Inability to Catabolize Rhamnose by Sinorhizobium meliloti Rm1021 Affects Competition for Nodule Occupancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Media
2.2. Genetic Techniques
2.3. DNA Manipulations
2.4. Rhamnose Transport Assay
2.5. Enzyme Assays
2.6. β-Galactosidase Assays
2.7. Plant Assays
3. Results
3.1. Identification of a Rhamnose Catabolic Operon in S. meliloti
3.2. Complementation of S. meliloti Rhamnose Mutants with R. leguminosarum Rhamnose Catabolic Genes
3.3. Growth of S. meliloti rhaDI Mutants Are Not Inhibited on Rhamnose/Glycerol Media
3.4. S. meliloti rhaK (rhaKSm) and rhaI (rhaISm) Can Complement R. leguminosarum Mutants
3.5. RhaKSm Does Not Possess Measurable Rhamnose Kinase Activity
3.6. The Rhamnose Transporter RhaSTPQ Is Required for Growth on Rhamnose
3.7. Cosmids Carrying the R. leguminosarum Rhamnose Locus Show Reduced Expression in Rm1021
3.8. S. meliloti Rhamnose Mutants Are Less Competitive for Nodule Occupancy on Alfalfa
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Triplett, E.W.; Sadowsky, M. Genetics of competition for nodulation of legumes. Annu. Rev. Micorbiol. 1992, 46, 399–428. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Suárez, M.; Andersen, S.U.; Poole, P.S.; Sánchez-Cañizares, C. Competition, nodule occupancy, and persistence of inoculant strains: Key factors in the Rhizobium-legume symbioses. Front. Plant Sci. 2021, 12, 690567. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume-Rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-Rhizobia symbiosis. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geddes, B.A.; Oresnik, I.J. The mechanism of symbiotic nitrogen fixation. In The Mechanistic Benefits of Microbial Symbionts; Hurst, C.J., Ed.; Springer International Publishing: New York, NY, USA, 2016; Volume 2, pp. 69–97. [Google Scholar]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Geddes, B.A.; Oresnik, I.J. Physiology, genetics and biochemistry of carbon metabolism in the α-proteobacterium Sinorhizobium meliloti. Can. J. Microbiol. 2014, 60, 491–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiebo, J.; Marek-Kozaczuk, M.; Kubik-Komar, A.; Skorupska, A. Increased metabolic potential of Rhizobium spp. is associated with bacterial competitiveness. Can. J. Microbiol. 2007, 53, 957–967. [Google Scholar]
- Ramachandran, V.K.; East, A.K.; Karunakaran, R.; Downie, J.A.; Poole, P. Adaptation of Rhizobium leguminosarum to peas, alfalfa, and sugar beet rhizospheres investigated by comparative transcriptomics. Genome Biol. 2011, 12, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauchline, T.H.; Fowler, J.E.; East, A.K.; Sartor, A.L.; Hosie, A.H.F.; Poole, P.S.; Finan, T.M. Mapping the Sinorhizobium meliloti 1021 solute-binding protein-dependent transportome. Proc. Natl. Acad. Sci. USA 2006, 103, 17933–17938. [Google Scholar] [CrossRef] [Green Version]
- Fry, J.; Wood, M.; Poole, P.S. Investigation of myo-inositol catabolism in Rhizobium leguminosarum bv. viciae and its effect on nodulation competitiveness. Mol. Plant-Microbe Interact. 2001, 14, 1016–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.; Yip, C.B.; Geddes, B.A.; Oresnik, I.J.; Hynes, M.F. Glycerol utilization by Rhizobium leguminosarum requires an ABC transporter and affects competition for nodulation. Microbiology 2012, 158, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinde, E.M.; Hynes, M.F.; Yost, C.K. Homoserine catabolism by Rhizobium leguinosarum bv. viciae 3841 requires a plasmid-borne gene cluster that also affects competitiveness for nodulation. Environ. Microbiol. 2014, 16, 205–217. [Google Scholar] [PubMed]
- Yost, C.K.; Rath, A.M.; Noel, T.C.; Hynes, M.F. Characterization of genes involved in erythritol catabolism in Rhizobium leguminosarum bv. viciae. Microbiology 2006, 152, 2061–2074. [Google Scholar] [CrossRef]
- Oresnik, I.J.; Pacarynuk, L.A.; O’Brien, S.A.P.; Yost, C.K.; Hynes, M.F. Plasmid encoded catabolic genes in Rhizobium leguminosarum bv. trifolii: Evidence for a plant-inducible rhamnose locus involved in competition for nodulation. Mol. Plant-Microbe Intract. 1998, 11, 1175–1185. [Google Scholar]
- Gordon, D.M.; Ryder, M.H.; Heinrich, K.; Murphy, P.J. An experimental test of the rhizopine concept in Rhizobium meliloti. Appl. Environ. Microbiol. 1996, 62, 3991–3996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, G.; Krishnan, A.H.; Kim, Y.W.; Wacek, T.J.; Krishnan, H.B. A functional myo-inositol dehydrogenase gene is required for efficient nitrogen fixation and competitivenes of Sinorhizobium fredii USDA191 to nodulate soybean (Glycine max [l] Merr.). J. Bacteriol. 2001, 183, 2595–2604. [Google Scholar] [CrossRef] [Green Version]
- Kohler, P.R.A.; Zheng, J.Y.; Schoffers, E.; Rossbach, S. Inositol catabolism, a key pathway in Sinorhizobium meliloti for competitive host nodulation. Appl. Environ. Microbiol. 2010, 76, 7972–7980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, M.; Darvill, A.G.; Fry, S.C.; Albersheim, P. Structure and function of the primary cell walls of plants. Annu. Rev. Biochem. 1984, 53, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Baldani, J.I.; Weaver, R.W.; Hynes, M.F.; Eardly, B.D. Utilization of carbon substrates, electrophoretic enzyme patterns, and symbiotic performance of plasmid-cured rhizobia. Appl. Environ. Microbiol. 1992, 58, 2308–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.S.; Carpena, X.; Switalta, J.; Perez-Luque, R.; Donald, L.J.; Loewen, P.C.; Oresnik, I.J. RhaU of Rhizobium leguminosarum is a rhamnose mutarotase. J. Bacteriol. 2008, 190, 2903–2910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.S.; Hynes, M.F.; Oresnik, I.J. A genetic locus necessary for rhamnose uptake and catabolism in Rhizobium leguminosarum bv. trifolii. J. Bacteriol. 2004, 186, 8433–8442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.S.; Oresnik, I.J. L-rhamnose transport in Rhizobium leguminosarum is dependent upon RhaK, a sugar kinase. J. Bacteriol. 2007, 189, 8437–8446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivers, D.; Oresnik, I.J. RhaK dependant ABC-transport of rhamnose in R. leguminosarum: Genetic separation of kinase and transport activities. J. Bacteriol. 2013, 195, 3424–3432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivers, D.M.; Oresnik, I.J. The sugar kinase that is necessry for the catabolism of rhamnose in Rhizobium leguminosarum directly interacts with the ABC transporter necessry for rhamnose transport. J. Bacteriol. 2015, 197, 3812–3821. [Google Scholar] [CrossRef] [Green Version]
- Sambrook, J.; Russell, D.W. Molecular Cloning A Laboratory Manual, 3rd ed.; Cold Spring Harbour Laboratory Press: Cold Spring Harbour, NY, USA, 2001. [Google Scholar]
- Beringer, J.E.; Beynon, J.L.; Buchanan-Wollason, A.V.; Johnston, A.W.B. Transfer of the drug resistance transposon Tn5 to Rhizobium. Nature 1978, 276, 633–634. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root-Nodule Bacteria; Blackwell Scientific Publications: Oxford, UK, 1970. [Google Scholar]
- Meade, H.M.; Long, S.R.; Ruvkin, G.B.; Brown, S.E.; Ausubel, F.M.R. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 1982, 149, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–570. [Google Scholar] [CrossRef]
- Finan, T.M.; Kunkel, B.; de Vos, G.F.; Signer, E.R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J. Bacteriol. 1986, 167, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.G.; Gutterson, N. An efficient mobilizable cosmid vector and its use in rapid marker exchange in Pseudomonas fluorescens strain HV37a. Gene 1987, 61, 299–306. [Google Scholar] [CrossRef]
- Jacob, A.I.; Adhamn, S.A.I.; Capstick, D.S.; Clark, S.R.D.; Spence, T.; Charles, T.C. Mutational analysis of the Sinorhizobium meliloti short-chain dehydrogenase/reductase family reveals substantial contribution to symbiosis and catabolic diversity. Mol. Plant-Microbe Interact. 2008, 21, 979–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poysti, N.J.; Oresnik, I.J. Characterization of Sinorhizobium meliloti triose phosphate isomerase genes. J. Bacteriol. 2007, 189, 3445–3451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finan, T.M.; Hartwieg, E.; Lemieux, K.; Bergman, K.; Walker, G.C.; Signer, E.R. General transduction in Rhizobium meliloti. J. Bacteriol. 1984, 159, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Finan, T.M.; Oresnik, I.; Bottacin, A. Mutants of Rhizobium meliloti defective in succinate metabolism. J. Bacteriol. 1988, 170, 3396–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, B.K.; House, B.L.; Mortimer, M.W.; Yurgel, S.N.; Maloney, S.C.; Ward, K.L.; Kahn, M.L. Development of a functional genomics platform for Sinorhizobium meliloti: Construction of an ORFeome. Appl. Environ. Microbiol. 2005, 71, 5858–5864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- House, B.L.; Mortimer, M.W.; Kahn, M.L. New recombination methods for Sinorhizobium meliloti genetics. Appl. Environ. Microbiol. 2004, 70, 2806–2815. [Google Scholar] [CrossRef] [Green Version]
- Geddes, B.A.; Pickering, B.S.; Poysti, N.J.; Yudistira, H.; Collins, H.; Oresnik, I.J. A locus necessary for the transport and catabolism of erythritol in Sinorhizobium meliloti. Microbiology 2010, 156, 2970–2981. [Google Scholar] [CrossRef] [Green Version]
- Poysti, N.J.; Loewen, E.D.; Wang, Z.; Oresnik, I.J. Sinorhizobium meliloti pSymB carries genes necessary for arabinose transport and catabolism. Microbiology 2007, 153, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Oresnik, I.J.; Layzell, D.B. Composition and distribution of adenylates in soybean (Glycine max L.) nodule tissue. Plant Physiol. 1994, 104, 217–225. [Google Scholar] [CrossRef]
- Takagi, Y.; Sawada, H. The metabolism of L-rhamnose in E. coli I. L-rhamnose isomerase. Biochim. Biophys. Acta 1964, 92, 10–17. [Google Scholar]
- Dische, Z.; Borenfreund, E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J. Biol. Chem. 1951, 192, 583–587. [Google Scholar] [CrossRef]
- Anderson, R.L.; Sapico, V.L. D-fructose (D-mannose) kinase. Methods Enzymol. 1975, 42, 39–43. [Google Scholar]
- Miller, J.H. Experiments in Molecular Genetics; Cold Springs Harbor Laboratory: Cold Springs Harbor, NY, USA, 1972. [Google Scholar]
- Oresnik, I.J.; Charles, T.C.; Finan, T.M. Second site mutations specifically suppress the Fix- phenotype of Rhizobium meliloti ndvF mutations on alfalfa: Identification of a conditional ndvF-dependent mucoid colony phenotype. Genetics 1994, 136, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Geddes, B.A.; Oresnik, I.J. Inability to catabolize galactose leads to increased ability to compete for nodule occupancy in Sinorhizobium meliloti. J. Bacteriol. 2012, 194, 5044–5505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, J.P.; Geddes, B.A.; Oresnik, I.J. Succinoglycan production contributes to acidic pH tolerance in Sinorhizobium meliloti Rm1021. Mol. Plant-Microbe Interact. 2017, 30, 1009–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geddes, B.A.; Gonzalez, J.E.; Oresnik, I.J. Exopolysaccharide production in response to medium acidification is correlated with an increase in competition for nodule occupancy. Mol. Plant-Microbe Interact. 2014, 27, 1307–1317. [Google Scholar] [CrossRef] [Green Version]
- Power, J. The L-rhamnose genetic system of E. coli K-12. Genetics 1967, 55, 557–568. [Google Scholar] [CrossRef]
- Takagi, Y.; Sawada, H. The metabolism of L-rhamnose in E. coli II. L-rhamnulose kinase. Biochim. Biophys. Acta 1964, 64, 18–25. [Google Scholar]
- Sawada, H.; Takagi, Y. The metabolism of L-rhamnose in E. coli III. L rhamnulose-phosphate aldolase. Biochim. Biophys. Acta 1964, 64, 26–32. [Google Scholar]
- Rodinova, I.A.; Li, X.; Thiel, V.; Stolyar, S.; Stanton, K.; Fredrickson, J.K.; Bryant, D.A.; Osterman, A.L.; Best, A.A.; Rodionov, D.A. Comparative genomics and functional analysis of rhamnose catabolic pathways and regulons in bacteria. Front. Microbiol. 2013, 4, 407. [Google Scholar] [CrossRef] [Green Version]
- Hirooka, K.; Kodoi, Y.; Satomura, T.; Fujita, Y. Regulation of the rhaEWRBMA operon involved in l-rhamnose catabolism through two transcritional factors, RhaR and CcpA, in Bacillus subtilus. J. Bacteriol. 2015, 198, 830–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheatley, R.W.; Lo, S.; Jancewica, L.J.; Dugdale, M.L.; Huber, R.E. Structural explanation for allolactose (lac operon inducer) synthesis by lacZ Beta-galactosidase and the evolutinary relationship between allolactose synthesis and the lac repressor. J. Biol. Chem. 2013, 288, 2993–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheatley, R.W.; Ford, B.L.; Li, L.; Aroney, S.T.N.; Knights, H.E.; Ledermann, R.; East, A.K.; Ramachandran, V.; Poole, P.S. Lifestyle adaptations of Rhizobium from rhizosphere to symbiosis. Proc. Natl. Acad. Sci. USA 2020, 117, 23823–23834. [Google Scholar] [CrossRef]
- Knee, E.M.; Gong, F.; Gao, M.; Teplitski, M.; Jones, A.R.; Foxworthy, A.; Mort, A.J.; Bauer, W.D. Root mucilage from pea and its utilization by rhizosphere bacteria as a sole carbon source. Mol. Plant-Microbe Interact. 2001, 14, 775–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
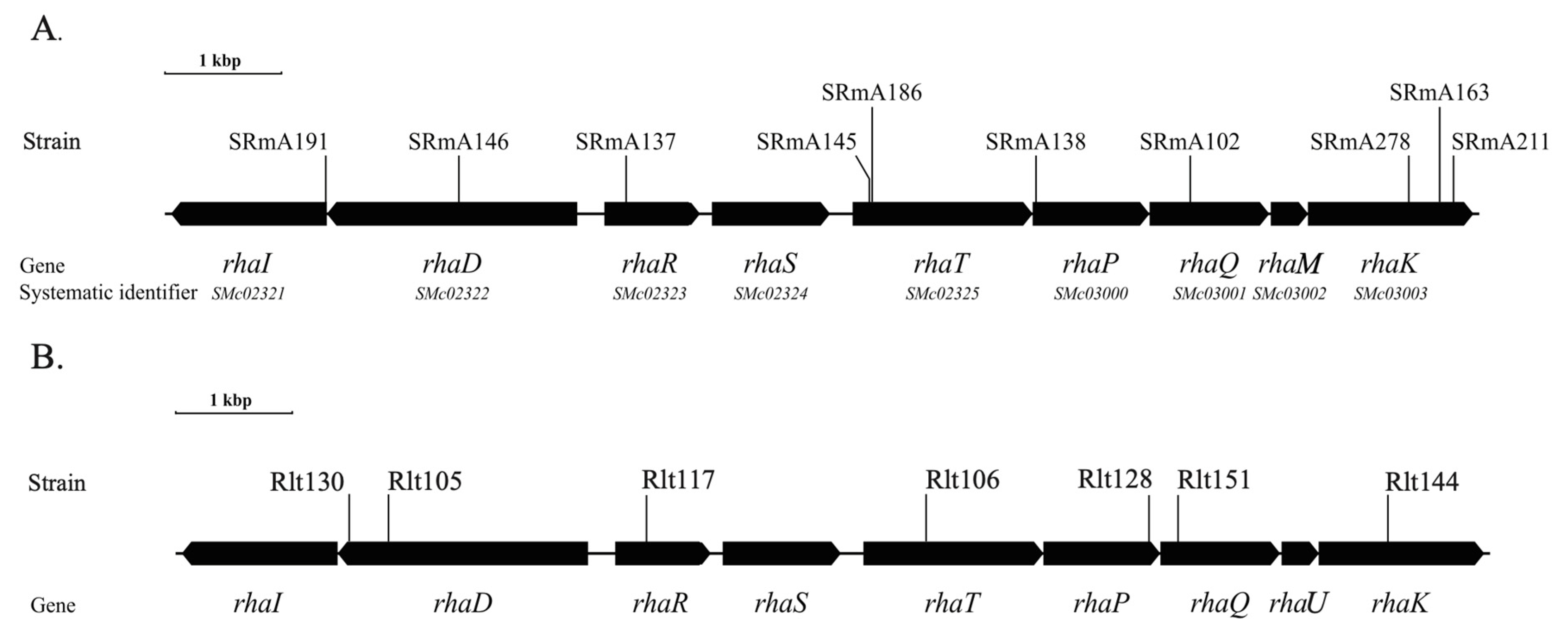
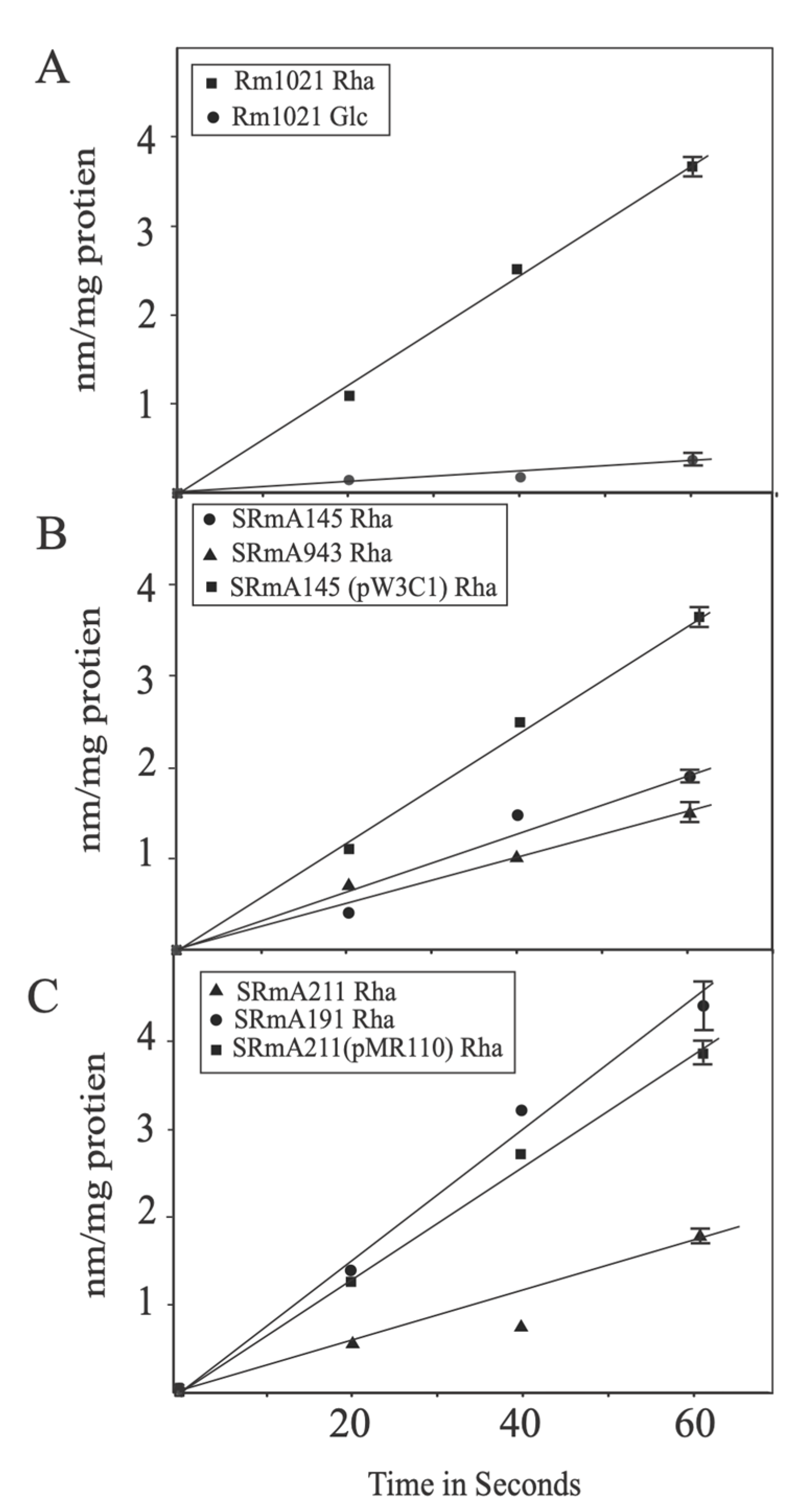
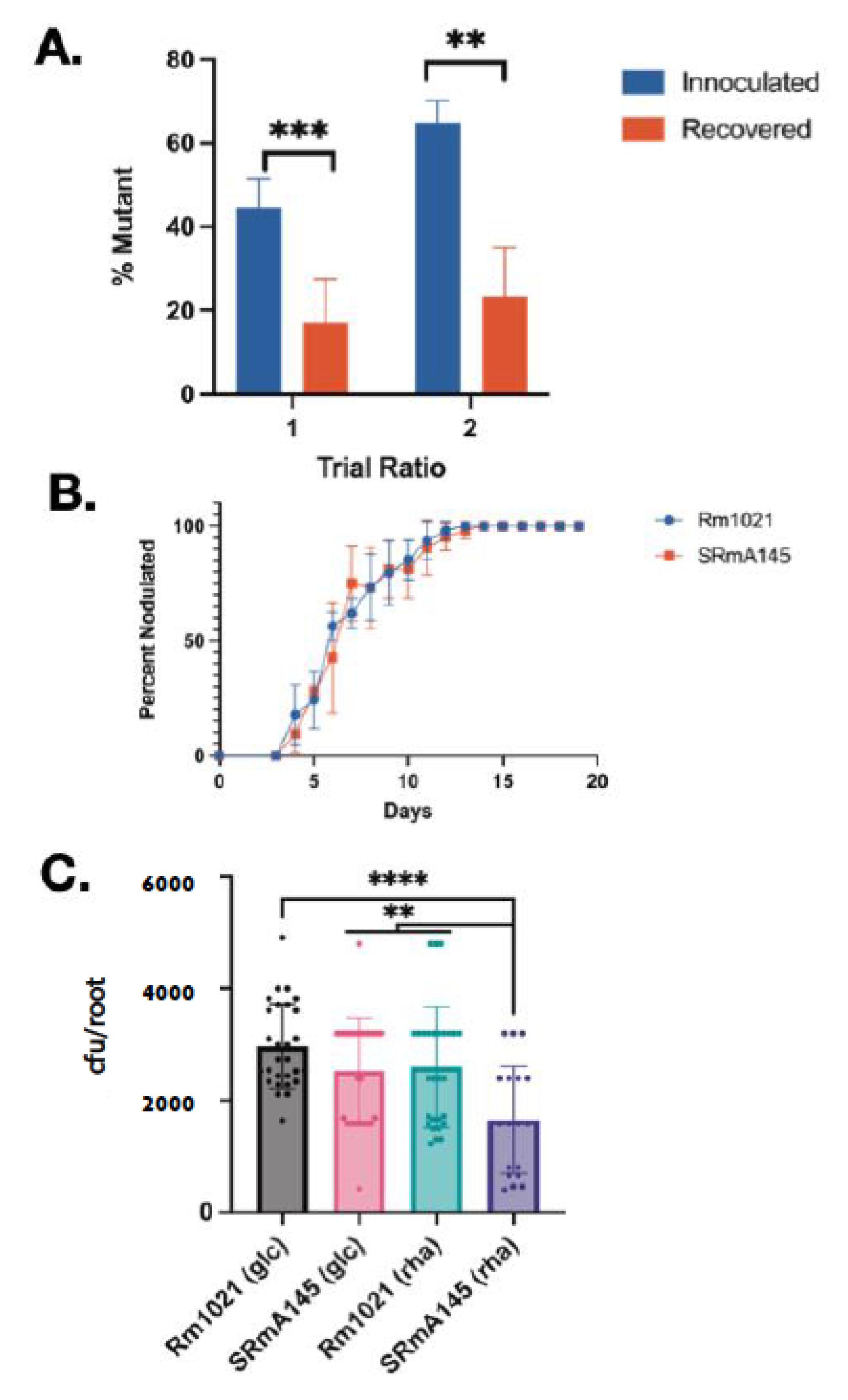
| Stain or Plasmid | Genotype or Phenotype | Reference or Source |
|---|---|---|
| Strains | ||
| S. meliloti | ||
| Rm1021 | SU47 str-21; Smr | [29] |
| SRmA102 | Rm1021, rhaQ::Tn5, (Nmr) | (This work) |
| SrmA137 | Rm1021, rhaR::Tn5, (Nmr) | (This work) |
| SrmA138 | Rm1021, rhaP::Tn5, (Nmr) | (This work) |
| SrmA145 | Rm1021, rhaT::Tn5, (Nmr) | (This work) |
| SrmA146 | Rm1021, rhaD::Tn5, (Nmr) | (This work) |
| SrmA163 | Rm1021, rhaK::Tn5, (Nmr) | (This work) |
| SrmA186 | Rm1021, rhaT::Tn5, (Nmr) | (This work) |
| SrmA191 | Rm1021, rhaI::Tn5, (Nmr) | (This work) |
| SrmA211 | Rm1021, rhaK::Tn5, (Nmr) | (This work) |
| SrmA278 | Rm1021, rhaK::Tn5, (Nmr) | (This work) |
| SrmA943 | Rm1021, ΔrhaP | (This work) |
| R. leguminosarum | ||
| Rlt100 | W14-2, wild-type (Smr) | [15] |
| Rlt105 | Rlt100 rhaD1::Tn5-B20, (Nmr) | [15] |
| Rlt106 | Rlt100 rhaT2::Tn5-B20, (Nmr) | [15] |
| Rlt117 | Rlt100 rhaR25::Tn5-B20, (Nmr) | [22] |
| Rlt128 | Rlt100 rhaP36::Tn5-B20, (Nmr) | [22] |
| Rlt130 | Rlt100 rhaI39::Tn5-B20, (Nmr) | [22] |
| Rlt144 | Rlt100 rhaK50::Tn5-B20, (Nmr) | [22] |
| Rlt151 | Rlt100 rhaQ38::Tn5-B20, (Nmr) | [22] |
| E. coli | ||
| DH5α | endA hsdR17 supE44 thi-1 recA1 gyrA96 relA1 (argF-lacZYA) U169 80 dlacZ M15 | [30] |
| MT616 | MT607 (pRK600) | [31] |
| Plasmids | ||
| pRK7813 | Broad host range vector, Tcr | [32] |
| pCO37 | pRK7813 containing attB sites; Gateway-compatible destination vector | [33] |
| pRK600 | pRK2013 npt::Tn9, Cmr | [31] |
| pW3A | R. leguminosarum rhamnose locus in pRK7813 | [15] |
| pW3C1 | R. leguminosarum rhamnose locus in pRK7813 | [15] |
| pW3AR1 | pW3A1, rhaD1::Tn5-B20 | [15] |
| pW3AR2 | pW3A1, rhaT2::Tn5-B20 | [15] |
| pMR84 | pW3C1, rhaP36::Tn5-B20 | [22] |
| pMR110 | R. leguminosarum rhaK+ in pRK7813 | [23] |
| pMR53 | R. leguminosarum rhaR+ in pRK7813 | [22] |
| pDR32 | S. meliloti rhaI+ in pCO37 | (This Work) |
| pDR35 | S. meliloti rhaK+ in pCO37 | (This Work) |
| pDR190 | S. meliloti rhaR+ in pCO37 | (This Work) |
| Strain. | Relevant Characteristics Chromosomal (Plasmid) | Glyc | Rham | Rham/Glyc |
|---|---|---|---|---|
| Rlt100 | wild-type | + | + | + |
| Rlt144 | rhaK | + | − | + |
| Rlt105 | rhaDI | + | − | − |
| Rm1021 | wild-type | + | + | + |
| SRmA211 | rhaK | + | − | + |
| SRmA146 | rhaDI | + | − | + |
| SRmA191 | rhaI | + | − | + |
| SRmA191 (pDR32) | rhaI (rhaI+) | + | + | + |
| SRmA146 (pDR32) | rhaDI (rhaI+) | + | − | ± |
| Strain | Relevant Characteristics | pDR32 (rhaISm) a | pDR35 (rhaKSm) | pMR110 (rhaKRl) |
|---|---|---|---|---|
| Rlt100 | R. leguminosarum, wild-type | + | + | + |
| Rlt144 | R. leguminosarum, rhaK− | − | + | + |
| Rlt130 | R. leguminosarum, rhaI− | + | − | − |
| Rm1021 | S. meliloti, wild-type | + | + | + |
| SRmA211 | S. meliloti, rhaK− | − | + | + |
| SRmA191 | S. meliloti, rhaI− | + | − | − |
| Kinase Activity a | Isomerase Activity b | ||||
|---|---|---|---|---|---|
| Strain | Relevant Characteristics | Glc | Rha c | Glc | Rha c |
| Rlt100 | R. leguminosarum, wild-type | 23 ± 3 | 211 ± 3 | 48 ± 4 | 180 ± 6 |
| Rlt144 | Rlt100, rhaK50 | 44 ± 6 | 50 ± 27 | e | |
| Rlt144 (pMR110) | Rlt100, rhaK50 (rhaK+Rl) | 640 ± 40 d | |||
| Rlt144 (pDR35) | Rlt100, rhaK50 (rhaK+Sm) | 50 ± 15 d | |||
| Rlt130 | Rlt100, rhaI | ND f | 43 ± 9 | ||
| Rlt130 (pDR32) | Rlt100, rhaI (rhaI+Sm) | 269 ± 8 d | |||
| Rm1021 | S. meliloti, wild-type | 18 ± 9 | 19 ± 6 | 65 ± 7 | 227 ± 32 |
| SRmA211 | Rm1021, rhaK | 13 ± 3 | 19 ± 3 | ||
| SRmA211 (pMR110) | Rm1021, rhaK (rhaK+Rl) | 383 ± 18 d | |||
| SRmA211 (pDR35) | Rm1021, rhaK (rhaK+Sm) | 33 ± 8 d | |||
| SRmA191 | Rm1021, rhaI12 | ND | 12 ± 1 | ||
| SRmA191(pDR32) | Rm1021, rhaI12 (rhaI+Sm) | ND | 152 ± 31 | ||
| Strain | Relevant Characteristics | Glc | Rha | Induction a |
|---|---|---|---|---|
| Rlt100 | R. leguminosarum, wild-type | 30 ± 8 | 77 ± 12 | - |
| Rm1021 | S. meliloti, wild-type | 7 ± 3 | 11 ± 4 | - |
| Rlt128 | Rlt100 rhaP36::Tn5-B20 | 74 ± 18 | 1336 ± 16 | 18.1 |
| Rlt151 | Rlt100 rhaQ38::Tn5-B20 | 46 ± 4 | 880 ± 94 | 19.1 |
| SRmA137 | Rm1021 rhaR::Tn5 | 6 ± 2 | 9 ± 4 | - |
| Rlt100 (pMR84) | Rlt100 (rhaP36::Tn5-B20) | 54 ± 3 | 362 ± 20 | 6.7 |
| Rm1021 (pMR84) | Rm1021 (rhaP36::Tn5-B20) | 122 ± 20 | 217 ± 20 | 1.8 |
| Rlt100 (pW3CR1) | Rlt100 (rhaD1::Tn5-B20) | 153 ± 10 | 832 ± 27 | 5.4 |
| Rm1021 (pW3CR1) | Rm1021 (rhaD1::Tn5-B20) | 207 ± 18 | 208 ± 34 | 1.0 |
| SRmA137 (pW3CR1) | SrmA137 (rhaD1::Tn5-B20) | 602 ± 31 | 1934 ± 18 | 3.2 |
| Rlt128(pMR53) | Rlt128 (rhaRRl) | 75 ± 5 | 1591 ± 19 | 21.2 |
| Rlt128(pDR190) | Rlt128 (rhaRSm) | 74± 5 | 202 ± 36 | 2.7 |
| Rlt151(pMR53) | Rlt151(rhaRRl) | 56 ± 3 | 795 ± 109 | 14.2 |
| Rlt51(pDR190) | Rlt151(rhaRSm) | 63 ± 2 | 192 ± 12 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivers, D.M.R.; Kim, D.D.; Oresnik, I.J. Inability to Catabolize Rhamnose by Sinorhizobium meliloti Rm1021 Affects Competition for Nodule Occupancy. Microorganisms 2022, 10, 732. https://doi.org/10.3390/microorganisms10040732
Rivers DMR, Kim DD, Oresnik IJ. Inability to Catabolize Rhamnose by Sinorhizobium meliloti Rm1021 Affects Competition for Nodule Occupancy. Microorganisms. 2022; 10(4):732. https://doi.org/10.3390/microorganisms10040732
Chicago/Turabian StyleRivers, Damien M. R., Derek D. Kim, and Ivan J. Oresnik. 2022. "Inability to Catabolize Rhamnose by Sinorhizobium meliloti Rm1021 Affects Competition for Nodule Occupancy" Microorganisms 10, no. 4: 732. https://doi.org/10.3390/microorganisms10040732





