Applications of Essential Oils as Antibacterial Agents in Minimally Processed Fruits and Vegetables—A Review
Abstract
:1. Introduction
1.1. Minimally Processed Fruits and Vegetables
1.2. Major Pathogens Related to Foodborne Diseases in MP Foods
1.3. Current Decontamination Methodologies for MP Foods and Related Problems
Current Decontamination Methodologies for MP Foods and Related Problems
1.4. Natural Alternatives for Decontamination of MP Fruits and Vegetables
2. Essential Oils as Alternative Food Disinfectants
2.1. Composition of Essential Oils
Secondary Effects Induced by EO Components
| Common Name | Scientific Name | Major Constituent | 2nd Constituent | 3rd Constituent | 4th Constituent | 5th Constituent | References |
|---|---|---|---|---|---|---|---|
| Amaryllidaceae | |||||||
| Garlic | Allium sativum | Diallyl disulfide | Allyl methyl trisulfide | Diallyl trisulfide | Diallyl sulfide | Allyl methyl disulfide | [98] |
| Onion | Allium cepa | Dipropyl disulfide | Dipropyl trisulfide | Propenyl propyl disulfide | Methyl propyl trisulfide | Allyl propyl trisulfide | [99] |
| Asteraceae | |||||||
| Chamomile | Matricaria chamomilla | Bisabolol oxide | Camphene | Sabinene | Limonene | Cineole | [100] |
| Cupressaceae | |||||||
| Juniper | Juniperus communis | Pinene | Myrcene | Sabinene | Limonene | Caryophyllene | [101] |
| Lauraceae | |||||||
| Cinnamon | Cinnamomum zeilanicum | Eugenol | α-Himachalene | Bicyclogermacrene | Linalool | Nerolidol | [76] |
| Lamiaceae | |||||||
| Basil | Ocimum basilicum | Linalool | Geraniol | Eugenol | Eucalyptol | Humulene | [102] |
| English Lavender | Lavandula angustifolia | Linalool | Linalyl acetate | Geraniol | Caryophyllene | Lavandulyl acetate | |
| Lavender | Lavandula hybrida | Octyl Acetate | Linalool | Isobornyl acetate | Camphor | α-Himachalene | [76] |
| Lemon Balm | Melissa officinalis | Neral | Nerol | Geranial | Geraniol | Caryophyllene | [103] |
| Marjoram | Origanum majorana | Terpineol | Sabinene | Cymene | Terpinene | Limonene | [104] |
| Oregano | Origanum vulgare | Thymol | Terpinene | Cymene | Carvacrol | Myrcene | [104] |
| Peppermint | Mentha piperita | Menthol | Menthone | Menthyl acetate | α-Himachalene | Eucalyptol | [76] |
| Rosemary | Rosmarinus officinalis | Eucalyptol | Camphor | Pinene | Camphene | α-Terpineol | [76] |
| Sage | Salvia officinalis | Camphor | Thujone | Cineole | Camphene | Borneol | [105] |
| Thyme | Thymus vulgaris | α-Terpinene | Cymene | Thymol | Linalool | Carvacrol | [106] |
| Myrtaceae | |||||||
| Eucalyptus | Corymbia citriodora | Citronelal | 7-Octen-1-ol | Isopulegol | Fenchyl acetate | Eucalyptol | [76] |
| Tea Tree | Melaleuca alternifolia | Terpinenol | γ-Terpinene | Eucalyptol | α-Terpinene | Cymene | [76] |
| Clove Tree | Syzygium aromaticum | Eugenol | α-Humulene | δ-Cadinene | Caryophyllene oxide | Eugenyl acetate | [76] |
| Piperaceae | |||||||
| Black Pepper | Piper nigrum | α-Pinene | β-Phellandrene | Terpinene | Cubebene | Farnesene | [76] |
| Poaceae | |||||||
| Lemon grass | Cymbopogon citratus | Geranial | Neral | Myrcene | Geraniol | Verbenol | [76] |
| Citronella | Cymbopogon nardus | Citronelal | Geraniol | Octenol | Elemol | Citronellyl isobutyrate | [76] |
| Palmarosa | Cymbopogon martini | Geraniol | Geranyl Acetate | Linalool | β-Ocimene | α-Himachalene | [76] |
| Rutaceae | |||||||
| Bergamot | Citrus bergamia | Linalool | Limonene | Linalyl acetate | Terpinene | Pinene | [107] |
| Citron | Citrus medica var. sarcodactylis | Limonene | γ-Terpinene | Terpineol | Bisabolene | Cymene | [108] |
| Grapefruit | Citrus paradisi | Limonene | Myrcene | Pinene | Sabinene | Carvone | [109] |
| Lemon | Citrus lemon | Limonene | Pinene | Linalool | Terpineol | Linalyl acetate | [110] |
| Orange | Citrus sinensis var. dulcis | Limonene | Myrcene | Pinene | Caproaldehyde | Sabinene | [76] |
| Tangerine | Citrus nobilis var. tangerine | Limonene | Linalool | Pinene | Myrcene | Terpineol | [111] |
| Zingiberaceae | |||||||
| Cardamom | Elettaria cardamomum | Terpinyl acetate | Cineole | Sabinene | Terpineol | Limonene | [112] |
| Ginger | Zingiber officinale | Zingiberene | Citronellyl | Phellandrene | Camphene | A-Pinene | [113] |
2.2. Antibacterial Activities of EOs in Food Safety
3. Challenges of the Application of EOs in MP Foods: Are They as Good as They Are Claimed to Be?
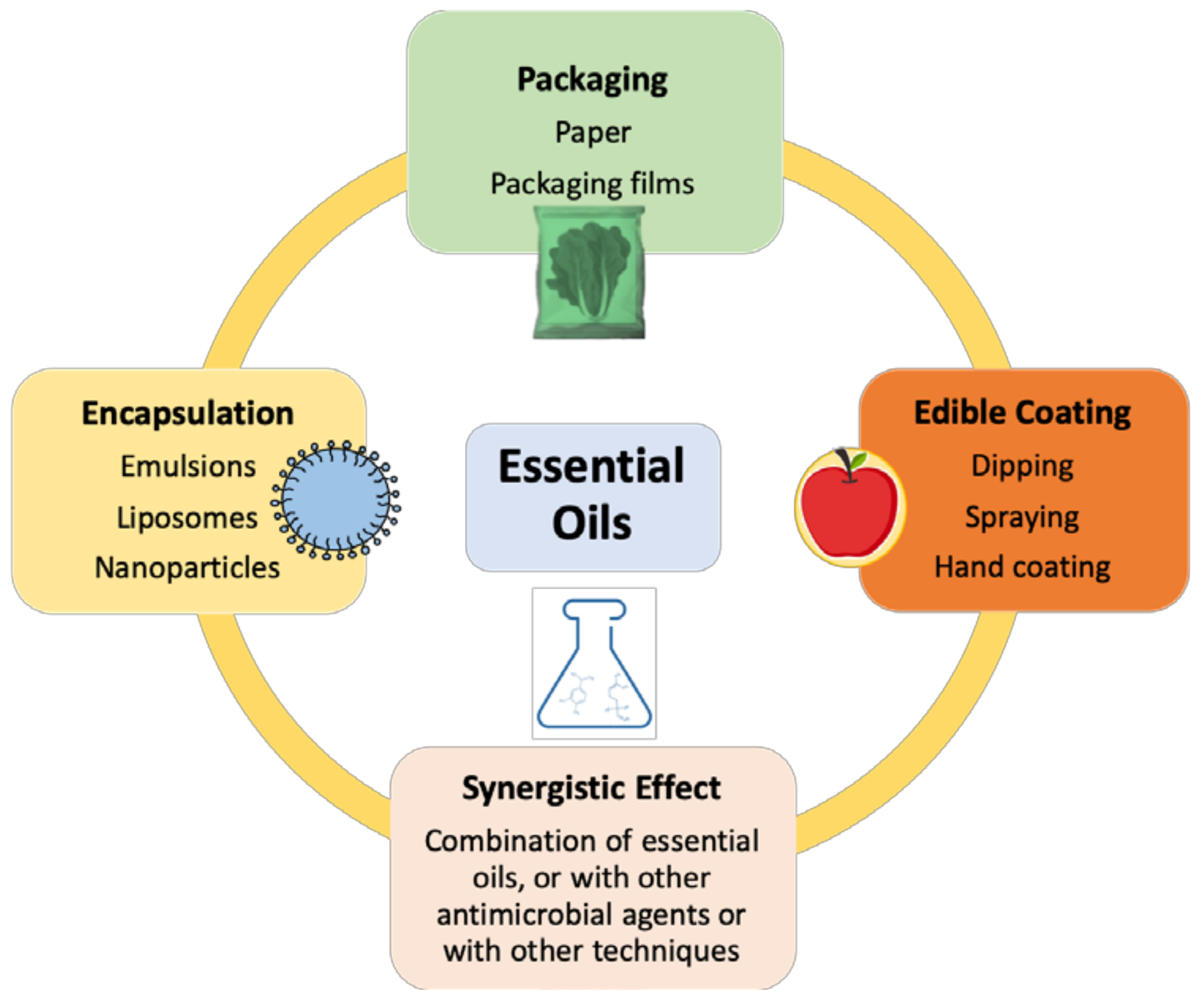
4. Realistic Applications of EOs in MP Foods
5. Conclusions
- (1)
- Be effective at the indicated doses;
- (2)
- Not be toxic, corrosive, or irritating;
- (3)
- Be easy to prepare and apply, at a large scale;
- (4)
- Be cost-effective;
- (5)
- Not negatively affect the product’s organoleptic characteristics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragaert, P.; Verbeke, W.; Devlieghere, F.; Debevere, J. Consumer perception and choice of minimally processed vegetables and packaged fruits. Food Qual. Prefer. 2004, 15, 259–270. [Google Scholar] [CrossRef]
- Buckley, M.; Cowan, C.; McCarthy, M. The convenience food market in Great Britain: Convenience food lifestyle (CFL) segments. Appetite 2007, 49, 600–617. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Oliveira, M.B.P.P. Alimentos frescos minimamente processados embalados em atmosfera modificada. Braz. J. Food Technol. 2012, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K. Sous-Vide Processed Foods: Safety Hazards and Control of Microbial Risks. In Microbial Safety of Minimally Processed Foods, 1st ed.; Novak, J.S., Sapers, G.M., Juneja, V.K., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 97–124. [Google Scholar]
- Gómez-López, V.; Ragert, P.; Debevere, J.; Dvlieghere, F. Descontaminantion methods to prolong the shelf-life of minimally processed vegetables, State-of-the-art. Crit. Rev. Food Sci. Nutr. 2008, 48, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Cenci, S.A. Processamento Mínimo de Frutas e Hortaliças. In Processamento Mínimo de Frutas e Hortaliças: Tecnologia, Qualidade e Sistemas de Embalagem, 1st ed.; Cenci, S.A., Ed.; Embrapa Agroindústria de Alimentos: Rio de Janeiro, Brasil, 2011; pp. 9–17. [Google Scholar]
- Mieszczakowska-Frąc, M.; Celejewska, K.; Płocharski, W. Impact of Innovative Technologies on the Content of Vitamin C and Its Bioavailability from Processed Fruit and Vegetable Products. Antioxidants 2021, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Minter, A.M.; Foley, D.M. Electron Beam and Gamma Irradiation Effectively reduce Listeria monocytogenes populations on chopped romaine lettuce. J. Food Prot. 2006, 69, 570–574. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Garner, E.; Martin-Belloso, O. The Fresh-Cut Fruit and Vegetables Industry Current Situations and Market Trends. In Advances in Fresh-Cut Fruits and Vegetables Processing, 1st ed.; Martin-Belloso, O., Soliva-Fortuny, R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–12. [Google Scholar]
- Martinez-Sanchez, A.; Allende, A.; Bennett, R.N.; Ferreres, F.; Gil, M.I. Microbial, Nutritional and Sensory Quality of Rocket Leaves as Affected by Different Sanitizers. Postharvest Biol. Technol. 2006, 42, 86–97. [Google Scholar] [CrossRef]
- Holden, N.; Pritchard, L.; Toth, I. Colonization outwith the colon: Plants as an alternative environmental reservoir for human pathogenic enterobacteria. FEMS Microbiol. Rev. 2009, 33, 689–703. [Google Scholar] [CrossRef]
- Karagözlü, N.; Ergönül, B.; Özcan, C. Determination of antimicrobial effect of mint and basil essential oils on survival of E. coli O157:H7 and S. typhimurium in fresh-cut lettuce and purslane. Food Control 2011, 22, 1851–1855. [Google Scholar] [CrossRef]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated With Fresh Produce From 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [Green Version]
- Hadjilouka, A.; Tsaltas, D. Cyclospora Cayetanensis—Major Outbreaks from Ready to Eat Fresh Fruits and Vegetables. Foods 2020, 9, 1703. [Google Scholar] [CrossRef]
- Brandl, M.T. Fitness of human enteric pathogens on plants and implications for food safety. Annu. Rev. Phytopathol. 2006, 44, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, J.M.; Ravishankar, S. Safer salads: Contaminated fruits and vegetables are more common than ever. why? and what can consumers do to protect themselves? Am. Sci. 2007, 95, 494–501. [Google Scholar] [CrossRef]
- Sagong, H.; Lee, S.; Chang, P.; Heu, S.; Ryu, S.; Choi, Y.; Kang, D.-H. Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. Int. J. Food Microbiol. 2011, 145, 287–292. [Google Scholar] [CrossRef]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef]
- Macieira, A.; Joana Barbosa, J.; Teixeira, P. Food Safety in Local Farming of Fruits and Vegetables. Int. J. Environ. Res. Public Health 2021, 18, 9733. [Google Scholar] [CrossRef]
- Heard, G.M. Microbiology of Fresh-Cut Produce. In Fresh-Cut Fruits and Vegetables: Science, Technology, and Market, 1st ed.; Lamikanra, O., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 187–248. [Google Scholar]
- Francis, G.A.; Gallone, A.; Nychas, G.J.; Sofos, J.N.; Colelli, G.; Amodio, M.L.; Spano, G. Factors affecting quality and safety of fresh-cut produce. Crit. Rev. Food Sci. Nutr. 2012, 52, 595–610. [Google Scholar] [CrossRef]
- Jung, Y.; Jang, H.; Matthews, K.R. Effect of the food production chain from farm practices to vegetable processing on outbreak incidence. Microb. Biotechnol. 2014, 7, 517–527. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Diana, I.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of bio-control agents to improve the safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Tech. 2015, 46, 302–310. [Google Scholar] [CrossRef]
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Frank, C.; Werber, D.; Cramer, J.P.; Askar, M.; Faber, M.; der Heiden, M.; Bernard, H.; Fruth, A.; Prager, R.; Spode, A.; et al. Epidemic profile of shiga-toxin–producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Outbreaks of E. coli O104:H4 Infection: Update 30. 2011. Available online: http://www.euro.who.int/en/health-topics/emergencies/international-health-regulations/news/news/2011/07/outbreaks-of-e.-coli-o104h4-infection-update-30 (accessed on 5 January 2022).
- King, L.A.; Nogareda, F.; Weill, F.X.; Mariani-Kurkdjian, P.; Loukiadis, E.; Gault, G.; Jourdan-DaSilva, N.; Bingen, E.; Macé, M.; Thevenot, D.; et al. Outbreak of Shiga toxin-producing Escherichia coli O104:H4 associated with organic fenugreek sprouts, France, June 2011. Clin. Infect. Dis. 2012, 54, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Gossner, C.M.; de Jong, B.; Hoebe, C.J.; Coulombier, D. Event-based surveillance of food- and waterborne diseases in Europe: Urgent inquiries (outbreak alerts) during 2008 to 2013. Eurosurveillance 2015, 20, 2116. [Google Scholar] [CrossRef] [Green Version]
- Erickson, M.C. Internalization of fresh produce by foodborne pathogens. Annu. Rev. Food Sci. Technol. 2012, 3, 283–310. [Google Scholar] [CrossRef]
- Banach, J.L.; Van Der Fels-Klerx, H.J. Microbiological Reduction Strategies of Irrigation Water for Fresh Produce. J. Food Prot. 2020, 83, 1072–1087. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef]
- Raffo, A.; Paoletti, F. Fresh-Cut Vegetables Processing: Environmental Sustainability and Food Safety Issues in a Comprehensive Perspective. Front. Sustain. Food Syst. 2022, 5, 681459. [Google Scholar] [CrossRef]
- Nguyen-the, C.; Carlin, F. Fresh and Processed Vegetables. In The Microbiological Safety and Quality of Food; Lund, B.M., Baird-Parker, T.C., Gould, G.W., Eds.; Aspen Publication: Gathersburg, MD, USA, 2000; Volume 1, pp. 622–684. [Google Scholar]
- Rico, D.; Martín-Diana, A.; Barat, J.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Meireles, A.; Giaouris, E.; Simões, M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res. Int. 2016, 82, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.I.S.; Fradinho, P.; Martins, S.; Lima, A.I.G.; Ferreira, R.M.S.B.; Pedroso, L.; Ferreira, M.A.S.S.; Sousa, I. A Novel Way for Whey: Cheese Whey Fermentation Produces an Effective and Environmentally-Safe Alternative to Chlorine. Appl. Sci. 2019, 9, 2800. [Google Scholar] [CrossRef] [Green Version]
- Cardador, M.J.; Gallego, M. Effect of the chlorinated washing of minimally processed vegetables on the generation of haloacetic acids. J. Agric. Food Chem. 2012, 60, 7326–7332. [Google Scholar] [CrossRef]
- Wu, V.C.H.; Kim, B. Effect of a simple chlorine dioxide method for controlling five foodborne pathogens, yeasts and molds on blueberries. Food Microbiol. 2007, 24, 794–800. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Adler, B.B.; Lang, M.M. Efficacy of chlorine and a peroxyacetic acid sanitizer in killing Listeria monocytogenes on iceberg and romaine lettuce using simulated commercial processing conditions. J. Food Prot. 2004, 67, 1238–1242. [Google Scholar] [CrossRef]
- Bari, M.L.; Ukuku, D.O.; Kawasaki, R.; Inatsu, Y.; Isshiki, K.; Kawamoto, S. Combined efficacy of nisin and pediocin with sodium lactate, citric acid, phytic acid, and potassium sorbate and EDTA in reducing the Listeria monocytogenes population of inoculated fresh-cut produce. J. Food Prot. 2005, 68, 1381–1387. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT-Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, L.; Phelan, V.H.; Doyle, M.P. Efficacy of antimicrobial agents in lettuce leaf processing water for control of Escherichia coli O157:H7. J. Food Prot. 2009, 72, 1392–1397. [Google Scholar] [CrossRef]
- Lin, C.M.; Moon, S.S.; Doyle, M.P.; McWatters, K.H. Inactivation of E. coli O157:H7, Salmonella enterica serotype Enteritidis, and Listeria monocytogenes on lettuce by hydrogen peroxide and lactic acid and by hydrogen peroxide with mild heat. J. Food Prot. 2002, 65, 1215–1220. [Google Scholar] [CrossRef]
- Samadi, N.; Abadian, N.; Bakhtiari, D.; Fazeli, M.R.; Jamalifar, H. Efficacy of detergents and fresh produce disinfectants against microorganisms associated with mixed raw vegetables. J. Food Prot. 2009, 72, 1486–1490. [Google Scholar] [CrossRef]
- Gopal, A.; Coventry, J.; Wan, J.; Roginski, H.; Ajlouni, S. Alternative disinfection techniques to extend the shelf-life of minimally processed iceberg lettuce. Food Microbiol. 2010, 27, 210–219. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Ragaert, P.; Ryckeboer, J.; Jeyachchandran, V.; Debevere, J.; Devlieghere, F. Shelf-life of minimally processed cabbage treated with neutral electrolysed oxidising water and stored under equilibrium modified atmosphere. Int. J. Food Microbiol. 2007, 117, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R. Surface Decontamination of Fruits and Vegetables Eaten Raw. In Food Safety Issues, WHO/FSF/FOS/98.2; Food Safety Unit, World Health Organization: Geneva, Switzerland, 1998; Available online: http://apps.who.int/iris/bitstream/10665/64435/1/WHO_FSF_FOS_98.2.pdf?ua=1 (accessed on 15 December 2021).
- Singh, N.; Singh, R.K.; Bhunia, A.K. Stroshine RL. Effect of inoculation and washing methods on the efficacy of different sanitizers against Escherichia coli O157:H7 on lettuce. Food Microbiol. 2002, 19, 183–193. [Google Scholar] [CrossRef]
- Rodgers, S.T.; Cash, J.N.; Siddiq, M.; Ryser, E.T. A comparation of different chemical sanitizers for inactivating Escherichia coli O157:H7 and Listeria monocytogenes in solution and on apples, lettuce, strawberries, and cantaloupe. J. Food Prot. 2004, 67, 721–731. [Google Scholar] [CrossRef]
- Ölmez, H.; Akbas, M.Y. Optimization of ozone treatment of fresh-cut green leaf lettuce. J. Food Eng. 2009, 90, 487–494. [Google Scholar] [CrossRef]
- Ölmez, H. Effect of different sanitizing methods and incubation time and temperature on inactivation of Escherichia coli on lettuce. J. Food Saf. 2010, 30, 288–299. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Heneham, G.T.M.; Frias, J.M.; Barry-Ryan, C. Effect of ozone and calcium lactate treatments on browning and textured properties of fresh cut lettuce. J. Sci. Food Agric. 2006, 86, 2179–2188. [Google Scholar] [CrossRef]
- Birmpa, A.; Sfika, V.; Van-tarakis, A. Ultraviolet light and Ultrasound as non-thermal treatments for the inactivation of microorganisms in fresh ready-to-eat foods. Int. J. Food Microbiol. 2013, 167, 96–102. [Google Scholar] [CrossRef]
- Goodburn, C.; Wallace, C. The microbiological efficacy of decontamination methodologies for fresh produce: A review. Food Control 2013, 32, 418–427. [Google Scholar] [CrossRef]
- Gachkar, L.; Yadegari, D.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007, 102, 898–904. [Google Scholar] [CrossRef]
- Souza, L.I.; Stamford, T.L.M.; Lima, E.O.; Trajano, V.N. Effectiveness of Origanum vulgare L. essential oil to inhibit the growth of food spoiling yeasts. Food Control 2007, 18, 409–413. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Rall, V.L.M.; Fernandes, A.A.H.F.; Ushimaru, P.I.; Probst, I.S.; Junior, F.A. Essential oils against foodborne pathogens and spoilage bacteria in minced meat. Foodborne Pathog. Dis. 2009, 6, 725–728. [Google Scholar] [CrossRef]
- Hayek, S.A.; Gyawali, R.; Ibrahim, S.A. Antimicrobial Natural Products. In Microbial Pathogens and Strategies for Combating Them: Science, Technology, and Education; Mendéz-Villas, A., Ed.; Formatex: Badajoz, Spain, 2013; pp. 910–921. [Google Scholar]
- Gyawalia, R.; Ibrahimb, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Martin-Diana, A.; Rico, D.; Frias, J.; Mulcahy, J. Whey permeate as a bio-preservative for shelf-life maintenance. Innov. Food Sci. Emerg. Technol. 2006, 7, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Rizello, C.G.; Losito, I.; Gobbetti, M.; Carbonara, T.; Bari, M.D.; Zambonin, P.G. Antibacterial activities of peptides from the wares-soluble extracts of Italian cheese varieties. J. Dairy Sci. 2005, 88, 2348–2360. [Google Scholar] [CrossRef] [Green Version]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 2006, 73, 236–244. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Formisano, C.; Mignola, E.; Senatore, F. Chemical composition and antimicrobial activity of the essential oils from three chemotypes of Origanum vulgare L. ssp. hirtum Ietswaart growing wild in Campania (Southern Italy). Molecules 2009, 14, 2735–2746. [Google Scholar] [CrossRef] [Green Version]
- Rožman, T.; Jeršek, B. Antimicrobial activity of rosemary extracts (Rosmarinus officinalis L.) against different species of Listeria. Acta Agric. Slov. 2009, 93, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Ban, X.; Zeng, H.; Huang, B.; He, J.; Wang, Y. In vitro and In Vivo activity of essential oil from dill (Anethum graveolens L.) against fungal spoilage of cherry tomatoes. Food Control 2011, 22, 1992–1999. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic plants as a source of bioactive compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Costa, A.G.; Deschamps, C.; Côcco, L.C.; Scheer, A.P. Desenvolvimento vegetativo, rendimento e composição do óleo essencial do patchouli submetido a diferentes doses de nitrogênio no plantio e manutenção. Biosci. J. 2014, 30, 387–392. [Google Scholar]
- Faleiro, M.L.; Miguel, M.G.; Ladeiro, F.; Venâncio, F.; Tavares, R.; Brito, J.C.; Figueiredo, A.C.; Barroso, J.G.; .Pedro, L.G. Antimicrobial activity of essential oils isolated from Portuguese endemic species of Thymus. Lett. Appl. Microbiol. 2003, 36, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Amiri, A.; Mottaghipisheh, J.; Jamshidi-Kia, F.; Saeidi, K.; Vitalini, S.; Iriti, M. Antimicorbial Potency of Major Functional Foods’ Essential Oils in Liquid and Vapor Phases: A Short Review. Appl. Sci. 2020, 10, 8103. [Google Scholar] [CrossRef]
- Lorenzetti, E.R.; Monteiro, F.P.; Souza, P.E.; Souza, R.J.; Scalice, H.K.; Diogo, R.; Pires, M.S.O. Bioatividade de óleos essenciais no controle de Botrytis cinerea isolado de morangueiro. Ver. Bras. De Plantas Med. 2011, 13, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and antifungal properties of essential oil components. J. Essen. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Sikkema, J.; De Bont, J.A.M.; Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; Von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.; Alberda, M.; Hoekstra, F.A.; Smid, E.J. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 2000, 174, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Turina, A.V.; Nolan, M.V.; Zygadlo, J.A.; Perillo, M.A. Natural terpenes: Self-assembly and membrane partitioning. Biophys. Chem. 2006, 122, 101–113. [Google Scholar] [CrossRef]
- Gustafson, J.E.; Liew, Y.C.; Chew, S.; Markham, J.L.; Bell, H.C.; Wyllie, S.G.; Warmington, J.R. Effects of tea tree oil on Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 194–198. [Google Scholar] [CrossRef]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef]
- Burkey, J.L.; Sauer, J.M.; McQueen, C.A.; Sipes, I.G. Cytotoxicity and genotoxicity of methyleugenol and related congeners—A mechanism of activation for methyleugenol. Mutat. Res. 2000, 453, 25–33. [Google Scholar] [CrossRef]
- Muller, L.; Kasper, P.; Muller-Tegethoff, K.; Petr, T. The genotoxic potential in vitro and in vivo of the allyl benzene etheric oils estragole, basil oil and trans-anethole. Mutat. Res. 1994, 325, 129–136. [Google Scholar] [CrossRef]
- Guba, R. Toxicity myths—Essential oils and their carcinogenic potential. Int. J. Aromather. 2001, 11, 76–83. [Google Scholar] [CrossRef]
- Miller, E.C.; Swanson, A.B.; Phillips, D.H.; Fletcher, T.L.; Liem, A.; Miller, J.A. Structure–activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 1983, 43, 1124–1134. [Google Scholar]
- Liu, C.J.; Chen, C.L.; Chang, K.W.; Chu, C.H.; Liu, T.Y. Safrole in betel quid may be a risk factor for hepatocellular carcinoma: Case report. Can. Med. Ass. J. 2000, 162, 359–360. [Google Scholar]
- Anthony, A.; Caldwell, G.; Hutt, A.G.; Smith, R.L. Metabolism of estragole in rat and mouse and influence of dose size on excretion of the proximate carcinogen 10-hydroxyestragole. Food Chem. Toxicol. 1987, 25, 799–806. [Google Scholar] [CrossRef]
- Averbeck, D.; Averbeck, S.; Dubertret, L.; Young, A.R.; Morlière, P. Genotoxicity of bergapten and bergamot oil in Saccharomyces cerevisiae. J. Photochem. Photobiol. 1990, 7, 209–229. [Google Scholar] [CrossRef]
- Averbeck, D.; Averbeck, S. DNA photodamage, repair, gene induction and genotoxicity following exposures to 254 nm UV and 8-methoxypsoralen plus UVA in a eukaryotic cell system. Photochem. Photobiol. 1998, 68, 289–295. [Google Scholar] [CrossRef]
- Satyal, P.; Craft, J.D.; Dosoky, N.S.; Setzer, W.N. The Chemical Compositions of the Volatile Oils of Garlic (Allium sativum) and Wild Garlic (Allium vineale). Foods 2017, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Mnayer, D.; Fabiano-Tixier, A.S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules 2014, 19, 20034–20053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsen, K. Chemical Composition and Antimicrobial Activity of Essential Oil of Matricaria Recutita. Int. J. Food Prop. 2015, 18, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, R.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka. India. Anc. Sci. Life 2014, 33, 151–156. [Google Scholar] [CrossRef]
- Smigielski, K.; Raj, A.; Krosowiak, K.; Gruska, R. Chemical composition of the essential oil of Lavandula angustifolia cultivated in Poland. J. Essent. Oil-Bear. Plants 2009, 12, 338–347. [Google Scholar] [CrossRef]
- Fokou, J.B.H.; Dongmo, P.M.J.; Boyom, F.F. Essential Oil’s Chemical Composition and Pharmacological Properties. In Essential Oils—Oils of Nature; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Raina, A.P.; Negi, K.S. Essential oil composition of Origanum majorana and Origanum vulgare ssp. hirtum growing in India. Chem. Nat. Compd. 2012, 47, 1015–1017. [Google Scholar] [CrossRef]
- El Euch, S.K.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar]
- Caputo, L.; Cornara, L.; Bazzicalupo, M.; De Francesco, C.; De Feo, V.; Trombetta, D.; Smeriglio, A. Chemical Composition and Biological Activities of Essential Oils from Peels of Three Citrus Species. Molecules 2020, 25, 1890. [Google Scholar] [CrossRef] [Green Version]
- Xing, C.; Qin, C.; Li, X.; Zhang, F.; Linhardt, R.J.; Sun, P.; Zhang, A. Chemical composition and biological activities of essential oil isolated by HS-SPME and UAHD from fruits of bergamot. LWT-Food Sci. Technol. 2019, 104, 38–44. [Google Scholar] [CrossRef]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef] [Green Version]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef] [Green Version]
- Aripin, D.; Julaeha, E.; Dardjan, M.; Cahyanto, A. Chemical composition of Citrus spp. and oral antimicrobial effect of Citrus spp. peels essential oils against Streptococcus mutans. Padjadjaran J. Dent. 2015, 27. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Vellaikumar, S.; Murugan, M.; Dhanya, M.K.; Ariharasutharsan, G.; Aiswarya, S.; Akilan, M.; Warkentin, T.D.; Karthikeyan, A. Essential oil profile diversity in cardamom accessions from southern India. Front. Sustain. Food Syst. 2021, 5, 137. [Google Scholar] [CrossRef]
- Sharma, P.K.; Singh, V.; Ali, M. Chemical composition and antimicrobial activity of fresh rhizome essential oil of Zingiber officinale Roscoe. Pharmacogn. J. 2016, 8, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef] [Green Version]
- Mith, H.; Duré, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Frangos, F.; Pyrgotou, N.; Giatrakou, V.; Ntzimani, A.; Savvaidis, I.N. Combined effects of salting, oregano oil and vacuum-packaging on the shelf-life of refrigerated trout fillets. Food Microbiol. 2010, 27, 115–121. [Google Scholar] [CrossRef]
- Lima, C.F.; Carvalho, F.; Fernandes, E.; Bastos, M.L.; Santos-Gomes, P.C.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Evaluation of toxic/protective effects of the essential oil of Salvia officinalis on freshly isolated rat hepatocytes. Toxicol. Vitr. 2004, 18, 457–465. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskarana, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [Green Version]
- Carson, C.F.; Cookson, B.D.; Farrelly, H.D.; Riley, T.V. Susceptibility of methicillin-resistant Staphylococcus aureus to the essential oil of Melaleuca alternifolia. J. Antimicrob. Chemother. 1995, 35, 421–424. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Canillac, N.; Mourey, A. Antibacterial activity of the essential oil of Picea excelsa on Listeria, Staphylococcus aureus and coliform bacteria. Food Microbiol. 2001, 18, 261–268. [Google Scholar] [CrossRef]
- Misra, R.; Sahoo, S.R. Antibacterial Activity of Doxycycline-Loaded Nanoparticles. In Methods in Enzymology; Düzgüneş, N., Ed.; Academic Press: San Diego, CA, USA, 2012; Volume 509, pp. 61–85. [Google Scholar] [CrossRef]
- Sykes, J.E.; Rankin, S.C. Isolation and Identification of Aerobic and Anaerobic Bacteria. In Canine and Feline Infectious Diseases; Sykes, J.E., Ed.; Saunders: Riverport Lane, MO, USA, 2014; pp. 17–28. [Google Scholar]
- Onawunmi, G.O. Evaluation of the antimicrobial activity of citral. Lett. Appl. Microbiol. 1989, 9, 105–108. [Google Scholar] [CrossRef]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998, 26, 118–122. [Google Scholar] [CrossRef]
- Herman, R.A.; Ayepa, E.; Shittu, S.; Fometu, S.S.; Wang, J. Essential Oils and Their Applications—A Mini Review. Adv. Nutr. Food Sci. 2019, 4, 1–13. [Google Scholar]
- Chávez-González, M.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Essential Oils: A Natural Alternative to Combat Antibiotics Resistance. In Antibiotic Resistance, Mechanisms and New Antimicrobial Approaches; Kon, K., Rai, M., Eds.; Academic Press: London, UK, 2016; pp. 227–337. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.I.S.; Martins, S.; Veríssimo, C.; Nunes, M.J.; Lima, A.I.G.R.; Ferreira, R.M.S.B.; Pedroso, L.; Sousa, I.; Ferreira, M.A.S.S. Essential oils as antibacterial agents against food-borne pathogens: Are they really as useful as they are claimed to be? J. Food Sci. Technol. 2017, 54, 4344–4352. [Google Scholar] [CrossRef]
- Mejlholm, O.; Dalgaard, P. Antimicrobial effect of essential oils on the seafood spoilage micro-organism Photobacterium phosphoreum in liquid media and fish products. Lett. Appl. Microbiol. 2002, 34, 27–31. [Google Scholar] [CrossRef]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial activity of essential oil of Baccharis dracunculifolia DC (Asteraceae) aerial parts at flowering period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Oulkheir, S.; Aghrouch, M.; El Mourabit, F.; Dalha, F.; Graich, H.; Amouch, F.; Ouzaid, K.; Moukale, A.; Chadli, S. Antibacterial activity of essential oils extracts from cinnamon, thyme, clove and geranium against a Gram negative and Gram-positive pathogenic bacteria. J. Med. Plants Res. 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Thosar, N.; Basak, S.; Bahadure, R.N.; Rajurkar, M. Antimicrobial efficacy of five essential oils against oral pathogens: An in vitro study. Eur. J. Dent. 2013, 7, S071–S077. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, H.; Economou, V.; Gousia, P.; Bozidis, P.; Sakkas, V.A.; Petsios, S.; Mpekoulis, G.; Ilia, A.; Papadopoulou, C. Antibacterial efficacy of commercially available essential oils tested against drug-resistant Gram-positive pathogens. Appl. Sci. 2018, 8, 2201. [Google Scholar] [CrossRef] [Green Version]
- Yasin, M.; Younis, A.; Javed, T.; Akram, A.; Ahsan, M.; Shabbir, R.; Ali, M.M.; Tahir, A.; El-Ballat, E.M.; Sheteiwy, M.S.; et al. River Tea Tree Oil: Composition, Antimicrobial and Antioxidant Activities, and Potential Applications in Agriculture. Plants 2021, 10, 2105. [Google Scholar] [CrossRef]
- El Moussaoui, N.; Sanchez, G.; Idaomar, M.; Mansour, A.I.; Abrini, J.; Aznar, R. Antibacterial and antiviral activities of essential oils of Northern Moroccan plants. Biotechnol. J. Int. 2013, 3, 318–331. [Google Scholar] [CrossRef]
- Radaelli, M.; Silva, P.; Weidlich, L.; Hoehneb, L.; Flach, A.; Costa, L.A.M.A.; Ethur, E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Mathlouthi, N.; Bouzaienne, T.; Oueslati, I.; Recoquillay, F.; Hamdi, M.; Urdaci, M.; Bergaoui, R. Use of rosemary, oregano, and a commercial blend of essential oils in broiler chickens: In vitro antimicrobial activities and effects on growth performance. J. Anim. Sci. 2015, 90, 813–823. [Google Scholar] [CrossRef]
- Boskovic, M.; Zdravkovic, N.; Ivanovic, J.; Janjic, J.; Djordjevic, J.; Starcevic, M.; Baltic, M.Z. Antimicrobial activity of thyme (Thymus vulgaris) and oregano (Origanum vulgare) essential oils against some food-borne microorganisms. Procedia Food Sci. 2015, 5, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Miladi, H.; Mili, D.; Slama, R.B.; Zouari, S.; Ammar, E.; Bakhrouf, A. Antibiofilm formation and anti-adhesive property of three mediterranean essential oils against a foodborne pathogen Salmonella strain. Microb. Pathog. 2016, 93, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Tserennadmid, R.; Takó, M.; Galgóczy, L.; Papp, T.; Pesti, M.; Vágvölgyi, C.; Almássy, K.; Krisch, J. Anti-yeast activities of some essential oils in growth medium fruit juices and milk. Int. J. Food Microbiol. 2011, 144, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Pateiro, M.; Rodríguez-Lázaro, D.; Domínguez, R.; Zhong, J.; Lorenzo, J.M. The Role of Essential Oils against Pathogenic Escherichia coli in Food Products. Microorganisms 2020, 8, 924. [Google Scholar] [CrossRef]
- Cava-Roda, R.; Taboada-Rodríguez, A.; Valverde-Franco, M.; Marín-Iniesta, F. Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157:H7 in milk. Food Bioprocess Technol. 2010, 5, 2120–2131. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 2010, 110, 614–619. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Cerrutti, P.; Alzamora, S.M. Inhibitory effects of vanillin on some food spoilage yeasts in laboratory media and fruit purées. Int. J. Food Microbiol. 1996, 29, 379–386. [Google Scholar] [CrossRef]
- Somolinos, M.; García, D.; Condón, S.; Mackey, B.; Pagán, R. Inactivation of Escherichia coli by citral. J. Appl. Microbiol. 2010, 108, 1928–1939. [Google Scholar] [CrossRef]
- Gill, A.O.; Delaquis, P.; Russo, P.; Holley, R.A. Evaluation of antilisterial action of cilantro oil on vacuum packed ham. Int. J. Food Microbiol. 2002, 73, 83–92. [Google Scholar] [CrossRef]
- Azevedo, A.N.; Buarque, P.R.; Cruz, E.M.O.; Blank, A.F.; Alves, P.B.; Nunes, M.L.; Santana, L.C.L.A. Response surface methodology for optimization of edible chitosan coating formulations incorporating essential oil against several foodborne pathogenic bacteria. Food Control 2014, 43, 1–9. [Google Scholar] [CrossRef]
- Pirie, D.G.; Clayson, D.H.F. Some Causes of Unreliability of Essential Oils as Microbial Inhibitors in Foods. In Microbial Inhibitors in Foods; Molin, N., Ed.; Almqvist & Wiksell: Stockholm, Sweden, 1964; pp. 145–150. [Google Scholar]
- Dehkordi, S.S.S.; Rohani, S.M.R.; Tajik, H.; Moradi, M.; Aliakbarlou, J. Antimicrobial effects of lysozyme in combination with Zataria multiflora boiss. Essential oil at different pH and NaCl concentrations on E. coli O157:H7 and Staphylococcus aureus. J. Anim. Vet. Adv. 2008, 7, 1458–1463. [Google Scholar]
- Sahin, F.; Güllüce, M.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M.; Agar, G.; Ozer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. Raspberry fresh fruit quality as affected by pectin and alginate-based edible coatings enriched with essential oils. Sci. Hortic. 2015, 194, 138–146. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The effect of alginate-based edible coatings enriched with essential oils constituents on Arbutus unedo L. fresh fruit storage. Postharvest Biol. Technol. 2015, 100, 226–233. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf-life of strawberries. Postharvest Biol. Technol. 2015, 110, 51–60. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F.; Pined, E.A.G. Antimicrobial, mechanical, and barrier properties of cassava starch-chitosan films incorporated with oregano essential oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef]
- Donsí, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Nguefack, J.; Tamgue, O.; Dongmo, J.B.L.; Dakole, C.D.; Leth, V.; Vismer, H.F.; Amvam Zollo, P.H.; Nkengfack, A.E. Synergistic action between fractions of essential oils from Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris against Penicillium expansum. Food Control 2012, 23, 377–383. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef]
- García-García, R.; López-Malo, A.; Palou, E. Bactericidal action of binary and ternary mixtures of carvacrol, thymol, and eugenol against Listeria innocua. J. Food Sci. 2011, 76, M95–M100. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int. J. Food Microbiol. 2001, 67, 187–195. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus Var. chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [Green Version]
- Valero, D.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. The combination of modified atmosphere packaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol. Technol. 2006, 41, 317–327. [Google Scholar] [CrossRef]
- Guillén, F.; Zapata, P.J.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Improvement of the Overall Quality of Table Grapes Stored under Modified Atmosphere Packaging in Combination with Natural Antimicrobial Compounds. J. Food Sci. 2007, 72, S185–S190. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Martinez-Romero, D.; Castillo, S.; Guillén, F.; Valero, D. The use of natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innov. Food Sci. Emerg. Technol. 2005, 6, 115–123. [Google Scholar] [CrossRef]
- Li, J.; Chang, J.W.; Saenger, M.; Deering, A. Thymol nanoemulsions formed via spontaneous emulsification: Physical and antimicrobial properties. Food Chem. 2017, 232, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Lee, H.; Kim, J.E.; Song, K.B.; Lee, Y.S.; Chung, D.S.; Min, S.C. Plum coatings of lemongrass oil-incorporating carnauba wax-based nanoemulsion. J. Food Sci. 2013, 78, E1551–E1559. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Mafune, M.; Sivakumar, D.; Soundy, P. Thyme oil vapour and modified atmosphere packaging reduce anthracnose incidence and maintain fruit quality in avocado. J. Sci. Food Agric. 2013, 93, 3024–3031. [Google Scholar] [CrossRef]
- Amiri, A.; Ramezanian, A.; Mortazavi, S.M.H.; Hosseini, S.M.H.; Yahia, E. Shelf-life extension of pomegranate arils using chitosan nanoparticles loaded with Satureja hortensis essential oil. J. Sci. Food Agric. 2021, 101, 3778–3786. [Google Scholar] [CrossRef]
- Roller, S.; Seedhar, P. Carvacrol and cinnamic acid inhibit microbial growth in fresh-cut melon and kiwifruit at 4 degrees and 8 degrees C. Lett. Appl. Microbiol. 2002, 35, 390–394. [Google Scholar] [CrossRef]
- Lanciotti, R.; Belletti, N.; Patrignani, F.; Gianotti, A.; Gardini, F.; Guerzoni, M.E. Application of hexanal, E-2-hexenal, and hexyl acetate to improve the safety of fresh-sliced apples. J. Agric. Food Chem. 2003, 51, 2958–2963. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Tapia, M.S.; Martín-Belloso, O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT-Food Sci. Technol. 2008, 41, 139–147. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Tappi, S.; Rocculi, P.; Gardini, F.; Lanciotti, R. Efficacy of natural antimicrobials to prolong the shelf-life of minimally processed apples packaged in modified atmosphere. Food Control 2014, 46, 403–411. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Tappi, S.; Rocculi, P.; Gardini, F.; Lanciotti, R. Potential of natural antimicrobials for the production of minimally processed fresh-cut apples. J. Food Sci. Technol. 2015, 6, 415. [Google Scholar] [CrossRef] [Green Version]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Vieira, A.I.; Guerreiro, A.; Antunes, M.D.; Miguel, M.G.; Faleiro, M.L. Edible Coatings Enriched with Essential Oils on Apples Impair the Survival of Bacterial Pathogens through a Simulated Gastrointestinal System. Foods 2019, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Almela, C.; Castelló, M.L.; Tarrazó, J.; Ortolá, M.D. Washing of cut persimmon with thyme or lemon essential oils. Food Sci. Technol. Int. 2014, 20, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J. Agric. Food Chem. 2004, 52, 6042–6048. [Google Scholar] [CrossRef]
- Belletti, N.; Lanciotti, R.; Patrignani, F.; Gardini, F. Antimicrobial efficacy of citron essential oil on spoilage and pathogenic microorganisms in fruit-based salads. J. Food Sci. 2008, 73, M331–M338. [Google Scholar] [CrossRef] [PubMed]
- De Azeredo, G.A.; Stamford, T.L.M.; Nunes, P.C.; Neto, N.J.G.; De Oliveira, M.E.G.; De Souza, E.L. Combined application of essential oils from Origanum vulgare L. and Rosmarinus officinalis L. to inhibit bacteria and autochthonous microflora associated with minimally processed vegetables. Food Res. 2011, 44, 1541–1548. [Google Scholar] [CrossRef] [Green Version]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tappi, S.; Rocculi, P.; Gardini, F.; Lanciotti, R. Natural antimicrobials to prolong the shelf-life of minimally processed lamb’s lettuce. Postharvest Biol. Technol. 2015, 103, 35–44. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Vernocchi, P.; Del Chierico, F.; Russo, A.; Torriani, S.; Putignani, L.; Gardini, F.; Lanciotti, R. Effect of thyme essential oil and Lactococcus lactis CBM21 on the microbiota composition and quality of minimally processed lamb’s lettuce. Food Microbiol. 2017, 68, 61–70. [Google Scholar] [CrossRef]
- Gündüz, G.T.; Gönül, Ş.A.; Karapınar, M. Efficacy of oregano oil in the inactivation of Salmonella typhimurium on lettuce. Food Control 2010, 21, 513–517. [Google Scholar] [CrossRef]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.; Zhang, Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Tzortzakis, N. The Combined and Single Effect of Marjoram Essential Oil, Ascorbic Acid, and Chitosan on Fresh-Cut Lettuce Preservation. Foods 2021, 10, 575. [Google Scholar] [CrossRef]
- Sessa, M.; Ferrari, G.; Donsì, F. Novel edible coating containing essential oil nanoemulsions to prolong the shelf life of vegetable products. Chem. Eng. Trans. 2015, 43, 55–60. [Google Scholar] [CrossRef]
- Awad, A.H.R.; Parmar, A.; Ali, M.R.; El-Mogy, M.M.; Abdelgawad, K.F. Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils. Foods 2021, 10, 1103. [Google Scholar] [CrossRef]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157: H7 and Salmonella Typhimurium on green beans. Food Control 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Severino, R.; Vu, K.D.; Donsì, F.; Salmieri, S.; Ferrari, G.; Lacroix, M. Antibacterial and physical effects of modified chitosan based-coating containing nanoemulsion of mandarin essential oil and three non-thermal treatments against Listeria innocua in green beans. Int. J. Food Microbiol. 2014, 191, 82–88. [Google Scholar] [CrossRef]
- Gniewosz, M.; Kraśniewska, K.; Woreta, M.; Kosakowska, O. Antimicrobial activity of a pullulan-caraway essential oil coating on reduction of food microorganisms and quality in fresh baby carrot. J Food Sci. 2013, 78, M1242–M1248. [Google Scholar] [CrossRef]
- Donsì, F.; Cuomo, A.; Marchese, E.; Ferrari, G. Infusion of essential oils for food stabilization: Unraveling the role of nanoemulsion-based delivery systems on mass transfer and antimicrobial activity. Innov. Food Sci. Emerg. Technol. 2014, 22, 212–220. [Google Scholar] [CrossRef]
- Ruengvisesh, S.; Loquercio, A.; Castell-Perez, E.; Taylor, T.M. Inhibition of Bacterial Pathogens in Medium and on Spinach Leaf Surfaces using Plant-Derived Antimicrobials Loaded in Surfactant Micelles. J Food Sci. 2015, 80, M2522–M2529. [Google Scholar] [CrossRef]
- Taştan, Ö.; Pataro, G.; Donsì, F.; Ferrari, G.; Baysal, T. Decontamination of fresh-cut cucumber slices by a combination of a modified chitosan coating containing carvacrol nanoemulsions and pulsed light. Int. J. Food Microbiol. 2017, 260, 75–80. [Google Scholar] [CrossRef]
- Jovanovic, G.D.; Klaus, A.S.P.; Niksic, M. Antimicrobial Activity of Chitosan Films with Essential Oils Against Listeria monocytogenes on Cabbage. Jundishapur J. Microbiol. 2016, 9, e34804. [Google Scholar] [CrossRef] [Green Version]
- Severino, R.; Vu, K.D.; Donsì, F.; Salmieri, S.; Ferrari, G.; Lacroix, M. Antimicrobial effects of different combined non-thermal treatments against Listeria monocytogenes in broccoli florets. J. Food Eng. 2014, 124, 1–10. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control 2013, 30, 137–143. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Nychas, G.J. Development and evaluation of a model predicting the survival of Escherichia coli O157:H7 NCTC 12900 in homemade eggplant salad at various temperatures, pHs, and oregano essential oil concentrations. Appl. Environ. Microbiol. 2000, 66, 1646–1653. [Google Scholar] [CrossRef] [Green Version]
- Kraśniewska, K.; Kosakowska, O.; Pobiega, K.; Gniewosz, M. The Influence of Two-Component Mixtures from Spanish Origanum Oil with Spanish Marjoram Oil or Coriander Oil on Antilisterial Activity and Sensory Quality of a Fresh Cut Vegetable Mixture. Foods 2020, 9, 1740. [Google Scholar] [CrossRef]
- Scollard, J.; Francis, G.A.; O’Beirne, D. Some conventional and latent anti-listerial effects of essential oils, herbs, carrot and cabbage in fresh-cut vegetable systems. Postharvest Biol. 2013, 77, 87–93. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Mazzucotelli, C.A.; Moreira, M.R. The impact of biopreservatives and storage temperature in the quality and safety of minimally processed mixed vegetables for soup. J. Sci. Food Agric. 2015, 95, 962–971. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Lara, M.; Gavara, R.; Hernández-Muñoz, P. Development of antimicrobial films for microbiological control of packaged salad. Int. J. Food Microbiol. 2012, 157, 195–201. [Google Scholar] [CrossRef]
- Landry, K.S.; Micheli, S.; McClements, D.J.; McLandsborough, L. Effectiveness of a spontaneous carvacrol nanoemulsion against Salmonella enterica Enteritidis and Escherichia coli O157: H7 on contaminated broccoli and radish seeds. Food Microbiol. 2015, 51, 10–17. [Google Scholar] [CrossRef]


| Terms | Definitions | References |
|---|---|---|
|
Minimal inhibitory concentration | Lowest concentration resulting in maintenance or reduction of inoculum viability of the tested organism. | [122] |
| Lowest concentration inducing a significant decrease in inoculum viability (>90%). | [123] | |
| Lowest concentration inducing a complete inhibition of the tested organism, up to 48 h of incubation. | [124] | |
| Lowest concentration inducing visible growth reduction of the tested organism. | [77] | |
| Lowest concentration reducing visible growth of the tested organism | [125] | |
| Lowest concentration inhibiting visible growth of the tested organism over 18 to 24 h. | [126] | |
|
Minimal bactericidal concentration | Lowest concentration at which no growth is observed after subculture. | [127] |
| Concentration inducing death of 99.9% or more of the initial inoculum. | [123] | |
| Lowest concentration that results in the death of 99.9% of the tested organism. | [125] | |
| Minimum concentration that induces a bactericidal effect, determined by re-culturing broth dilutions that inhibit bacterial growth (i.e., those at or above the MIC). | [126] | |
|
Bacteriostatic concentration | Lowest concentration stopping bacterial growth in broth, but cultured when broth is plated onto agar. | [128] |
|
Bactericidal concentration | Lowest concentration stopping bacterial growth in broth; not cultured when broth is plated onto agar. | [128] |
| Essential Oil | Microbial Strains Tested | MIC | MBC | References |
|---|---|---|---|---|
| Baccharis dracunculifolia | Enterobacter cloacae (clinical isolate) Escherichia coli ATCC 35218 | 6.3 mg/mL | 8.4 mg/mL | [134] |
| Listeria monocytogenes NCTC 7973 Salmonella Typhimurium ATCC 13311 | 12.7 mg/mL | 16.9 mg/mL | ||
| Micrococcus flavus ATCC 10240 | 3.15 mg/mL | 4.2 mg/mL | ||
| Pseudomonas aeruginosa ATCC 27853 | 1.05 mg/mL | 2.1 mg/mL | ||
| Cinnamomum cassia | Listeria monocytogenes NCTC 11994 | 0.5 µL/mL | 0.5 µL/mL | [116] |
| Listeria monocytogenes S0580 Escherichia coli O157:H7 S0575 | 0.3 µL/mL | 0.3 µL/mL | ||
| Salmonella Typhimurium ATCC 14028 Salmonella Typhimurium S0584 | 0.25 µL/mL | 1 µL/mL | ||
| Klebsiella pneumoniae ATCC 10031 | 2.5 mg/mL | 2.5 mg/mL | [135] | |
| Pseudomonas aeruginosa ATCC 27853 | 5 mg/mL | 5 mg/mL | ||
| Cinnamomum verum | Listeria monocytogenes NCTC 11994 Salmonella Typhimurium ATCC 14028 Escherichia coli O157:H7 ATCC 35150 | 0.5 µL/mL | 0.5 µL/mL | [116] |
| Escherichia coli O157:H7 S0575 Listeria monocytogenes S0580 | 0.5 µL/mL | 1 µL/mL | ||
| Eugenia caryophyllus | Listeria monocytogenes NCTC 11994 Listeria monocytogenes S0580 | 1 µL/mL | >1.5 µL/mL | |
| Salmonella Typhimurium ATCC 14028 Salmonella Typhimurium S0584 | 1 µL/mL | 1.5 µL/mL | ||
| Escherichia coli O157:H7 S0575 Escherichia coli O157:H7 ATCC 35150 | 1 µL/mL | 1 µL/mL | ||
| Lavandula angustifolia | Enterococcus faecalis ATCC 29212 Staphylococcus aureus ATCC 25923 | 32 µL/mL | 64 µL/mL | [136] |
| Escherichia coli ATCC 25922 | 128 µL/mL | 512 µL/mL | ||
| Matricaria chamomilla | Staphylococcus aureus ATCC 29213 Staphylococcus aureus ATCC 43300 Staphylococcus epidermidis ATCC 12228 Enterococcus faecalis ATCC 51299 | >4 µL/mL | >4 µL/mL | [137] |
| Melaleuca alternifolia | Lactobacillus spp. | 1 µL/mL | 2 µL/mL | [138] |
| Enterococcus faecalis ATCC 29212 | 64 µL/mL | 64 µL/mL | [136] | |
| Escherichia coli ATCC 25922 | 2 µL/mL | 2 µL/mL | ||
| Staphylococcus aureus ATCC 25923 | 1 µL/mL | 2 µL/mL | ||
| Mentha suaveolens | Salmonella CECT 915 | 0.5 µL/mL | 1 µL/mL | [139] |
| Mentha × piperita | Clostridium perfringens | 10 mg/mL | 10 mg/mL | [140] |
| Ocimum basilicum | 5 mg/mL | 5 mg/mL | ||
| Staphylococcus aureus ATCC 29213 Staphylococcus epidermidis ATCC 12228 | 0.25 µL/mL | 0.25 µL/mL | [137] | |
| Enterococcus faecalis ATCC 51299 | 4 µL/mL | 4 µL/mL | ||
| Pimpinella anisum | Clostridium perfringens | 10 mg/mL | 20 mg/mL | [140] |
| Origanum sp. | Escherichia coli Salmonella Indiana Listeria innocua Staphylococcus aureus | 0.9 mg/mL | 1.1 mg/mL | [141] |
| Origanum elongatum | Escherichia coli 0157:H7 | 0.5 µL/mL | 0.5 µL/mL | [139] |
| Origanum majorana | Clostridium perfringens | 5 mg/mL | 5 mg/mL | [140] |
| Origanum vulgare | Salmonella Enteritidis ATCC 13076 Escherichia coli ATCC 25922 | 320 µg/mL | 320 µg/mL | [142] |
| Salmonella Typhimurium ATCC 14028 | 160 µg/mL | 320 µg/mL | ||
| Staphylococcus aureus ATCC 25923 | 640 µg/mL | >2560 µg/mL | ||
| Methicillin resistant Staphylococcus aureus ATCC 43300 | 320 µg/mL | 1280 µg/mL | ||
| Bacillus cereus ATCC 11778 | 160 µg/mL | 1280 µg/mL | ||
| Origanum vulgare ecotype S | Proteus mirabilis ATCC 25933 Proteus vulgaris ATCC 13315 | 100 µg/mL | 100 µg/mL | |
| Origanum vulgare ecotype SG | Streptococcus faecalis ATTC 29212 | 100 µg/mL | 100 µg/mL | |
| Rosmarinus officinalis | Salmonella spp. (strains: 6554, 6877, 6907, 7643, 9487, 9340, 9681, 9812) # | 12.5 mg/mL | 25 mg/mL | [143] |
| Clostridium perfringens | 10 mg/mL | 10 mg/mL | [140] | |
| Escherichia coli | 4.4 mg/mL | 4.4 mg/mL | [139] | |
| Salmonella Indiana | 8.8 mg/mL | NA | ||
| Listeria innocua | 8.8 mg/mL | NA | ||
| Satureja montana | Salmonella spp. (strains: 6554, 6877, 6907, 7215, 7466, 9487, 9681) # | 0.4 mg/mL | 39 mg/mL | [143] |
| Thymus vulgaris | Salmonella Typhimurium LT2 DT104 Salmonella spp. (strains: 6877, 6907, 7466, 7643, 9487, 9681, 9983) | 1.6 mg/mL | 1.6 mg/mL | [143] |
| Thymus vulgaris thymoliferum | Listeria monocytogenes S0580 Escherichia coli O157:H7 ATCC 35150 | 0.25 µL/mL | 0.25 µL/mL | [116] |
| Salmonella Typhimurium (ATCC 14028; S0584) Escherichia coli O157:H7 S0575 | 0.25 µL/mL | 0.5 µL/mL | ||
| Thymus daenensis | Escherichia coli | 4 mg/mL | 4 mg/mL | [144] |
| Food Group | Food | Essential Oil (or Component) | Targeted Bacteria | Type of Application | References |
|---|---|---|---|---|---|
| Fruits | Table grapes | Eugenol and thymol | Natural microbiota | MAP | [170] |
| Table grapes | Eugenol, thymol, and carvacrol | Natural microbiota | MAP | [171] | |
| Sweet cherries | Eugenol, thymol, menthol, eucalyptol | Natural microbiota | MAP | [172] | |
| Blueberries | Thymol | Escherichia coli O157:H7, Salmonella Typhimurium, Listeria monocytogenes | Washing solution | [173] | |
| Plums | Lemongrass | Escherichia coli, Salmonella Typhimurium | Coating | [174] | |
| Avocado | Thyme | Natural microbiota | MAP | [175] | |
| Pomegranate arils | Satureja hortensis | Natural microbiota | Dipping solution with encapsulation of EO in chitosan nanoparticles | [176] | |
| Fresh cut honeydew melon | Carvacrol, cinnamic acid | Natural microbiota | Dipping solution | [177] | |
| Fresh cut kiwi | Carvacrol, cinnamic acid | Natural microbiota | Dipping solution | [177] | |
| Fresh sliced apples | Hexanal, hexyl acetate, E(2)hexenal | Salmonella enteritidis, Escherichia coli, Listeria monocytogenes | Dipping solution | [178] | |
| Fresh sliced apples | Oregano, lemongrass, |
Natural microflora and inoculated Listeria innocua | Edible coating | [179] | |
| Fresh cut apples | Citron EO, hexanal, E(2)hexenal, Citral, carvacrol | Natural microbiota Listeria monocytogenes, Escherichia coli, Salmonella enteritidis | Dipping solution | [180,181] | |
| Apple pieces | Lemongrass | Escherichia coli, endogenous microflora | Coating | [182] | |
| Fresh cut apples | Vanillin | Escherichiacoli O157:H7, Listeria spp. | Dipping solution | [179] | |
| Fresh cut apples | Eugenol and citral | Listeria monocytogenes and Salmonella Typhimurium | Edible coating | [183] | |
| Cut persimmon | Thyme and lemon EO | Natural microbiota | Washing solution | [184] | |
| Apple juice | Carvacrol, oregano oil, geraniol, eugenol, cinnamon leaf oil, citral, clove bud oil, lemongrass oil, cinnamon bark oil and lemon oil | Escherichia coli O157:H7 | Suspensions of oils in apple juices | [185] | |
| Apple juice | Melissa oil, carvacrol, oregano oil, terpineol, geraniol, lemon oil, citral, lemongrass oil, cinnamon leaf oil, and linalool | Salmonella enterica | Suspensions of oils in apple juices | [185] | |
| Fruit salads | Citral Citron EO | Salmonella Enteritidis, Escherichia coli, Listeria monocytogenes | EO added in the syrup | [186] | |
| Vegetables | Romaine lettuce | Thyme | Escherichia coli O157:H7 | EO added to washing water | [49] |
| Romaine lettuce | Thymol | Escherichia coli O157:H7, Salmonella Typhimurium, Listeria monocytogenes | Washing solution | [172] | |
| Iceberg lettuce | Basil methyl chavicol | Natural microbiota | Washing solution | [63] | |
| Iceberg lettuce | Oregano and rosemary | Listeria monocytogenes, Yersinia enterocolitica, and Aeromonas hydrophila | Dipping solution | [187] | |
| Lamb’s lettuce | Oregano and thyme EO | Natural microbiota Listeria monocytogenes, Escherichia coli | Dipping solution | [188] | |
| Lamb’s lettuce | Oregano and thyme EO | Listeria monocytogenes,
Salmonella enteritidis, Escherichia coli, Staphylococcus aureus | Washing solution | [189] | |
| Lettuce | Oregano EO | Salmonella Typhimurium | Washing solution | [190] | |
| Fresh lettuce | Oregano oil | Escherichia coli , Listeria monocytogenes, Salmonella Typhimurium | Washing in nanoemulsions | [191] | |
| Fresh-cut lettuce | Origanum majorana EO | Natural microbiota | Dipping solutions in combination with ascorbic acid and chitosan | [192] | |
| Rucola leaves | Lemon oil | Natural microbiota | Coating | [193] | |
| Green beans | Tea tree and peppermint EO | Natural microbiota | Dipping solution | [194] | |
| Green beans | Carvacrol | Escherichia coli , Salmonella Typhimurium | MAP | [195] | |
| Green beans | Mandarin oil | Listeria innocua | Combined coating and γ-irradiation treatment | [196] | |
| Carrots | Thyme | Escherichia coli O157:H7 | EO added to washing water | [49] | |
| Fresh Baby carrot | Pullulan–caraway | Salmonella Enteritidis, Staphylococcus aureus | Coating with pullulan films containing EO | [197] | |
| Zucchini | Carvacrol | Escherichia coli | Washing with nanoemulsions | [198] | |
| Spinach leaves | Carvacrol/ Eugenol | Escherichia coli , Salmonella enterica | Washing with nanoemulsions | [199] | |
| Cucumber slices | Carvacrol | Escherichia coli | Coating and combined with pulsed light | [200] | |
| Fresh shredded cabbage | Mint or thyme | Listeria monocytogenes | MAP with EO imbibed in chitosan film | [201] | |
| Broccoli florets | Mandarin | Listeria monocytogenes | Coating | [202] | |
| Four season salad | Oregano EO and citral | Natural microbiota | MAP | [203] | |
| Eggplant salad | Oregano oil | Escherichia coli O157:H7 | EO mixed added directly to the food product | [204] | |
| Fresh leafy vegetables with red beet | Spanish origanum, Spanish marjoram, and coriander | Listeria monocytogenes | Dipping solution | [205] | |
| Fresh-cut vegetables | Thyme, oregano, and rosemary | Listeria monocytogenes | MAP + shredded fresh herbs (thyme, oregano and rosemary) | [206] | |
| Fresh-cut mixed celery, leek and butternut squash | Tea tree | Escherichia coli O157:H7 | Combination of bioactive agents (tea tree EO, propolis extract, and gallic acid) and storage temperature | [207] | |
| Lettuce, carrot and red cabbage | Oregano and citral | Escherichia coli , Salmonella enterica, Listeria monocytogenes and natural microflora | MAP | [208] | |
| Broccoli and radish sprouts | Carvacrol | Salmonella Enteritidis and Escherichia coli O157:H7 | Nanoemulsified carvacrol washing solution | [209] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.I.S.; Marques, C.; Mota, J.; Pedroso, L.; Lima, A. Applications of Essential Oils as Antibacterial Agents in Minimally Processed Fruits and Vegetables—A Review. Microorganisms 2022, 10, 760. https://doi.org/10.3390/microorganisms10040760
Santos MIS, Marques C, Mota J, Pedroso L, Lima A. Applications of Essential Oils as Antibacterial Agents in Minimally Processed Fruits and Vegetables—A Review. Microorganisms. 2022; 10(4):760. https://doi.org/10.3390/microorganisms10040760
Chicago/Turabian StyleSantos, Maria Isabel S., Cátia Marques, Joana Mota, Laurentina Pedroso, and Ana Lima. 2022. "Applications of Essential Oils as Antibacterial Agents in Minimally Processed Fruits and Vegetables—A Review" Microorganisms 10, no. 4: 760. https://doi.org/10.3390/microorganisms10040760
APA StyleSantos, M. I. S., Marques, C., Mota, J., Pedroso, L., & Lima, A. (2022). Applications of Essential Oils as Antibacterial Agents in Minimally Processed Fruits and Vegetables—A Review. Microorganisms, 10(4), 760. https://doi.org/10.3390/microorganisms10040760






