Cyclic b-1,2-Glucans in Brucella-Mammalian Infections. Int. J. Microbiol. 2010, 2010, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souwer, Y.; Griekspoor, A.; Jorritsma, T.; de Wit, J.; Janssen, H.; Neefjes, J.; van Ham, S.M. B Cell Receptor-Mediated Internalization of Salmonella: A Novel Pathway for Autonomous B Cell Activation and Antibody Production. J. Immunol. 2009, 182, 7473–7481. [Google Scholar] [CrossRef] [Green Version]
- Meikle, P.J.; Perry, M.B.; Cherwonogrodzky, J.W.; Bundle, D.R. Fine structure of A and M antigens from Brucella biovars. Infect. Immun. 1989, 57, 2820–2828. [Google Scholar] [CrossRef] [Green Version]
- Zaccheus, M.V.; Ali, T.; Cloeckaert, A.; Zygmunt, M.S.; Weintraub, A.; Iriarte, M.; Moriyón, I.; Widmalm, G. The Epitopic and Structural Characterization of Brucella suis Biovar 2 O-Polysaccharide Demonstrates the Existence of a New M-Negative C-Negative Smooth Brucella Serovar. PLoS ONE 2013, 8, e53941. [Google Scholar] [CrossRef]
- Kubler-Kielb, J.; Vinogradov, E. Reinvestigation of the structure of Brucella O-antigens. Carbohydr. Res. 2013, 378, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Kubler-Kielb, J.; Vinogradov, E. The study of the core part and non-repeating elements of the O-antigen of Brucella lipopolysaccharide. Carbohydr. Res. 2013, 366, 33–37. [Google Scholar] [CrossRef] [Green Version]
- OIE. Brucellosis (Chapter 3.1.4). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2016. [Google Scholar]
- Alonso-Urmeneta, B.; Marin, C.; Aragon, V.; Blasco, J.M.; Diaz, R.; Moriyon, I. Evaluation of lipopolysaccharides and polysaccharides of different epitopic structures in the indirect enzyme-linked immunosorbent assay for diagnosis of brucellosis in small ruminants and cattle. Clin. Diagn. Lab. Immunol. 1998, 5, 749–754. [Google Scholar] [CrossRef] [Green Version]
- Bundle, D.R.; Cherwonogrodzky, J.W.; Gidney, M.A.; Meikle, P.J.; Perry, M.B.; Peters, T. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect. Immun. 1989, 57, 2829–2836. [Google Scholar] [CrossRef] [Green Version]
- Laurent, T.C.; Mertens, P.; Dierick, J.F.; Letesson, J.J.; Lambert, C.; De Bolle, X. Functional, molecular and structural characterisation of five anti-Brucella LPS mAb. Mol. Immunol. 2004, 40, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- McGiven, J.; Howells, L.; Duncombe, L.; Stack, J.; Ganesh, N.V.; Guiard, J.; Bundle, D.R. Improved Serodiagnosis of Bovine Brucellosis by Novel Synthetic Oligosaccharide Antigens Representing the Capping M Epitope Elements of Brucella O-Polysaccharide. J. Clin. Microbiol. 2015, 53, 1204–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.S.; Duncombe, L.; Ganesh, N.V.; Sarkar, S.; Howells, L.; Hogarth, P.J.; Bundle, D.R.; McGiven, J. Novel Solutions for Vaccines and Diagnostics To Combat Brucellosis. ACS Cent. Sci. 2017, 3, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerbier, G.; Garin-Bastuji, B.; Pouillot, R.; Very, P.; Cau, C.; Berr, V.; Dufour, B.; Moutou, F. False positive serological reactions in bovine brucellosis: Evidence of the role of Yersinia enterocolitica serotype O:9 in a field trial. Vet. Res. 1997, 28, 375–383. [Google Scholar] [PubMed]
- Garin-Bastuji, B.; Hummel, N.; Gerbier, G.; Cau, C.; Pouillot, R.; Da Costa, M.; Fontaine, J.-J. Non specific serological reactions in the diagnosis of bovine brucellosis: Experimental oral infection of cattle with repeated doses of Yersinia enterocolitica O:9. Vet. Microbiol. 1999, 66, 223–233. [Google Scholar] [CrossRef]
- O’Grady, D.; Kenny, K.; Power, S.; Egan, J.; Ryan, F. Detection of Yersinia enterocolitica serotype O:9 in the faeces of cattle with false positive reactions in serological tests for brucellosis in Ireland. Vet. J. 2016, 216, 133–135. [Google Scholar] [CrossRef]
- Caroff, M.; Bundle, D.R.; Perry, M.B. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur. J. Biochem. 1984, 139, 195–200. [Google Scholar] [CrossRef]
- Villeneuve, S.; Souchon, H.; Riottot, M.M.; Mazie, J.C.; Lei, P.; Glaudemans, C.P.; Kovac, P.; Fournier, J.M.; Alzari, P.M. Crystal structure of an anti-carbohydrate antibody directed against Vibrio cholerae O1 in complex with antigen: Molecular basis for serotype specificity. Proc. Natl. Acad. Sci. USA 2000, 97, 8433–8438. [Google Scholar] [CrossRef] [Green Version]
- Bishop, A.L.; Schild, S.; Patimalla, B.; Klein, B.; Camilli, A. Mucosal Immunization with Vibrio cholerae Outer Membrane Vesicles Provides Maternal Protection Mediated by Antilipopolysaccharide Antibodies That Inhibit Bacterial Motility. Infect. Immun. 2010, 78, 4402–4420. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Rynkiewicz, M.J.; Yang, C.-Y.; Madico, G.; Perkins, H.M.; Wang, Q.; Costello, C.E.; Zaia, J.; Seaton, B.A.; Sharon, J. The binding sites of monoclonal antibodies to the non-reducing end of Francisella tularensis O-antigen accommodate mainly the terminal saccharide. Immunology 2013, 140, 374–389. [Google Scholar] [CrossRef]
- Tamigney Kenfack, M.; Mazur, M.; Nualnoi, T.; Shaffer, T.L.; Ngassimou, A.; Blériot, Y.; Marrot, J.; Marchetti, R.; Sintiprungrat, K.; Chantratita, N.; et al. Deciphering minimal antigenic epitopes associated with Burkholderia pseudomallei and Burkholderia mallei lipopolysaccharide O-antigens. Nat. Commun. 2017, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Westphal, O.; Luderitz, O.; Bister, F. Uber die Extraction von Bakterien mit Phenol/Wasser. Z. Naturforsch. 1952, 7, 148–155. [Google Scholar] [CrossRef]
- Guiard, J.; Paszkiewicz, E.; Sadowska, J.; Bundle, D.R. Design and Synthesis of a Universal Antigen to Detect Brucellosis. Angew. Chem. Int. Ed. 2013, 52, 7181–7185. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, N.V.; Sadowska, J.M.; Sarkar, S.; Howells, L.; McGiven, J.; Bundle, D.R. Molecular Recognition of Brucella A and M Antigens Dissected by Synthetic Oligosaccharide Glycoconjugates Leads to a Disaccharide Diagnostic for Brucellosis. J. Am. Chem. Soc. 2014, 136, 16260–16269. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.S.; Ganesh, N.V.; Sadowska, J.M.; Bundle, D.R. Synthetic glycoconjugates characterize the fine specificity of Brucella A and M monoclonal antibodies. Org. Biomol. Chem. 2017, 15, 3874–3883. [Google Scholar] [CrossRef]
- Alton, G.G.; Jones, L.M.; Angus, R.D.; Verger, J.M. Techniques for the Brucellosis Laboratory (Techniques et Pratiques); INRA: Paris, France, 1994; p. 190. [Google Scholar]
- McGiven, J.; Sawyer, J.; Perrett, L.; Brew, S.; Commander, N.; Fisher, A.; McLarnon, S.; Harper, K.; Stack, J. A new homogeneous assay for high throughput serological diagnosis of brucellosis in ruminants. J. Immunol. Methods 2008, 337, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, R.H. Validation of serological assays for diagnosis of infectious diseases. Rev. Sci. Tech. Off. Int. Epiz. 1998, 17, 469–526. [Google Scholar] [CrossRef]
- Hou, S.-j.; Saksena, R.; Kováč, P. Preparation of glycoconjugates by dialkyl squarate chemistry revisited. Carbohydr. Res. 2008, 343, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Holland, G.P.; Steward, M.W. The influence of epitope density on the estimation of the affinity of antibody for complex antigens. J. Immunol. Methods 1991, 138, 245–255. [Google Scholar] [CrossRef]
- Ducrotoy, M.J.; Conde-Álvarez, R.; Blasco, J.M.; Moriyón, I. A review of the basis of the immunological diagnosis of ruminant brucellosis. Vet. Immunol. Immunopathol. 2016, 171, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Bundle, D.R.; Gidney, M.A.; Perry, M.B.; Duncan, J.R.; Cherwonogrodzky, J.W. Serological confirmation of Brucella abortus and Yersinia enterocolitica O:9 O-antigens by monoclonal antibodies. Infect. Immun. 1984, 46, 389–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller-Loennies, S.; Rund, S.; Ervelä, E.; Skurnik, M.; Holst, O. The structure of the carbohydrate backbone of the core-lipid A region of the lipopolysaccharide from a clinical isolate of Yersinia enterocolitica O:9. Eur. J. Biochem. 1999, 261, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottone, E.J. Yersinia enterocolitica: The charisma continues. Clin. Microbiol. Rev. 1997, 10, 257–276. [Google Scholar] [CrossRef]
- Skurnik, M.; Bengoechea, J.A. The biosynthesis and biological role of lipopolysaccharide O-antigens of pathogenic Yersiniae. Carbohydr. Res. 2003, 338, 2521–2529. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Najdenski, H.; Skurnik, M. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol. Microbiol. 2004, 52, 451–469. [Google Scholar] [CrossRef]
- Uliczka, F.; Pisano, F.; Schaake, J.; Stolz, T.; Rohde, M.; Fruth, A.; Strauch, E.; Skurnik, M.; Batzilla, J.; Rakin, A.; et al. Unique cell adhesion and invasion properties of Yersinia enterocolitica O:3, the most frequent cause of human Yersiniosis. PLoS Pathog. 2011, 7, e1002117. [Google Scholar] [CrossRef]
- Gu, W.P.; Wang, X.; Qiu, H.Y.; Luo, X.; Xiao, Y.C.; Tang, L.Y.; Kan, B.; Xu, J.G.; Jing, H.Q. Comparison of lipopolysaccharide and protein immunogens from pathogenic Yersinia enterocolitica bio-serotype 1B/O:8 and 2/O:9 using SDS-PAGE. Biomed. Environ. Sci. BES 2012, 25, 282–290. [Google Scholar] [CrossRef]
- Domínguez-Medina, C.C.; Pérez-Toledo, M.; Schager, A.E.; Marshall, J.L.; Cook, C.N.; Bobat, S.; Hwang, H.; Chun, B.J.; Logan, E.; Bryant, J.A.; et al. Outer membrane protein size and LPS O-antigen define protective antibody targeting to the Salmonella surface. Nat. Commun. 2020, 11, 851. [Google Scholar] [CrossRef] [Green Version]
- Perry, M.B.; Bundle, D.R. Antigenic relationships of the lipopolysaccharides of Escherichia hermannii strains with those of Escherichia coli O157:H7, Brucella melitensis, and Brucella abortus. Infect. Immun. 1990, 58, 1391–1395. [Google Scholar] [CrossRef] [Green Version]




| Antigen Number | Antigen Name | Oligosaccharide Structure | Linker Type | Oligo per BSA | Primary Reference |
|---|---|---|---|---|---|
| (1) | Mono | α-D-Rha4NFo | Sq | 10-15 | [14] |
| (2) | 3-Di | α-D-Rha4NFo-(1→3)-α-D-Rha4NFo | Sq | 15.2 | [25] |
| (3) | 2,3-Tri | α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→3)-α-D-Rha4NFo | Sq | 15.9 | [25] |
| (4) | 3,2-Tri | α-D-Rha4NFo-(1→3)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo | Sq | 15.7 | [25] |
| (5) | 2,2-Tri | α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo | Sq | 16.2 | [14] |
| (6) | 2,3,2-Tetra | α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→3)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo | Sq | 13.4 | [25] |
| (7) | 2,3,2,2-Penta | α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→3)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)- α-D-Rha4NFo | Sq | ~16 | [24] |
| (8) | 2,3,2,2,2-Hexa | α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→3)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)- α-D-Rha4NFo-(1→2)-α-D-Rha4NFo | Sq | 11.6 | [25] |
| (9) | 2,2,2,2,2-Hexa | α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)- α-D-Rha4NFo-(1→2)-α-D-Rha4NFo | Sq | 13.8 | [25] |
| (10) | 2,2,2,3,2,2,2,2-Nona | α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→3)- α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo | Sq | ~16 | [24] |
| (11) | Man-Mono | α-D-Man-(1→2)-α-D-Rha4NFo | Sq | 16 | [26] |
| (12) | Man-3,2-Tri | α-D-Man-(1→2)-α-D-Rha4NFo-(1→3)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo | Sq | 19.6 | [26] |
| (13) | Man-2,2,2,2-Penta | α-D-Man-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)- α-D-Rha4NFo | Sq | 19 | [26] |
| (14) | Tc-2,2,2,2,2-Hexa * | BSA-Sq-α-D-Rha-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)- α-D-Rha4NFo-(1→2)-α-D-Rha4NFo | Sq | 10.3 | [14] |
| (15) | 3-Di-dsg | α-D-Rha4NFo-(1→3)-α-D-Rha4NFo | DSG | Not done | Oligo as [14] conjugate not previously published |
| (16) | 2,3-Tri-dsg | α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→3)-α-D-Rha4NFo | DSG | 7.6 | [26] |
| (17) | 2,3,2-Tetra-dsg | α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→3)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo | DSG | 7.3 | [26] |
| (18) | 2Me-3-Di-dsg | α-D-4-6-dideoxy-4-formamido-2-O-methyl-mannopyranosyl-(1→3)-α-D-Rha4NFo | DSG | 9.2 | [26] |
| (19) | 2,3Me-3-Di-dsg | α-D-4-6-dideoxy-4-formamido-2-3-O-methyl-mannopyranosyl-(1→3)-α-D-Rha4NFo | DSG | 8.7 | [26] |
| (20) | 2Me-2,3-Tr-dsg | α-D-4-6-dideoxy-4-formamido-2-O-methyl-mannopyranosyl-(1→2)-α-D-Rha4NFo-(1→3)-α-D-Rha4NFo | DSG | 9.5 | [26] |
| (21) | Tc-2,2,2,2,2-Hexa-dsg | BSA-DSG-α-D-Rha-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)-α-D-Rha4NFo-(1→2)- α-D-Rha4NFo-(1→2)-α-D-Rha4NFo | DSG | Oligo as [14] conjugate not previously published |
| Antigen | Specificity (Experimental) | AUC (Experimental) | AUC Field FPSRs (n = 16) vs. Brucella (n = 21) | AUC Field FPSRs (n = 39) vs. Brucella (n = 42) |
|---|---|---|---|---|
| Mono (1) | 75 | 0.974 | 0.720 | |
| 3-Di (2) | 85 | 0.957 | 0.807 | 0.834 |
| 2,3-Tri (3) | 65 | 0.863 | ||
| 3,2-Tri (4) | 85 | 0.934 | ||
| 2,2-Tri (5) * | 65 | 0.916 | 0.908 | 0.888 |
| 2,3,2-Tetra (6) | 55 | 0.882 | 0.795 | 0.730 |
| 2,2-Tri (5) + 2,3,2-Tetra (6) | 0.839 | |||
| 2,3,2,2-Penta (7) | 40 | 0.867 | ||
| 2,3,2,2,2-Hexa (8) * | 40 | 0.916 | ||
| 2,2,2,2,2-Hexa (9) | 45 | 0.900 | 0.878 | |
| 2,2,2,3,2,2,2,2-Nona (10) | 60 | 0.897 | ||
| Man-Mono (11) | Not done | |||
| Man-3,2-Tri (12) | 5 | 0.538 | ||
| Man-2,2,2,2-Penta (13) | 45 | 0.870 | 0.795 | |
| Tc-2,2,2,2,2-Hexa (14) * | 40 | 0.816 | ||
| 3-Di-dsg (15) * | 85 | 0.976 | ||
| 2,3-Tri-dsg (16) | Not done | |||
| 2,3,2-Tetra-dsg (17) * | 85 | 0.976 | ||
| 2Me-3-Di-dsg (18) * | 55 | 0.892 | ||
| 22Me-3-Di-dsg (19) | Not done | |||
| 2Me-2,3-Tri-dsg (20) | Not done | |||
| Tc-2,2,2,2,2-Hexa-dsg (21) | Not done | |||
| B. abortus S99 sLPS * | 25 | 0.868 | 0.711 | 0.701 |
| B. abortus S99 mOPS * | 60 | 0.890 | 0.714 | 0.763 |
| B. melitensis 16M mOPS * | 70 | 0.897 | 0.607 | 0.650 |
| B. suis (biovar 2) mOPS * | 30 | 0.890 | ||
| Y. enterocolitica O:9 mOPS * | 15 | 0.747 | 0.597 | 0.542 |
| SAT (Sensitivity 85%, 30 I.U.s) | 30 (50) | 0.740 | ||
| CFT (Sensitivity 90%, 20 I.U.s) | 0 (55) | 0.848 | ||
| cELISA (B. melitensis 16M sLPS) (Sensitivity 90%) | 15 (35) | 0.853 | ||
| FPA (Sensitivity 90%) | 45 (50) | 0.843 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duncombe, L.; Howells, L.; Haughey, A.; Taylor, A.V.; Kaveh, D.; Erdenliğ Gϋrbilek, S.; Dell, A.; Hitchen, P.G.; Haslam, S.M.; Mandal, S.S.; et al. The Tip of Brucella O-Polysaccharide Is a Potent Epitope in Response to Brucellosis Infection and Enables Short Synthetic Antigens to Be Superior Diagnostic Reagents. Microorganisms 2022, 10, 708. https://doi.org/10.3390/microorganisms10040708
Duncombe L, Howells L, Haughey A, Taylor AV, Kaveh D, Erdenliğ Gϋrbilek S, Dell A, Hitchen PG, Haslam SM, Mandal SS, et al. The Tip of Brucella O-Polysaccharide Is a Potent Epitope in Response to Brucellosis Infection and Enables Short Synthetic Antigens to Be Superior Diagnostic Reagents. Microorganisms. 2022; 10(4):708. https://doi.org/10.3390/microorganisms10040708
Chicago/Turabian StyleDuncombe, Lucy, Laurence Howells, Anna Haughey, Andrew V. Taylor, Daryan Kaveh, Sevil Erdenliğ Gϋrbilek, Anne Dell, Paul G. Hitchen, Stuart M. Haslam, Satadru Sekhar Mandal, and et al. 2022. "The Tip of Brucella O-Polysaccharide Is a Potent Epitope in Response to Brucellosis Infection and Enables Short Synthetic Antigens to Be Superior Diagnostic Reagents" Microorganisms 10, no. 4: 708. https://doi.org/10.3390/microorganisms10040708
APA StyleDuncombe, L., Howells, L., Haughey, A., Taylor, A. V., Kaveh, D., Erdenliğ Gϋrbilek, S., Dell, A., Hitchen, P. G., Haslam, S. M., Mandal, S. S., Ganesh, N. V., Bundle, D. R., & McGiven, J. (2022). The Tip of Brucella O-Polysaccharide Is a Potent Epitope in Response to Brucellosis Infection and Enables Short Synthetic Antigens to Be Superior Diagnostic Reagents. Microorganisms, 10(4), 708. https://doi.org/10.3390/microorganisms10040708








